Abstract
The dibenzofuran usnic acid is an important secondary lichen metabolite, having many postulated biological roles, but evidence from field surveys is scanty. Trends in usnic acid concentrations and lobe width in the arctic lichen Flavocetraria nivalis were analysed along local longitudinal and altitudinal gradients in the Kongsfjorden area, northwestern Spitsbergen. Temperature was measured along the same gradients during 1 year. Other environmental variables were also estimated. The model that best explains the variability in usnic-acid levels includes the parameters effective temperature sum, frost sum and temperature range. Temperature range indicates a relationship between high usnic acid levels and humidity, whereas the two first parameters indicate a relationship with low temperatures, which could be direct or indirect. Much of the variability in usnic acid levels and lobe width could not be explained by the selected models. Thus, the secondary metabolism and lobe growth in this lichen is a complex matter, involving numerous environmental and possibly also intrinsic factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lichens constitute a prominent part of terrestrial arctic ecosystems and contribute considerably to carbon sequestration, nitrogen assimilation and nutrient turnover. Many arctic lichens are coloured yellow, orange, brown or black by secondary metabolites that accumulate in the upper cortex. One of the most common lichen pigments is the yellow-green phenolic usnic acid, belonging to the dibenzofurans (Huneck and Yoshimura 1996), which is found in species from a wide range of genera and families. It is a highly interesting metabolite, because it accumulates in high quantities, normally making up from 1% to 9% of the total dry weight of the lichen (Huovinen et al. 1985; Quilhot et al. 1987; 1989; Bjerke et al. 2002; Bjerke 2003). Other lichen metabolites are also often found in such quantities. It is therefore reasonable to believe that usnic acid, and other secondary metabolites, serve important biological functions. Usnic acid is known to have high activity against viruses, microbes, protozoans, insects and fungi (Ingólfsdóttir 2002). It also absorbs efficiently in the ultraviolet (UV) range of the spectrum (Rancan et al. 2002), and therefore probably renders some protection against UV-B radiation (Bjerke et al. 2002; Buffoni Hall et al. 2002).
Lichen metabolites vary in concentration within and between individual thalli, and between populations of the same species. Usnic acid is generally found with the highest concentrations in the youngest thallus zones (Fahselt 1984; Quilhot et al. 1987; Bjerke et al. 2002; Buffoni Hall et al. 2002; Hyvärinen et al. 2002). In some cases, usnic acid concentrations of young thallus zones show high variation between thalli of the same uniform population (Mirando and Fahselt 1978; Fahselt 1984), whereas in other cases, intra-population variability is low in comparison with the variability between populations growing under different climatic conditions (Rundel 1969; Quilhot et al. 1998; Bjerke et al. 2002). Spatial variability in concentration has been explained in various ways. Intrathalline variability is mostly explained by differences in metabolic activity and exposure to stress factors, especially solar radiation (Mirando and Fahselt 1978; Quilhot et al. 1998; Buffoni Hall et al. 2002). Variation between thalli and between populations is found to correlate with variation in some climatic factors, such as solar radiation (Rundel 1969), temperature (Hamada 1991; under laboratory conditions) and altitude (Fernández et al. 1998; Quilhot et al. 1998; Bjerke et al. 2002). When concentrations increase with altitude, UV-B radiation is often mentioned as a possible causal factor, since it is more strongly correlated with altitude than visible light is (Blumthaler et al. 1997), but other factors such as temperature and humidity certainly also vary along altitudinal gradients. Experiments with various bands of UV and visible radiation have shown that UV-B and/or UV-A radiation may in some cases induce the synthesis of usnic acid, but it has also been shown that usnic acid is degraded by shortwave radiation, so results are equivocal (Solhaug and Gauslaa 1996; BeGora and Fahselt 2001a, 2001b; Bjerke et al. 2002; Bjerke 2003; Buffoni Hall et al. 2002).
Thus, there are many uncertainties concerning which environmental variables regulate the biosynthesis and thereby the concentration of usnic acid in nature. Despite these uncertainties, it has been suggested that chemical signatures in lichens, in particular the concentration of usnic acid, may prove useful as a tool for monitoring spatial or temporal variations in UV-B fluxes (Galloway 1993; Rikkinen 1995; Quilhot et al. 1998; Bjerke and Dahl 2002). If this was possible, usnic acid concentrations should be closely correlated with incident radiation, but since lichen metabolites may be altered in response to a number of different stimuli and often do not show any correlation with solar radiation, this potential may be limited (BeGora and Fahselt 2001a; Bjerke 2003).
