Abstract
In the current investigation, for the first time, silver nanoparticles (AgNPs) were synthesized using Annona squamosa peel extract as reducing and stabilizing agents via microwave irradiation assisted method. The nanoparticles had been characterized using UV–Vis, FTIR, XRD, and TEM techniques. The biosynthesized nanoparticles exhibited a broad peak at about 430 nm due to the surface plasmon resonance of the AgNPs. From the TEM images, the AgNPs are almost spherical, with an average size ranging from 18 to 35 nm. FT–IR analysis and phytochemical screening indicated that phenolic compounds and proteins of A. squamosa might have an essential role in AgNPs synthesis and stabilization. The antioxidant activity observed was almost comparable to the standard antioxidant agent (ascorbic acid). In vitro antidiabetic studies demonstrated the significant capability of AgNPs to inhibit the α-amylase in a dose-dependent manner. Thus, this green approach of AgNPs synthesis via microwave irradiated heating could be a quick, cost-effective, and environmentally friendly method, with the synthesized AgNPs proving to be a possible anti-diabetic therapeutic candidate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is quickly expanding due to its wide range of applications in science and technology. Metallic nanoparticles like gold, silver, platinum, and palladium have attracted much attention due to their unique size and shape-dependent capabilities, which are useful for applications in various fields such as sensor technology, catalysis, optics, drug delivery, and data storage (Jayaprakash et al. 2017). Silver nanoparticles (AgNPs) are metallic nanoparticles that have a wide range of features, including significant catalytic and antibacterial activity. Nanoparticles from a wide range of materials can be prepared by several methods, including electrochemical, chemical reduction, and heat-induced evaporation (Nasretdinova et al. 2015; Bahiraei et al. 2017). However, most of these methods are costly; nanotechnology is quickly expanding due to its wide range of applications in science and technology. Metallic nanoparticles like gold, silver, platinum, and palladium have attracted much attention due to their unique size and shape-dependent capabilities, which are useful for applications in various fields such as sensor technology, catalysis, optics, drug delivery, and data storage (Jayaprakash et al. 2017). AgNPs are metallic nanoparticles that have a wide range of features, including catalytic and antibacterial activity. Several methods could be used to prepare nanoparticles from various materials and chemicals that require harsh reaction conditions such as high temperature and pressure. Moreover, the use and release of toxic chemicals and solvents could be a potential source of environmental contamination and ecological imbalance. In this regard, biological methods, using eco-friendly solvents and nontoxic reducing agents derived from plants, bacteria, and fungi, have been suggested as possible alternatives (Mohanpuria et al. 2008).
Plant extracts have proven to be more useful than other biological processes since they are easily available, safe to handle, avoid the time-consuming process of cell culture and produce crystalline nanoparticles of various shapes and sizes in a simple, efficient, and low-cost manner (Ahmed et al. 2016). In addition, various metabolites existing in the plants, including sugars, alkaloids, phenolic acids, terpenoids, polyphenols, and proteins, play an important role in the bioreduction of silver ions to AgNPs and their stabilization. As compared with chemical methods, biological methods for nanoparticle synthesis are generally very slow. This limitation can be overcome by integrating biosynthetic methods with microwave chemistry. Microwave-assisted synthesis using plant extracts as both reducing and capping agent is a feasible approach for the rapid and facile green synthesis of metal nanoparticles. It has many advantages, such as short reaction time, lower energy consumption, and better product yield (Nadagouda et al. 2011; Guadie Assefa et al. 2017). Moreover, microwave irradiation offers rapid and consistent heating of the reaction medium and thus results in homogeneous nucleation and growth conditions for nanoparticles (Joseph and Mathew 2015). Several reports on the synthesis of AgNPs-based on the microwave-assisted method have been published (Joseph and Mathew 2014; Kahrilas et al. 2014; El-Naggar et al. 2016).
