Abstract
A simple modification on commonly employed biological wastewater treatment process into microbial fuel cell (MFC) could become value added to the treatment unit for removing organic contaminant. The objective of this study was to evaluate the developed membrane-less fixed-bed upflow MFC for generating electricity from synthetic wastewater. The bioreactors were fabricated using cylinder column with 4.5 cm D × 25 cm H and gravel was loaded into the bioreactors for biofilm development. Carbon felts were used as anode and cathode at lower bed and upper bed, respectively, in the bioreactors. The effects of feeding pattern of wastewater, organic loading, biofilm on the cathode, pH at aerobic region of bioreactor, and concentration of KCl on the performance of the MFCs for power generation were evaluated. The optimum power output from the developed MFC was about 28 mW/m2 with 6.28 g/L of sodium acetate as carbon source, pH 6.5–6.6, and 9.5 h of HRT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bioenergy such as hydrogen produced from biomass (Cavattoni and Garbarino 2017) and bioethanol from algae (Hamouda Ragaa et al. 2016) has been studied intensively by researchers worldwide. Simultaneous wastewater treatment and electricity generation using various design of microbial fuel cell (MFC) has been actively researched currently (Zhang et al. 2014; Yoshizawa et al. 2014; Venkata Mohan et al. 2008; Mohanakrishna et al. 2010). In general, MFC is a bioreactor that converts the energy in organic compound to electricity by microbial activity. The main components of the MFC are anode and cathode with a separator such as proton-exchange membrane (PEM) to allow the transportation of proton which generated during the degradation of organic compound by anaerobic microbes. Most of the researchers employed PEM in their MFC to separate the anodic and cathodic chambers and using platinum loaded carbon as cathode electrode to optimize the power generation (Lin et al. 2014; Liu et al. 2013; Mansoorian et al. 2013). The using of platinum as cathode could enhance the reduction reaction of oxygen and subsequently increase the power generation.
The application of PEM and platinum could improve the performance of MFC for converting the chemical energy to electrical energy, but all of this implies high cost and the feasibility for applying the MFC in real wastewater treatment plant is questionable. Thus, the use of biomass as catalyst instead of carbon material loaded with platinum was investigated by some researchers (Bajracharya et al. 2016; Gajda et al. 2014). Despite recent design efforts to enhance the performance of MFC in electricity generation, the main concern should be focused on reducing development and operation costs to apply this technology in wastewater treatment plant. The configuration of MFC should be simple and in-line to the real wastewater treatment process. By simple modification of the wastewater treatment process to be able to convert the organic contaminant to electrical energy will become an added value to the wastewater treatment plant. On the other hand, the integration of wastewater treatment process and MFC may also enhance the organic contaminant removal efficiency due to the increase of bioactivity in the anaerobic region. The objective of this study is to develop an innovative design of membrane-less microbial fuel cell (MFC) for generating electricity continuously from synthetic wastewater. The configuration of the developed MFC is almost similar to the fixed-bed column which is commonly used as biological wastewater treatment process (Rajinikanth et al. 2010; Ghorbani and Eisazadeh 2013) and the effects of some operating conditions on the performance of the MFC were evaluated.
2 Materials and methods
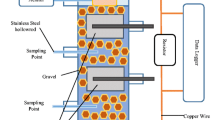
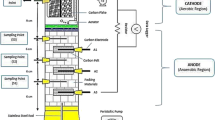
The microbial fuel cells employed in this study were developed using cylinder column with 4.5 cm D × 25 cm H and the schematic diagram of the MFC is shown in Fig. 1. The reactor was filled with gravel until 15 cm height from the bottom of reactor. The gravels used were immersed in anaerobic sludge collected from a municipal wastewater treatment plant. Carbon felt with size 2.5 cm × 3 cm (provided by Maido Corporation and Osaka Gas Chemica, Japan) was used as anode and cathode electrodes at the lower bed and upper bed, respectively, of the reactor. The carbon felts were immersed in aerobic and anaerobic sludge for a week before using them as electrodes in the MFC. The carbon felt at the upper bed was inserted with a small tube for air supply to support the aerobic microbial activity in the inner carbon felt. Besides, air diffuser was also placed below the carbon felt (cathode) to support the oxygen reduction reaction. The carbon rods were inserted into the carbon felts and then connected with copper wire to a 1000 Ω resistor. The voltage generated was recorded for every 30 min with a digital multi-meter (Keithley, 2700).
The MFC was fed with a synthetic wastewater containing carbon source, nutrients, and buffer, and the composition is as follows (in g/L): CH3COONa (1.569), KCl (0.13), NH4Cl (0.31), NaH2PO4 (2.544), and Na2HPO4 (4.0896). The water samples were collected daily for TOC, pH, and ORP analysis.The power density (mW/m2) was calculated according to the following equation:
Here, V is the voltage, R is the resistant, and A is the surface area of anode.