The objective of this study was to evaluate in detail how the concentrations of usnic acid vary along local climatic gradients in the Arctic, and assess whether there are trends that could be related to any climatic parameters, in particular, various temperature-related variables. Spatial variations in solar radiation and humidity were estimated from temperature-based indices. The results are of importance for evaluating the bioindicator values of chemical signatures in lichens and for explaining the impact of single climatic factors on the synthesis of usnic acid. A coast-inland gradient in the Kongsfjorden area, Svalbard, was selected, because here the same usnic acid-producing species are found in quantities from sea level to mountain summits and from the westernmost cape to the inland glaciers. The Kongsfjorden area offers an almost 1,000-m-high altitudinal gradient of continuous distribution of the studied species, undisturbed by a forest canopy, coupled with a strong oceanity-continentality gradient, from the west to the east. The Arctic is also of interest from an ecoclimatic point of view, since large climatic changes are expected to take place in polar regions (Weller 2000), which could affect lichen viability, distribution and physiology (see, for example, Cornelissen et al. 2001; Solheim et al. 2002; Bjerke et al. 2003), and thereby also usnic acid synthesis.
Materials and methods
Study area
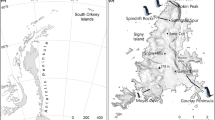
The fiord Kongsfjorden is situated in northwestern Spitsbergen (Fig. 1). The peninsula Brøggerhalvøya delimits the fiord to the south. In the northern side of the fiord, the large island Blomstrandhalvøya is situated close to the south-facing slopes of the major island. In the eastern parts of Kongsfjorden, several arms of the inland glacier reach the sea, but a relatively large and floristically rich ice-free hilly terrain, Ossian Sars-fjellet, is found between the glacier arms. The community Ny-Ålesund is situated halfway between the northwestern cape of Brøggerhalvøya (Kvadehuken) and the glacier Kongsvegen. It has a mean July temperature of 5.1°C and a mean annual precipitation of 385 mm (Førland et al. 1997), and is in the northern arctic tundra zone, but very close to the boundary with the middle arctic tundra zone, which is found to the east and in south-facing areas north of the fiord (Elvebakk 1997).
Temperature monitoring and interpolation
A network of temperature loggers (HOBO H8 Pro Temp/external logger, 64 Kb memory, with an external thermocouple sensor, Onset Computing, Bourne, Mass.) was established in the Kongsfjorden area in July 2000. The values obtained from the loggers were compared with the ones from standard meteorological sensors, and the standard deviation of the differences never exceeded 0.3°C (Météo-France 2000).
As the study focuses on the vegetation-temperature relationship, the sensors were positioned 10 cm above the ground. For that reason, the size of the shelters had to be small. A preliminary test was carried out to define their technical characteristics: white plastic cylinders, 20 cm long and 5 cm in diameter, in oblique positions with their upper ends facing to the north. The structure of the shelter allows air to circulate. The accuracy of the system was tested both in France and in Svalbard with satisfactory results (Météo-France, unpublished report).
The loggers were placed according to two major sampling purposes: an optimal regular sampling of the study area, and an optimal sampling of local environment variation in terms of morphotopography, altitudinal transects on both sides of the fiord from west to east and land cover (Joly et al. 2003). The exact position (longitude, latitude, altitude) of each logger was obtained by using Geographical Position Systems (GPS) (Differential bifrequence GPS, Leica Geosystems Heerbrugg, Switzerland). The primary objective with the temperature monitoring is to use the data for modelling climatic variation in the area (see Brossard et al. 2002), but the data are useful in other ecoclimatic studies as well. The temperature data were downloaded from the loggers in July 2001.
Because the values were observed in punctual field stations, an interpolation process was carried out to allow a cartographical presentation of temperatures in squares of 50 m×50 m resolution. The statistical correlations at meso- and microscales between temperature measurements and variables describing the environmental conditions are systematically explored for recognition of the most significant combination of factors for the spatial model of temperature variation (Joly et al. 2003). The interpolation procedure involves processing temperature values at each pixel, restoring the thermic field with a high accuracy in the entire area. The calculations are made both for the daily maximum and minimum temperatures of the complete observation period to calculate the thermic values that are used as predictor variables in the present study (see below).
Lichen sampling
The lichen cover in this area is extensive, although the reintroduction of reindeer in 1979 led to rapid reductions of lichen mats in certain areas, especially in lower-lying strandflats on Brøggerhalvøya (Wegener et al. 1992; Elvebakk 1997). The usnic acid-producing fruticose species Flavocetraria nivalis (L.) Kärnefelt and Thell is one of the most abundant species in the area (Lynge 1938; Elvebakk 1997). It typically grows on exposed sites that have a discontinuous snow cover in winter. This species was selected for this study, since previous vegetation analyses had shown that it was the most abundant and widespread usnic acid-producing species in the area (A. Elvebakk, personal communication).