The fruit peels are generally discarded into the environment, increasing pollution (Deng et al. 2015). However, these peels contain many important phytoconstituents which can be used for the synthesis of metal nanoparticles. There are few reports on using different fruit peels such as banana peel, papaya peel, and orange peel to synthesize nanoparticles (Ibrahim 2015; Balavijayalakshmi and Ramalakshmi 2017; Manal et al. 2014). This work aimed to explore the synthesis of AgNPs from Annona squamosa peel extract via microwave irradiation heating and then test their antioxidant and amylase inhibitory activities. The reason behind the selection of Annona squamosa for nano-synthesis is that this plant possesses several medicinal properties such as cardiotonic, antimicrobial, and insecticidal, and anti-cancerous activities (Gajalakshmi et al. 2011). It also contains various chemicals and secondary metabolites such as proteins, phenolic, flavonoids, triterpenoids, and others that catalyze the formation of tiny and stable AgNPs (Kumar et al. 2021). To the best of our knowledge, no report is available on utilizing microwave irradiation for AgNPs synthesis from Annona squamosa peels and on utilizing synthesized AgNPs in amylase inhibition.
2 Materials and methods
2.1 Materials
Amylase, starch, silver nitrate, DNAS, DPPH·, and acarbose were purchased from Sigma-Aldrich (USA) and Merck (Germany).
2.2 Methods
2.2.1 Synthesis and characterization of AgNPs using peel extract
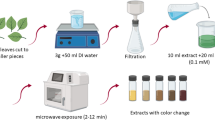
Fresh peels of Annona squamosa were collected, washed meticulously with deionized water, dried at 50°C in a hot air oven for 3 h, and powdered. About 10 g of the powdered peels was taken in a 150 mL beaker containing 100 mL Milli-Q water, mixed well, and boiled for 20 min at 80 °C. The obtained extract was filtered through Whatman No. 1 filter paper. The filtrate was collected and used for the microwave-assisted synthesis of AgNPs. In a typical experiment, 10 mL of the filtrate of the Annona squamosa peel were added to 90 mL of 1 mM AgNOɜ in 250 mL Erlenmeyer flasks to reduce Ag+ to Ag°. The reaction mixture was kept in a turntable domestic microwave oven (LG) operating at a power of 850 W and frequency 2450 MHz for a plus of 180 S, followed by 15 min incubation at room temperature. The resulting solution of AgNPs was purified by repeated centrifugation at 1500 rpm for 15 min. The centrifuging and re-dispersing process was repeated two to three times using Milli-Q water. Finally, the pellet was dried at 50 °C in a hot air oven for 3 h.
2.2.2 Characterization of synthesized AgNPs
The reduction of pure Ag+ ions by peel extract was monitored by measuring the UV–Vis spectrum from 200 to 800 nm of the reaction medium using a UV-Vis spectrophotometer (Shimadzu model UV-1601). FT-IR spectra of peel extract and AgNPs were recorded on Mattson Satellite spectrometer using pressed KBr pellets to determine the type of biomolecules present in the sample extract responsible for synthesis and stabilization of AgNPs. TEM analysis was employed to visualize the shape of AgNPs. A drop of aqueous suspension (50 µL) containing the synthesized AgNPs was placed on the carbon-coated copper grids after allowing the water to evaporate, drying in air. TEM micrographs were taken by analyzing the prepared grids on the JEOL TEM 2100 high-resolution transmission electron microscope. Dispersive X-ray spectra (EDX) EDX was recorded using JEOL- JSM 6360 L A (Japan) to detect elemental silver in AgNPs. The particle size distribution was measured by using ImageJ software analysis of TEM micrographs. X-ray diffraction (XRD) was performed on an X-ray diffractometer (X’pert Pro MRD X-ray diffractometer (PANalytical BV, The Netherlands) operated at 30 kV, and 100 mA and the spectrum was recorded by CuKα radiation with a wavelength of 1.5406 Å in the 2θ range of 10°–80°. The Zeta potential of the synthesized AgNPs was done using dynamic light scattering (Malvern instrument Ltd., Malvern, UK) to check the size distribution and stability of AgNPs.