3 Results and discussion
3.1 Effects of the feeding pattern on voltage output
The MFC was fed with synthetic wastewater at flowrate 0.174 mL/min continuously at the initial stage. It was observed that the OCV (open circuit voltage) increased up to 470 mV after operated for 6 h and then decreased to about 350 mV. This might be due to the low HRT (2.4 h) at the anaerobic region of the MFC. As a result, the feeding pump was set with 30 min on and 60 min off, consequently increased the HRT at the anaerobic region to 9.5 h.
As shown in Fig. 2, the OCV dropped as the feeding pump was on and vice versa indicating the alternative on and off of the feeding pump resulted in the increase of OCV. The anaerobic microbial at the bottom of MFC required longer time to degrade the sodium acetate and subsequently released the electron to the anode. Rahimnejad et al. (2011) recorded optimum OCV (880 mV) for their study in the double chamber MFC at HRT of 6.7 h and this indicated that HRT is an important operating parameter for a continuous MFC system which affecting on electrical generation.
Furthermore, as the feeding pump was on, some of the carbon sources from the anaerobic region will be transferred to aerobic region, and the degradation of carbon sources at the aerobic region may lower the OCV output. As the feeding pump was off, the release of electron through anaerobic degradation at the bottom of the MFC increased the different potential between anode and cathode regions. Moreover, the initial stage in biodegradation process is the adsorption of the organic mattes onto biomass followed by degradation of the adsorbed organic matters. In this experiment, the OCV was increased and stabilized at about 635 mV, and thus, the feeding pattern was maintained in the following experiments.
3.2 Effects of the growth of biofilm at cathode on voltage output
The using of oxygen as electron acceptor at cathode is a common practice in MFC (Rahimnejad et al. 2011; Ghangrekar and Shinde 2007; Wei et al. 2012). The oxygen reduction reaction rate can be enhanced using catalyst such as platinum, but it is not economically feasible for application in real wastewater treatment. The biofilm developed on the cathode could act as biocatalyst and mediators to transfer the electron to the oxygen or other electron acceptors (Rismani-Yazdi et al. 2008). As reported by Logan and Regan (2006), sulfate reducing bacteria can transfer an electron directly between membrane and electrode. As shown in Fig. 3, as new carbon felt was used as cathode, the voltage output dropped significantly from about 220 to 145 mV. However, the voltage generated from the MFC improved significantly after 8 h of operation and then increased gradually as the operation proceeded. This could be ascribed to the growth of biofilm onto the surface of carbon felt and then accelerated the transfer of electron to the oxygen. The result shows that the aerobic microbes that attached on the carbon felt may act as biocatalyst to improve the oxygen reduction rate in the cathode. As the growth of biofilm increased until to certain level, the voltage output tended to decrease. This indicates that the over growth of biofilm at the surface of cathode could cause the potential losses which may ascribe to none conductive behavior of the biofilm. Furthermore, the anoxic condition or anaerobic condition at the inner biofilm may contribute to the loss of potential in the MFC. As a result, the control of the growth of biofilm onto the carbon felt is very important for optimization of oxygen reduction reaction rate at the cathode. The using of biocatalyst is more practical compared to other metal oxide as catalyst such as platinum and subsequently reduces the operational cost of MFC.
The power generated from the MFC was monitored after the changing of new carbon felt as cathode. As shown in Fig. 4, it was observed that the maximum power density was 4 mW/m2 as new carbon felt used as cathode. The power density increased as the operation of MFC proceeded and achieved 9 mW/m2 after 9 days of operation. It can be observed that the biofilm was developed on the carbon felt and this indicates the important of the aerobic microbes in cathode for power generation. After the carbon felt was used for 37 days, the power density dropped drastically (~ 50%) ascribed to the over growth of biofilm on the cathode. This shows that the growth of biofilm in cathode must be controlled to achieve optimum power output from the MFC.
3.3 Effects of sodium acetate concentration on power output
The effect of sodium acetate on power generation was investigated by increasing the concentration from 1.569 to 6.276 g/L. The sodium acetate will be oxidized by anaerobic microbes at the lower bed of the MFC and electron generated was transferred to electrode.
As shown in Fig. 5, the power generation from the membrane-less single chamber MFC was increased as the concentration of the sodium acetate increased until three times with approximately 88% improvement of power output. It is expected that the higher the dosage of sodium acetate, the greater the electrons could be generated through degradation from the anaerobic microbes at the lower bed of the reactor. However, the maximum power density generated did not show any significant changes as the concentration of sodium acetate increased to four times. This shows that other factors may become limitation on the power generation of the MFC. Further increase of substrate concentration could lower the power output as higher amount of unutilized sodium acetate may transport to the cathodic region and further mineralized by the aerobic microbes.
3.4 Effects of pH in cathode region on power output
The pH of the influent and effluent in this study was about 7.2 and 9.4, respectively, which shows a significantly increase of the pH in solution, although buffer was used for preparing the synthetic wastewater. This could be due to the reaction at cathode that produced the OH− ion. The imbalance of the pH within the bioreactor showed the different rate of proton and OH− generation at the anodic and cathodic regions, respectively. It was very obvious that the proton availability in the cathode was the limiting factor in the power generation from the MFC. Thus, 1N H2SO4 was added into the cathode region to adjust the pH to various ranges for measurement of power generation from the MFC. As shown in Fig. 6, the power generation was measured with five different pH ranges. As the pH decreased from about 9 to 6.5, the power generated from the MFC was increased 67%. This shows the importance of the pH adjustment in the cathode with external proton addition to improve the oxygen reduction reaction at cathode.