From each site where temperature loggers were placed out, ten thalli of F. nivalis were collected during a 2-week period in July 2000. They were randomly sampled from a circular area of 10 m diameter with the logger in the centre of the circle. At a few sites where F. nivalis was sparser, the diameter of the collection area was increased to 20 m. All sampled thalli grew in highly exposed microsites. Thalli growing in shade (e.g. hidden between rocks) were avoided. In total, lichens were collected from 25 sites from sea level to 970 m altitude (Fig. 1). F. nivalis was scattered in the westernmost part, and around one logger placed in a rocky scree at ca. 500 m altitude at Kvadehuken, no thalli of F. nivalis were found. In the remaining area, F. nivalis was abundant. The sites are divided into five geographical groups: western Brøggerhalvøya (sites A–C), Slåttofjellet (sites D–F), Blomstrandhalvøya (sites G–O), Dyrevika (sites P–S) and Ossian Sars-fjellet (sites T–Y). The predominant slope aspect and slope angle of each site were measured. The thalli were air-dried, kept in darkness and transported to the University of Tromsø within 2 weeks after sampling.
Quantitative analyses of usnic acid
Since previous analyses of F. nivalis from other areas had shown that the highest concentrations of usnic acid are found in the lobe tips (Bjerke et al. 2002), and since preliminary tests showed that this was the case for F. nivalis from Kongsfjorden as well, only the outermost 0.5 cm of the thalli were used. Usnic acid was extracted in three series, first in excess acetone for 24 h, then in a 1:1 mixture of acetone and methanol for another 24 h, and finally in pure methanol for 24 h. This was necessary to obtain a complete extraction. The combined extracts were mixed, filtered and diluted to 25 ml. Extracts were analysed quantitatively by reversed-phased high performance liquid chromatography (RP-HPLC) with a mobile phase consisting of methanol and 1% orthophosphoric acid in ultra-pure water, as described by Bjerke et al. (2002).
Measurements of lobe width
F. nivalis has a rather variable morphology, especially concerning height of thalli and cushions and width of single lobes. In areas with high grazing pressure, the lichen tends to be considerably smaller than in other areas. Microclimatic conditions can also affect size. We therefore measured the width of the lobes, which we considered as a more accurate estimate of size than thallus height. The width was measured on air-dried thalli under a stereo-microscope to the nearest ocular unit (0.06 mm), and the narrowest part right below the uppermost branching node was chosen, since this part showed little intrathalline variation.
Environmental variables
The temperature data were used to derive various parameters that contribute in different ways to describe the climatic properties of the studied sites. The following parameters were derived, the first four chiefly in accordance with Tuhkanen (1984):
-
1.
Length of the growing season. It is defined as the period of the year during which growth and other physiological functions in general are possible. Days with daily mean temperatures above 0°C were included here.
-
2.
Effective temperature sum. It is the cumulative number of degrees above a certain threshold (0°C selected here) recorded during a year. Daily mean temperatures were used.
-
3.
Frost sum. It is the “coldness sum” analogous to the effective temperature sum, and is here defined as the cumulative number of daily mean degrees below 0°C recorded during a year.
-
4.
The Conrad index of continentality (C). It is a conventional thermic index that is based on the fact that the annual temperature range is small in an oceanic climate and large in a continental one. For a delimited area as Kongsfjorden with negligible latitudinal differences, the index is practically identical (r=0.990) to the temperature range, i.e. the difference in mean temperature between the warmest and coldest months of the year. We therefore used the temperature range, and not C, in the analyses. Temperature range gives an indication of relative differences in water availability, since oceanic sites are characterised by low ranges and a humid climate, whereas sites that are more continental are characterised by larger ranges and a drier climate.
-
5.
Model-based index of thermophily. Botany-based indices of thermophily are generally used to detect areas on local and regional scales with a high number of temperature-demanding plants (including bryophytes and lichens) and to estimate temperature regimes based on botanical data (Elvebakk 1990; Karlsen and Elvebakk 2003). This approach was taken a step further by Brossard et al. (2002), who developed a model-based index of thermophily primarily used for the Kongsfjorden area. Model-based index values were estimated for each site according to Brossard et al. (2002).
-
6.
Radiation index. Temperature is often closely associated with fluxes of solar radiation. However, other factors such as wind, shading, proximity to cooling water bodies etc. contribute to reduce the correlation between temperature and solar radiation. An index for estimating solar radiation was developed. This parameter is a theoretical index calculated for a cloudless day. For estimating the most potential conditions of incoming energy, global solar radiation is given for the summer solstice (21 June). One value is calculated for each hour of the day, using both solar characteristics (height on the skyline and azimuth) and land characteristics (gradient and aspect) according to the equations given by Dumoulin and Parizet (1987). The mask effect due to the topography is also integrated. Finally, the 24 values are added to obtain a theoretical estimation of global radiation.