2.2.3 Phytochemical screening
The preliminary phytochemical screening of aqueous peels extract of Annona squamosal for the presence of phytochemicals viz. carbohydrates, alkaloids, saponins, proteins, amino acids, phenol, diterpenes, tannins, and phytosterols was performed according to the methods described earlier (Kaur 2016; Jayaprakash and Sangeetha 2015; Karthikeyan and Vidya 2019).
2.2.4 Amylase inhibition analysis
2.2.4.1 in vitro α‑amylase inhibition assay
The inhibition assay was performed using the DNSA method according to Olubomethin et al. (Olubomehin et al. 2013) with few modifications. In short, aliquots of 500 µL of each test sample at different concentrations were pre-incubated with 500 µL of α-amylase solution (3 U/mL) at room temperature for 30 min. Then 500 µL of starch solution (1%, w/v) as a substrate was added to each reaction tube, and the reaction mixture was incubated at room temperature for 10 min. The reaction was stopped by adding 1 mL of dinitrosalicylic acid reagent and then heated in a boiling water bath for 5 min. After cooling to room temperature, the reaction mixture in each tube was diluted with 5 mL of distilled water, and the absorbance was measured at 540 nm. The reference sample included all other reagents and the enzyme except the test sample. The α-amylase inhibitory activity was expressed as percentage inhibition. The α-amylase inhibitory activity was calculated according to the equation below
where A0 is the absorbance of the control (blank, without AgNPs) and A1 is the absorbance of the sample.
2.2.4.2 The mode of α-amylase inhibitory activity of synthesized AgNPs
The mode of α-amylase inhibitory activity of biosynthesized AgNPs was determined using Lineweaver and Burk double reciprocal plot. α-Amylase inhibition was determined over a range of starch concentrations (31.2–500 µg/mL) in the absence and the presence of AgNPs (120 µg/mL). α-Amylase enzyme activity was carried out in three independents set of duplicates, and data were presented as mean ± SD.
2.2.5 DPPH· radical-scavenging activity
DPPH· (2,2-diphenyl-1-picrylhydrazyl) scavenging assay was performed as described by Brand-Williams et al. (Brand-Williams et al. 1995). An aliquot of 3.9 mL aliquot of a 6 × 10–5 mol/L of DPPH· in methanol was added to 0.1 mL of samples (including AgNPs, extract, and ascorbic acid as the standard antioxidant) at different concentrations (25, 50, 100, and 200 μg/mL). Thirty min later, the absorbance was measured at 517 nm. The lower absorbance of the reaction mixture indicated higher radical scavenging activity. Radical scavenging activity was expressed as the inhibition percentage of free radicals by the sample and was calculated using the following formula:
where A0 is the absorbance of the control (blank, without AgNPs) and A1 is the absorbance in the presence of the AgNPs. Methanol was used as a blank. The IC50 values (indicate the concentration of the sample (µg/mL) providing 50% inhibition) were calculated from the graph-plotted scavenging percentage against sample concentrations.
2.2.6 Statistical analysis
All the assays were carried out in triplicate, and mean values were reported using the SPSS program (version 13; IBM, Armonk, NY, USA), analysis of variance (ANOVA), and Duncan’s multiple range test differences between the samples.