In addition, mean temperatures of the months and of the growing season were calculated and used as stand-alone variables in preliminary analyses, but later excluded due to apparent lack of informative data and a need for a reduction of the number of predictor variables included in the model selection analyses.
Longitude, latitude and altitude were also used as predictor variables. The same is the case for slope angle and aspect, but these two parameters were also combined to calculate the heat load index (Geiger 1966), which takes into account that southwest-facing slopes experience the greatest diurnal heat load because of delayed ground heating.
An estimate of grazing and trampling pressure was necessary in order to assess whether the presence of reindeer affects usnic acid concentrations. A five-level scale (0–4) was used, where 0 indicates no observed reindeer and the level 4 indicates high density of reindeer. Little published information on the number of reindeer in other areas than Brøggerhalvøya is available, but our own observations and personal communications indicate that very few reindeer graze in the northern and eastern parts of the study area. Collection date was included as the last predictor variable. Thus, no direct measurements of humidity and solar irradiance were made, but these factors can indirectly be derived from some of the various temperature-based parameters, e.g. effective temperature sum, radiation index and temperature range.
Statistical analyses
Usnic acid concentrations and lobe width data were explored graphically by means of boxplots showing the median, the interquartile distance, 1.5 times the interquartile distance and outliers. The ecological questions we attempted to answer were whether usnic acid concentrations are correlated to any environmental variables, and if so, which are the most important variables. Thus, to find the linear models that best fit the variation in response variables, Akaike’s information criterion (AIC) was used. AIC incorporates the log-likelihood with a penalty for added parameters, and thus aims at finding the best model(s) among the candidate models by making a compromise that few parameters may lead to high bias and that a complex model with many parameters may result in large sampling variance (Akaike 1973; Burnham and Anderson 2002). Since the ratio between number of observations and number of parameters is less than 40, the second-order variant of AIC (AIC c ) was used. Analyses were done by using S-PLUS 6.1 for Windows (Insightful, Seattle, Wash.) with the library MASS enabled (Venables and Ripley 2002). Interaction terms were not included in the analyses since no a-priori support exists for any particular interactions. AIC c values and maximised log-likelihood were subsequently calculated according to Burnham and Anderson (2002).
Results
Usnic acid concentrations
The overall mean concentration of usnic acid from the 25 sites is 6.03% of dry weight lichen. Mean site values range from 4.48% to 9.07%, whereas median values range from 4.25% to 9.03% (Fig. 2). The highest values were found at the summit of the mountain Slåttofjellet (Fig. 2, site F) and near sea level at Kvadehuken (site A). The loftiest site at 970 m (site S) has the third highest mean and median value. No sites have exceptionally low values. Scattered sites from the entire coast-inland gradient have mean and median concentrations between 4.25% and 5.16%. Remarkably, almost all of the easternmost sites (Ossian Sars-fjellet; sites T–Y) have values at the lower end of the range.
Usnic acid concentrations at the 25 sites in Kongsfjorden. The sites are sorted for each geographical group according to altitude. The five groups are: 1 western Brøggerhalvøya, 2 Slåttofjellet, 3 Blomstrandhalvøya, 4 Dyrevika, 5 Ossian Sars-fjellet. Altitude is shown as numbers on the horizontal axis, whereas usnic acid concentrations are shown as percentage of dry weight on the vertical axis. The boxplots show the median (white square within box), the interquartile distance (box), 1.5 times the interquartile distance (whiskers) and outliers (individual horizontal lines). The locations of the sites A–Y are shown on the map behind the boxplots
As the interquartile ranges of the boxplots in Fig. 2 show, some sites experience high variability, despite apparently uniform habitat properties within each site. In particular, site P, a south-facing site near sea level, has high intrasite variability with individual values ranging from the lowest to the highest on the overall range.
Models with the lowest AIC c values are given in Table 1. They explained between 34% and 35% of the variance in usnic acid concentrations. The least complex model of those with almost identical values includes effective temperature sum, temperature range (≈C) and frost sum. The slightly more complex models include the heat load index and inclination. The explanatory variables included in the simplest model indicate that usnic acid concentrations are related to areas that experience low temperatures in both winter and summer (Table 2). In fact, the three sites with the lowest temperature sums (sites S, F and A) have the highest mean usnic acid levels, whereas the three sites with the highest temperature sums (sites T, U and V; all in Ossian Sars-fjellet) have the lowest, the second lowest and the fifth lowest mean usnic acid values. Site F, the one with the highest usnic acid concentration, is also the coldest site in winter (i.e. it has the lowest frost sum). The summit of Blomstrandhalvøya (site K) has the second lowest frost sum, and has the highest mean usnic acid level of the sites on Blomstrandhalvøya. The negative estimate of temperature range (Table 2) indicates a tendency towards higher usnic acid levels in the oceanic sites than in the more continental sites. Most sites have a nonnormal (close to lognormal) distribution of usnic acid levels (Fig. 2). The use of log-transformed data resulted in the same selection of models. The maximised likelihood differs only slightly between the models (Table 1).
Lobe width
The overall mean and median lobe width values are 1.79 mm and 1.70 mm, respectively. Most sites have median values not far from the grand mean (Fig. 3). However, site R and to a lesser extent site Q, differ from the other sites by having broader lobes than elsewhere. In this southwest-facing slope at Dyrevika, F. nivalis is the dominating species, being very abundant from sea level to 1,000 m altitude. Close to site R was also the only place where fertile specimens of F. nivalis were observed. Thus, it appears that the conditions there are very suitable for the growth of this species.
Lobe width measurements from the 25 sites in Kongsfjorden. Sites, boxplots and geographical groups as for Fig. 2
The model with the lowest AIC c value includes 10 explanatory variables, and models with similar values have either 9 or 11 variables (Table 3). Thus, a simple relationship between lobe width and the measured environmental variables was not found. Being such complex models, they do not explain much of the variability in lobe width, only between 36 and 38%. The correlation between usnic acid concentration (response variable) and lobe width (predictor variable) was very low (R 2<0.01 both when all sites are included and when sites Q and R are left out), and not significantly different from 0 (not shown).
Discussion
We have shown here that usnic acid concentrations vary considerably between sites along local longitudinal and altitudinal transects, and that the variability is correlated, at least in part, with climatic factors, with the coldest sites having the highest concentrations. This is the first time that a detailed survey of the spatial variability of usnic acid levels, and levels of secondary lichen metabolites in general, is reported and therefore the first time that such a relationship has been demonstrated in nature. Previous studies along spatial gradients in the field have included comparatively fewer sites with lower control of environmental variables, and have therefore not been able to detect such relationships. The positive correlations with altitude found in some lichen studies, and considered an effect of higher UV-B radiation fluxes at high altitudes (e.g. Fernández et al. 1998; Quilhot et al. 1998; Bjerke et al. 2002; Rubio et al. 2002) may instead be related to the lower temperatures at higher altitudes. No direct relationships with solar radiation were found in the present study, as both altitude and the radiation index were among the first variables to be excluded during the selection analyses. The present results correspond to the results from some recent field experiments where the effects of enhanced UV-B radiation on secondary metabolism were shown to be very modest or absent (Bjerke et al. 2003, Bjerke 2003).
The physiological aspects concerning the impact of low temperatures on secondary metabolism in lichens are not much studied. Lichens from cold environments are generally very tolerant to freezing stress (Kappen et al. 1996). It may be that secondary metabolites contribute to the high tolerance, for example, by reducing ice formation within the cells, either because of hydrophobic properties, or because they can be suspended in the cell sap and thereby lower the freezing point (Avalos and Vicente 1987; Longton 1988; Rikkinen 1995; Huneck 1999). Thus, the trend towards higher concentrations of usnic acid in colder environments may be a protective response against freezing effects. Laboratory experiments have shown that usnic acid accumulates when nutrient availability is low, and degrades under high photosynthetic activity (Vicente et al. 1980; Herrero-Yudego et al. 1989). Another interpretation is therefore that usnic acid may accumulate under low temperatures because of lower photosynthetic rates. However, this interpretation is not supported by BeGora and Fahselt (2001a) whose results indicate that usnic acid is synthesised during periods with a positive carbon balance.
The coldest sites are all situated in the areas most regularly covered by sea fog or clouds (cf. Svendsen et al. 2002). Thus, the observed relationship between coldness and usnic acid concentrations could be indirect, and instead caused by increased dew formation (i.e. increased water availability) due to fog and clouds at the coldest sites. Lichens need to be wet and metabolically active over long periods to be able to synthesise large quantities of secondary metabolites as a response to a regulatory factor, such as solar radiation (Solhaug et al. 2003). Thus, the inclusion of temperature range in the model is probably a reflection of the direct and indirect impact humidity has on usnic acid synthesis, since oceanic (i.e. more humid) sites tend to have higher concentrations than more continental (i.e. drier) sites. Bjerke (2003) also indicated that water availability plays an important role for the usnic acid synthesis in F. nivalis.