3 Results and discussion
3.1 Synthesis and characterization of AgNPs
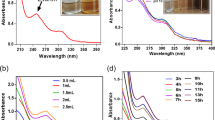
In the present study, the synthesis of AgNPs using aqueous Annona squamosal peel extract as a reducing agent was carried out under microwave irradiation. The resulting formation of AgNPs was monitored by measuring the UV–Vis spectrum of the reaction media at 30 s intervals. Upon microwave irradiation, the color of the reaction mixture gradually changes from colorless to dark brown within 180 s (Fig. 1A). The color change is due to the reduction of silver ions with the help of bio-molecules present in the peel extract (Sankar et al. 2014). Phenols and flavonoids present in Annona squamosal peels act as strong reducing agents by donating electrons to Ag+ ions and reduced to form Ag° nanoparticles (Kumar et al. 2018). The UV–visible spectra of the reaction mixture recorded at 30-s intervals are illustrated in Fig. (1B). After 30 s of microwave irradiation, the biosynthesized nanoparticles exhibited a broad peak at about 415 nm due to the surface plasmon resonance of the AgNPs (Hamelian et al. 2018), and the intensity of which increased with increasing reaction time with a significant change in wavelength to 430 nm. The hydrodynamic diameter and stability of the prepared AgNPs were determined using dynamic light scattering, as shown in Fig. 2A. The average diameter of the synthesized AgNPs and was determined to be 80.1 nm. The particle size distribution of the silver nanoparticles was determined by the polydispersity index (PDI), and it was found to be 0.362, suggesting a narrow size distribution. AgNPs, further, the zeta potential value of the synthesized AgNPs were found to be − 20.4 ± 3.92 mV. A negative charge on the surface of the produced nanoparticles indicates high stability (Kgatshe et al. 2019). The size and morphology of the particles were further determined by Transmission Electron Microscopy (TEM) (Fig. 3A, B). The TEM image of synthesizing AgNPs shows relatively uniform spherical particles with an average size ranging from 18 to 35 nm. The TEM pictures also revealed what appears to be a coating of organic material around the produced AgNPs, which might explain their excellent dispersion in solution. The identification of the elemental compositions for biosynthesized AgNPs was carried out by EDX analysis. EDX profile Fig. 2B reveals a strong elemental signal of silver at approximately 3 keV, which is a typical optical absorption peak of metallic silver nanocrystallites due to surface plasmon resonance (Saratale et al. 2017). The crystalline nature and purity of nanoparticles were also determined using the powder X-ray diffraction technique (Fig. 4B). The XRD patterns of the biosynthesized AgNPs show the crystalline nature of silver with four main characteristic diffraction peaks. The peaks at 38.12°, 44.23°, 64.51°, and 77.69° corresponds to the (111), (200), (220) and (311) planes of the face-centered cubic structure of silver planes, which are identical with those reported in the database of JCPDS card no. 04-0783 for the cubic silver. Additional peaks at 27.82° and 32.20° were also observed (Fig. 5). These peaks could be attributed to the peels extract containing organic compounds responsible for reducing silver ions and stabilizing AgNPs (Basavegowda et al. 2014). FTIR measurements were carried out to identify the functional groups in biomolecules of peel extract responsible for the bio-reduction of Ag+ and capping/stabilization of AgNPs (Fig. 4A). FTIR spectrum of Annona squamosa peel shows different major peak positions at 3386, 2927, 1731, 1612, 1419, 871, 620, and 420 cm−1. These groups were also found in AgNPs, and some peaks were shifted from their original wave number from the peel extract (3413, 2923, 1743, 1612, 1103, 860, 728, and 439 cm−1). The broad and intense peak at 3386 cm−1 corresponds to the OH stretching vibrations of the phenol/carboxylic group present in the extract, a peak at 2927 cm−1 can be assigned to the alkyne group present in phytoconstituents of extract. The peak located at 1612 cm−1 could be assigned to C=O stretching or amide bending. The peak at 1388 cm−1 was assigned to nitro N–O bending and 721 cm−1 assigned to the C-H alkenes stretch. The peak located at 1731 cm−1 could be assigned to C=O aldehyde. The observed peaks are mainly attributed to the presence of some secondary metabolites like flavonoids, triterpenes, tannins, steroids, and saponins excessively present in plant extract, as also suggested by other researchers (Banerjee et al. 2014; Prathna et al. 2011). The existence of flavonoids, proteins, alkaloids, steroids, saponins, and phenolic compounds was confirmed in this study by phytochemical analysis, as shown in Table 1.