If we, despite the results of the model selection analysis, still hold to the hypothesis that usnic acid has a photoprotective role, some support to this hypothesis should be given. It is known that UV repair mechanisms are enzymatic and therefore become increasingly inefficient with decreasing temperatures (Björn et al. 1999; Buffoni Hall et al. 2003). Polar lichens such as F. nivalis are metabolically active even at near-zero or subzero temperatures (Schipperges 1992; Kappen et al. 1996). Thus, F. nivalis is probably often metabolically active under conditions of low temperatures and relatively strong solar radiation. Excessive irradiation could then lead to irreversible damage on molecular structures, for instance, in algal thylakoids. The mycobiont response would therefore be to increase concentrations of usnic acid under low temperatures to compensate for the concurrent decrease in UV repair efficiency of the photobiont. Due to slower desiccation rates, lichens growing in oceanic habitats may be metabolically active more often than lichens from more continental habitats during periods of low temperatures and at least moderate levels of solar radiation. By using the same argument as above, one would therefore expect higher levels of usnic acid in the most oceanic sites. This situation is equivalent to open-shade conditions (see Rikkinen 1995), in which lichens stay metabolically active for much longer periods than in adjacent habitats with higher exposure to direct sun.
The selected models explain only a minor part of the variability in usnic acid concentrations. A large part of the overall variation is caused by intrasite variability, which could not be explained by the models, as we assumed that all thalli within each site grew under identical climatic conditions. Thus, we can only speculate on what causes the high variability within some sites. Fahselt (1984) suspected that high variability within a uniform population was because of innate, genetic differences among thalli, and not a reflection of microclimatic conditions. Biotic factors such as infection of pathogenic fungi and insect herbivory (Ingólfsdóttir 2002) would possibly also result in intrasite variability if the distribution and activity of the attackers were not spatially uniform. Although the collected lichen samples bear extremely few indications of biotic attacks, we should not exclude this possibility, as initial establishment of parasitic fungi and grazing insects are often difficult to detect.
The variability in lobe width was not much explained by the environmental variables included in the analysis. The best models include between 9 and 11 explanatory variables, and they explain not more than 38% of the variability. Site R, which has remarkably wider lobes than the other sites, is probably a site having a good combination of environmental variables, facilitating extensive thallus growth. It has very little grazing pressure, but lichens from other areas with little grazing pressure do not have exceptionally wide lobes. It is one of the sites with the lowest temperature sum, the lowest frost sum and the shortest growing season. Thus, the large thallus sizes at this site may be related to slower desiccation rates, and thereby longer periods of metabolic activity.
The relatively high intrasite variability in lobe width is an important factor for explaining the low correlation coefficients. As for usnic acid, there may be some abiotic or biotic differences on microscale causing the high variability. The lack of correlation between usnic acid concentration and lobe width implies that usnic acid is not highest in small thalli with a thin medullary layer and a larger proportion of the thallus made up by the usnic acid-containing outer cortex. Thus, the variability in usnic acid concentrations cannot be explained by morphological differences between and within sites.
Altogether, we conclude that usnic acid levels are altered by numerous stimuli, among which low temperatures and humidity are of some importance for usnic acid synthesis. It is therefore not likely that usnic acid levels can be used to biomonitor UV-B radiation fluxes as suggested by some authors (see Introduction). There is still much unexplained intrasite and between-site variability in usnic acid concentrations and lobe width that one should attempt to assess in detail through controlled field and laboratory experiments.
References
Akaike H (1973) Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second international symposium on information theory. Akademiai Kiado, Budapest, pp 267–281
Avalos A, Vicente C (1987) The occurrence of lichen phenolics in the photobiont cells of Evernia prunastri. Plant Cell Rep 6:74–76
BeGora MD, Fahselt D (2001a) Usnic acid and atranorin concentrations in lichens in relation to bands of UV irradiance. Bryologist 104:134–140
BeGora MD, Fahselt D (2001b) Photolability of secondary compounds in some lichen species. Symbiosis 31:3–22
Bjerke JW (2003) Arctic-alpine lichens and global change: how do ultraviolet-B radiation and warming affect secondary metabolism, morphological characters and physiological processes? Doctoral thesis, University of Tromsø, Tromsø, Norway
Bjerke JW, Dahl T (2002) Distribution patterns of usnic acid-producing lichens along local radiation gradients in West Greenland. Nova Hedwigia 75:487–506
Bjerke JW, Lerfall K, Elvebakk A (2002) Effects of ultraviolet radiation and PAR on the content of usnic and divaricatic acids in two arctic-alpine lichens. Photochem Photobiol Sci 1:678–685
Bjerke JW, Zielke M, Solheim B (2003) Long-term impacts of simulated climatic change on secondary metabolism, thallus strucure and nitrogen fixation activity in two cyanolichens from the Arctic. New Phytol 159:361–367
Björn LO, Callaghan TV, Gehrke C, Gwynn-Jones D, Lee JA, Johanson U, Sonesson M, Buck ND (1999) Effects of ozone depletion and increased ultraviolet-B radiation on northern vegetation. Polar Res 18:331–337
Blumthaler M, Ambach W, Ellinger R (1997) Increase in solar UV radiation with altitude. J Photochem Photobiol B Biol 39:130–134
Brossard T, Elvebakk A, Joly D, Nilsen L (2002) Modelling index of thermophily by means of a multi-source database on Brøggerhalvøya Peninsula (Svalbard). Int J Remote Sensing 23:4683–4698
Buffoni Hall RS, Bornman JF, Björn LO (2002) UV-induced changes in pigment content and light penetration in the fruticose lichen Cladonia arbuscula ssp. mitis. J Photochem Photobiol B Biol 66:13–20
Buffoni Hall RS, Paulsson M, Duncan K, Tobin AK, Widell S, Bornman JF (2003) Water- and temperature-dependence of DNA damage and repair in the fruticose lichen Cladonia arbuscular ssp. mitis exposed to UV-B radiation. Physiol Plant 118:371–379
Burnham KP, Anderson DR (2002) Model selection and multimodel inference, 2nd edn. Springer, Berlin Heidelberg New York
Cornelissen JHC, Callaghan TV, Alatalo JM, Michelsen A, Graglia E, Hartley AE, Hik DS, Hobbie SE, Press MC, Robinson CH, Henry GHR, Shaver GR, Phoenix GK, Gwynn-Jones D, Jonasson S, Chapin FS III, Molau U, Neill C, Lee JA, Melillo JM, Sveinbjörnsson B, Aerts R (2001) Global change and arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? J Ecol 89:984–994
Dumoulin C, Parizet JC (1987) Astronomie pratique et informatique. Masson, Paris
Elvebakk A (1990) A new method for defining biogeographical zones in the Arctic. In: Kotlyakov VM, Sokolov VE (eds) Arctic research. Advances and prospects. Proceedings of the conference of Arctic and Nordic countries on coordination of research in the Arctic, Leningrad, December 1988, part 2. Nauka, Moscow, pp 175–186
Elvebakk A (1997) Tundra diversity and ecological characteristics of Svalbard. In: Wielgolaski FE (ed) Polar and alpine tundra. Ecosystems of the Worlds, 3. Elsevier, Amsterdam, pp 347–359
Fahselt D (1984) Interthalline variability in levels of lichen products within stands of Cladina stellaris. Bryologist 87:50–56
Fernández E, Quilhot W, Rubio C, Barre E (1998) Lichen’s adaptation to altitude. In: Garab G (ed) Photosynthesis: mechanisms and effects, vol V. Kluwer, Dordrecht, pp 4093–4096
Førland EJ, Hanssen-Bauer I, Nordli PØ (1997) Climate statistics and longterm series of temperature and precipitation at Svalbard and Jan Mayen. Det Nor Meteorol Inst Rapp Klima 21/97:1–72
Galloway DJ (1993) Global environmental change: lichens and chemistry. Bibl Lichenol 53:87–95
Geiger R (1966) The climate near the ground. Harvard University Press, Cambridge, Mass
Hamada N (1991) Environmental factors affecting the content of usnic acid in the lichen mycobiont of Ramalina siliquosa. Bryologist 94:57–59
Herrero-Yudego P, Martin-Pedrosa M, Norato J, Vicente C (1989) Some features about usnic acid accumulation and its movement between symbionts of the lichen, Evernia prunastri. J Plant Physiol 135:170–174
Huneck S (1999) The significance of lichens and their metabolites. Naturwissenschaften 86:559–570
Huneck S, Yoshimura I (1996) Identification of lichen substances. Springer, Berlin Heidelberg New York
Huovinen K, Hiltunen R, von Schantz M (1985) A high performance liquid chromatographic method for the analysis of lichen compounds from the genera Cladina and Cladonia. Acta Pharm Fenn 94:99–112
Hyvärinen M, Walter B, Koopmann R (2002) Secondary metabolites in Cladina stellaris in relation to reindeer grazing and thallus nutrient content. Oikos 96:273–280
Ingólfsdóttir K (2002) Molecules of interest. Usnic acid. Phytochemistry 61:729–736
Joly D, Nilsen L, Fury R, Elvebakk A, Brossard T (2003) Temperature interpolation at large scale; test on a small area on Svalbard. Int J Climatol 23:1637–1654
Kappen L, Schroeter B, Scheidegger C, Sommerkorn M, Hestmark G (1996) Cold resistance and metabolic activity of lichens below 0°C. Adv Space Res 18:119–128