3.2 Antioxidant activity
The antioxidant activity of Annona squamosa fruit peels and Annona squamosa fruit peels-AgNPs was examined by DPPH· scavenging assay using ascorbic acid as a positive control. The results on the effect of different concentrations of peel extract biosynthesized nanoparticles, and ascorbic acid on DPPH radical scavenging activity is shown in Fig. 5. The DPPH· radical scavenging tends to increase as the concentration of all tested substances increases.
At doses ranging from 25 to 200 g/mL, the peel extract exhibited a scavenging rate ranging from 12.5 to 87.9%, compared to 19.55–90.04% and 30.96–97.63% for AgNPs and ascorbic acid, respectively. Compared to peel extract and AgNPs, ascorbic acid exhibited the greatest radical scavenging action with the lowest IC50 values (71.9 µg/mL).
Annona squamosa fruit peels extract exhibited the lowest radical scavenging activity with the greatest IC50 (111.2 µg/mL), whereas AgNPs had 104.1 µg/mL. This finding is consistent with the reported DPPH· scavenging activity in the literature (Dhayalan et al. 2017; Pérez et al. 2017). The higher free radical inhibition of nanoparticles may be attributed to the presence of phytochemicals adsorbed onto their surfaces, their excellent dispersion, and their small sizes (Srirranjani et al. 2016).
3.3 Amylase inhibition
3.3.1 In vitro a-amylase inhibition assay
The amylase inhibitory activity of the AgNPs against α-amylase as a representative enzyme was investigated and compared to those of acarbose (standard drug). The results are shown in Table 2 in the form of percent inhibition. The A. squamosa-AgNPs effectively inhibited α-amylase in a dose-dependent manner with the lowest inhibition (20.1 ± 0.5%) at 25 µg/mL and the maximum inhibition (90.4%) at the 200 µg/mL. Similarly, dose-dependent inhibition of α-amylase by acarbose revealed substantial inhibition at all dosages. It varied from 17.90.5 percent at the lowest concentration to 88.40.1 percent at the highest concentration of 200 g/mL. The IC50 value of AgNPs and acarbose was 80 and 88 µg/mL, respectively. The Km, V max values and mode of inhibition of AgNPs synthesized from Annona peel extract (120 µg/mL) on the activity of α-amylase were determined by Lineweaver–Burk plot analysis of data according to Michaelis–Menten kinetics. As shown in Fig. 6, the mode of inhibition of AgNPs synthesized from Annona peel extract on α-amylase activity displayed noncompetitive inhibition. Km of control and AgNPs' inhibited reaction remained constant at 87 µg/mL, whereas Vmax decreased to 0.41 µmol/min comparing with 0.888 µmol/min for control uninhibited enzyme. This finding of inhibition mode was similar to previous studies obtained using AgNPs synthesized using Annona muricata (Badmus et al. 2020; Thatoi et al. 2016a)], Lonicera japonica (Balan et al. 2016), Bauhinia variegata (Johnson et al. 2018), Heritiera fomes, Sonneratia apetala (Thatoi et al. 2016b), and Momordica charantia (Dhivya and Rajasimman 2015). The non-competitive manner of inhibition of AgNPs observed in this work implies that binding of these NPs may have changed the conformation of the α-amylase.
4 Conclusions
To the best of knowledge, this is the first scientific study to examine the usage of Annona squamosa fruit peels extract in the manufacture of stable AgNPs using microwave irradiation and the subsequent in vitro assessment of their anti-diabetic and antioxidant properties. The antioxidant activity of AgNPs was almost comparable to standard vitamin C as estimated through DPPH· test. Furthermore, in vitro, antidiabetic activity studies demonstrated the significant capability of AgNPs to inhibit the α-amylase in a dose-dependent manner. Thus, this green approach of silver nanoparticle synthesis via microwave irradiated heating could be a quick, cost-effective, and environmentally friendly method, with the synthesized silver nanoparticle proving to be a possible anti-diabetic therapeutic candidate.
References
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7(1):17–28
Badmus JA, Oyemomi SA, Adedosu OT, Yekeen TA, Azeez MA, Adebayo EA, Marnewick JL (2020) Photo-assisted bio-fabrication of silver nanoparticles using Annona muricata leaf extract: exploring the antioxidant, anti-diabetic, antimicrobial, and cytotoxic activities. Heliyon 6(11):e05413
Bahiraei M, Naghibzadeh SM, Jamshidmofid M (2017) Efficacy of an eco-friendly nanofluid in a miniature heat exchanger regarding to arrangement of silver nanoparticles. Energy Convers Manag 144:224–234
Balan K, Wang Y, Liu X et al (2016) Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv 2016(6):4016
Balavijayalakshmi J, Ramalakshmi V (2017) Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J Appl Res Technol 15(5):413–422
Banerjee, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresourc Bioprocess 1(3):1–10
Basavegowda N, Idhayadhulla A, Lee YR (2014) Tyrosinase inhibitory activity of silver nanoparticles treated with Hovenia dulcis fruit extract: an in vitro study. Mater Lett 129:28–30
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30
Deng GF, Xu DP, Li S, Li HB (2015) Optimization of ultrasound-assisted extraction of natural antioxidants from sugar apple (Annona squamosa L.) peel using response surface methodology. Molecules 20(11):20448–20459
Dhayalan M, Denison MIJ, Krishnan K (2017) In vitro antioxidant, antimicrobial, cytotoxic potential of gold and silver nanoparticles prepared using Embelia ribes. Nat Prod Res 31(4):465–468
Dhivya G, Rajasimman M (2015) Synthesis of silver nanoparticles using Momordica charantia and its applications. J Chem Pharm Res 107:113–117
El-Naggar ME, Shaheen TI, Fouda MM, Hebeish AA (2016) Eco-friendly microwave-assisted green and rapid synthesis of well-stabilized gold and core–shell silver–gold nanoparticles. Carbohyd Polym 136:1128–1136
Gajalakshmi S, Divya R, Divya V, Mythili S, Sathiavelu A (2011) Pharmacological activities of Annona squamosa: a review. Int J Pharm Sci Rev Res 10(2):24–29
Guadie Assefa A, Adugna Mesfin A, Legesse Akele M, Kokeb Alemu A, Gangapuram BR, Guttena V, Alle M (2017) Microwave-assisted green synthesis of gold nanoparticles using Olibanum gum (Boswellia serrate) and its catalytic reduction of 4-nitrophenol and hexacyanoferrate (III) by sodium borohydride. J Cluster Sci 28(3):917–935
Hamelian M, Zangeneh MM, Amisama A, Varmira K, Veisi H (2018) Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl Organomet Chem 32:e4458
Ibrahim HM (2015) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci 8(3):265–275
Jayaprakash A, Sangeetha R (2015) Phytochemical screening of Punica granatum Linn. peel extracts. J Acad Ind Res 4(5):160–162
Jayaprakash N, Vijaya JJ, Kaviyarasu K, Kombaiah K, Kennedy LJ, Ramalingam RJ, Al-Lohedan HA (2017) Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J Photochem Photobiol, B 169:178–185
Johnson P, Krishnan V, Loganathan C, Govindhan K, Raji V, Sakayanathan P, Vijayan S, Sathishkumar P, Palvannan T (2018) Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: an effective antioxidant scavenger and α-amylase inhibitor. Artif Cells Nanomed Biotechnol 46(7):1488–1494
Joseph S, Mathew B (2014) Microwave-assisted facile synthesis of silver nanoparticles in aqueous medium and investigation of their catalytic and antibacterial activities. J Mol Liq 197:346–352
Joseph S, Mathew B (2015) Microwave assisted facile green synthesis of silver and gold nanocatalysts using the leaf extract of Aerva lanata. Mol Biomol Spectrosc 136:1371–1379