Karlsen SR, Elvebakk A (2003) A method using indicator plants to map local climatic variation in the Kangerlussuaq/Scoresby Sund area, East Greenland. J Biogeogr 30:1469–1491
Longton RE (1988) The biology of polar bryophytes and lichens. Cambridge University Press, Cambridge
Lynge B (1938) Lichens from the west and north coasts of Spitsbergen and the North-East Land collected by numerous expeditions. I. The macrolichens. Skr Det Nor Vidensk-Akad Oslo 6:1–136
Météo-France (2000) Enregistreur de température Hobo. Note technique, documentation DSO, Réf. T010. Météo-France, Paris
Mirando M, Fahselt D (1978) The effect of thallus age and drying procedure on extractable lichen substances. Can J Bot 56:1499–1504
Quilhot W, Leighton G, Flores E, Fernández E, Peña W, Guzmán G (1987) Factores exógenos y endógenos determinantes de la acumulación de ácido úsnico en líquenes. Acta Pharma Bonaerense 6:15–22
Quilhot W, Garbarino JA, Piovano M, Chamy MC, Gambaro V, Oyarzún ML, Vinet C, Hormaechea V, Fiedler P (1989) Studies on Chilean lichens. XI. Secondary metabolites from Antarctic lichens. Ser Cient Inst Antárct Chil 39:75–89
Quilhot W, Fernández E, Rubio C, Goddard M, Hidalgo ME (1998) Lichen secondary products and their importance in environmental studies. In: Marcelli M, Seaward RDH (eds) Lichenology in Latin America: history, current knowledge and applications. Companhia de Tecnologia de Saneamento Ambiental, São Paulo, pp 171–179
Rancan F, Rosan S, Boehm K, Fernández E, Hidalgo ME, Quilhot W, Rubio C, Boehm F, Piazena H, Oltmanns U (2002) Protection against UVB irradiation by natural filters extracted from lichens. J Photochem Photobiol B Biol 68:133–139
Rikkinen J (1995) What’s behind the pretty colours? A study on the photobiology of lichens. Bryobrothera 4:1–239
Rubio C, Fernández E, Hidalgo ME, Quilhot W (2002) Effects of solar UV-B radiation in [sic] the accumulation of rhizocarpic acid in a lichen species from alpine zones of Chile. Bol Soc Chil Quím 47:67–72
Rundel PW (1969) Clinal variation in the production of usnic acid in Cladonia subtenuis along light gradients. Bryologist 72:40–44
Schipperges B (1992) Patterns of CO2 gas-exchange and thallus water content in Arctic lichens along a ridge profile near Ny-Ålesund, Svalbard. Polar Res 11:47–68
Solhaug KA, Gauslaa Y (1996) Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 108:412–418
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV-induction of sun-screening pigments in lichens. New Phytol 158:91–100
Solheim B, Johanson U, Callaghan TV, Lee JA, Gwynn-Jones D, Björn LO (2002) The nitrogen fixation potential of arctic cryptogram [sic] species is influenced by enhanced UV-B radiation. Oecologia 133:90–93
Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S, Ørbaek JB, Bischof K, Papucci C, Zajaczkowski M, Azzolini R, Bruland O, Wiencke C, Winther JG, Dallmann W (2002) The physical environment of Kongsfjorden-Krossfjorden, an Arctic fjord system in Svalbard. Polar Res 221:133–166
Tuhkanen S (1984) A circumboreal system of climatic-phytogeographical regions. Acta Bot Fenn 127:1–50
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, Berlin Heidelberg New York
Vicente C, Ruíz JL, Estévez MP (1980) Mobilization of usnic acid in Evernia prunastri under critical conditions of nutrient availability. Phyton 39:15–20
Wegener C, Hansen M, Bryhn Jacobsen L (1992) Vegetasjonsovervåkning på Svalbard 1991. Effekter av reinbeite ved Kongsfjorden, Svalbard. Nor Polarinst Medd 121:1–54
Weller G (2000) The weather and climate of the Arctic. In: Nutall M, Callaghan TV (eds) The Arctic: environment, people, policy. Harwood, Amsterdam, pp 143–160
Acknowledgements
The first author acknowledges financial support from Kong Haakon den 7des utdannelsesfond for norsk ungdom, from Polarfondet administered by The Committee for the conservation of the polar ship “Fram” at the Fram Museum, and from the Roald Amundsen Centre for Arctic Research at the University of Tromsø. We warmly thank our good colleague and friend Arve Elvebakk, University of Tromsø, for useful suggestions and information before field surveys were undertaken, and for comments on the manuscript, Siw Killengreen, Norwegian Institute for Nature Research, for advice on model selection procedures, and Silke Werth, Swiss Federal Institute for Forest, Snow and Landscape Research, for discussions on multiple regression analyses. The Governor of Svalbard is gratefully acknowledged for permission to collect samples in the Kongsfjorden area, and referees for suggesting topics that subsequently were included in the discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bjerke, J.W., Joly, D., Nilsen, L. et al. Spatial trends in usnic acid concentrations of the lichen Flavocetraria nivalis along local climatic gradients in the Arctic (Kongsfjorden, Svalbard). Polar Biol 27, 409–417 (2004). https://doi.org/10.1007/s00300-004-0590-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-004-0590-8