Kahrilas GA, Wally LM, Fredrick SJ, Hiskey M, Prieto AL, Owens JE (2014) Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustain Chem Eng 2(3):367–376
Karthikeyan G, Vidya A (2019) Phytochemical analysis, antioxidant and antibacterial activity of pomegranate peel. Res J Life Sci Bioinform Pharm Chem Sci 5(1)
Kaur N, Kishore L, Singh R (2018) Dillenia indica L. attenuates diabetic nephropathy via inhibition of advanced glycation end products accumulation in STZ-nicotinamide induced diabetic rats. Int J Tradit Complement Med 8(1):226–238
Kgatshe M, Aremu OS, Katata-Seru L, Gopane R (2019) Characterization and antibacterial activity of biosynthesized silver nanoparticles using the ethanolic extract of Pelargonium DC. J Nanomater 2019(2019):1–10
Kumar M, Dandapat S, Ranjan R, Kumar A, Sinha MP (2018) Plant mediated synthesis of silver nanoparticles using Punica granatum aqueous leaf extract. J Microbiol Exp 6(4):175
Kumar M, Changan S, Tomar M, Prajapati U, Saurabh V, Hasan M, Mekhemar M (2021) Custard apple (Annona squamosa L.) leaves: nutritional composition, phytochemical profile, and health-promoting biological activities. Biomolecules 11(5):614
Manal AA, Awatif AH, Khalid MOO, Dalia FAE, Nada EE, Lamia AA et al (2014) Silver nanoparticles biogenic synthesized using an orange peel extract and their use as an anti-bacterial agent. Int J Phys Sci 9(3):34–40
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10(3):507–517
Nadagouda MN, Speth TF, Varma RS (2011) Microwave-assisted green synthesis of silver nanostructures. Acc Chem Res 44(7):469–478
Nasretdinova GR, Fazleeva RR, Mukhitova RK, Nizameev IR, Kadirov MK, Ziganshina AY, Yanilkin VV (2015) Electrochemical synthesis of silver nanoparticles in solution. Electrochem Commun 50:69–72
Olubomehin OO, Abo KA, Ajaiyeoba EO (2013) Alpha-amylase inhibitory activity of two Anthocleista species and in vivo rat model anti-diabetic activities of Anthocleista djalonensis extracts and fractions. J Ethnopharmacol 146(3):811–814
Pérez ZEJ, Ramya Mathiyalagan JM, Kim YJ, Kang HM, Abbai R, Seo KH, Yang DC (2017) Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of them in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int J Nanomed 12:709
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A (2011) Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf, B 82(1):152–159
Sankar R, Manikandan P, Malarvizhi V, Fathima T, Shivashangari KS, Ravikumar V (2014) Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim Acta Part A Mol Biomol Spectrosc 121:746–750
Saratale GD, Saratale RG, Benelli G et al (2017) Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J Clust Sci 28:1709–1727
Srirranjani R, Srinithya B, Vellinngiri V et al (2016) Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J Mol Liq 220:926–930
Thatoi P, Kerry RG, Gouda S, Das G, Pramanik K, Thatoi H, Patra JK (2016a) Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J Photochem Photobiol B 163:311–318
Thatoi P, Gouda SO, Das G et al (2016b) J Photochem Photobiol b: Biol 163:311–318
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassan, AM.S., Mahmoud, A.S., Ramadan, M.F. et al. Microwave-assisted green synthesis of silver nanoparticles using Annona squamosa peels extract: characterization, antioxidant, and amylase inhibition activities. Rend. Fis. Acc. Lincei 33, 83–91 (2022). https://doi.org/10.1007/s12210-022-01049-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-022-01049-w