3.5 Effects of KCl concentration on power output
KCl can be used as electrolyte to improve the mass transfer of charged particles. The concentration of the KCl in the synthetic wastewater was increased from 1.7 to 17.5 mM to evaluate the effects on the power generation. As shown in Fig. 7, the increase of KCl from 1.7 to 8.5 mM did not show any significant effect on the power generation, but it hampered the performance once the KCl increased to 17.7 mM. It was reported that 0.15 MK+ could cause 50% inhibition of acetate-utilizing methanogens (Kugelman and McCarty 1964). Although the concentration of KCl employed in this study was only 17.7 mM, the sharp decrease of power density indicated that the reactivity on anaerobic microorganisms was highly affected.
4 Conclusions
The experimental results showed that the membrane-less fixed-bed upflow MFC could generate electricity from the synthetic wastewater. The biofilm developed on the carbon felt cathode could act as biocatalyst for accelerating the oxygen reduction reaction, but the growth must be controlled to optimize the power generation. The imbalance of pH between the anodic and cathodic regions could be adjusted by acid addition and as the pH at the cathodic region decreased from about 9 to 6.5, the power generated from the MFC was increased 67%. In the membrane-less fixed-bed upflow MFC, the concentration of the sodium acetate and HRT also played a very important role in power generation, because low HRT and high organic loading might cause the unutilized substrate being transported from the anodic to cathodic region and lower the power output. In overall, the maximum power output from this study was around 28 mW/m2 using membrane-less and carbon material without platinum loaded MFC. The power output is perfectly within the range (10–100 mW/m2) from literature and a more than satisfactory value.
References
Bajracharya S, Sharma M, Mohanakrishna G, Dominguez Benneton X, Strik DPBTB, Sarma PM, Pant D (2016) An overview on emerging bioelectrochemical systems (BESs): technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew Energy 98:153–170
Cavattoni T, Garbarino G (2017) Catalytic abatement of biomass tar: a technological perspective of Ni-based catalysts. Rend Fis Acc Lincei. https://doi.org/10.1007/s12210-017-0609-z
Gajda I, Greenman J, Melhuish C, Santoro C, Li B, Cristiani P, Ieropoulos I (2014) Water formation at the cathode and sodium recovery using microbial fuel cells (MFCs). Sustain Energy Technol Assess 7:187–194
Ghangrekar MM, Shinde VB (2007) Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour Technol 98:2879–2885
Ghorbani M, Eisazadeh H (2013) Fixed-bed column removal of chemical oxygen demand, anions, and heavy metals from paper mill wastewater by using polyaniline and polypyrrole nanocomposites on carbon nanotubes. J Vinyl Addit Technol 19(3):213–218
Hamouda Ragaa A, Sherif Shaimaa A, Dawoud Gouda TM, Ghareeb Mohammed M (2016) Enhancement of bioethanol production from Ulva fasciata by biological and chemical saccharification. Rendiconti Lincei 27(4):665–672
Kugelman IJ, McCarty PL (1964), Cation toxicity and stimulation in anaerobic waste treatment: II-daily feed studies. In: 19th annual purdue industrial waste conference
Lin CW, Wu CH, Chiu YH, Tsai SL (2014) Effects of different mediators on electricity generation and microbial structure of a toluene powered microbial fuel cell. Fuel 125(1):30–35
Liu L, Tsyganova O, Lee DJ, Chang JS, Wang A, Ren N (2013) Double-chamber microbial fuel cells started up under room and low temperatures. Int J Hydrog Energy 38(35):15574–15579
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(2):512–518
Mansoorian HJ, Mahvi AH, Jafari AJ, Amin MM, Rajabizadeh A, Khanjani N (2013) Bioelectricity generation using two chamber microbial fuel cell treating wastewater from food processing. Enzyme Microb Technol 52(6–7):352–357
Mohanakrishna G, Venkata Mohan S, Sarma PN (2010) Bio-electrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J Hazard Mater 177(1–3):487–494
Rahimnejad M, Ghoreyshi AA, Najafpour G, Jafary T (2011) Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl Energy 88:3999–4004
Rajinikanth R, Thanikal JV, Ramanujam RA, Torrijos M (2010) Anaerobic treatment of winery wastewater in fixed bed reactors. Bioprocess Biosyst Eng 33(5):619–628
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells: an overview. J Power Sources 180:683–694
Venkata Mohan S, Mohanakrishna G, Purushotham Reddy B, Saravanan R, Sarma PN (2008) Bioelectricity generation from chemical wastewater treatment in mediatorless (anode) microbial fuel cell (MFC) using selectively enriched hydrogen producing mixed culture under acidophilic microenvironment. Biochem Eng J 39(1):121–130
Wei L, Yuan Z, Cui M, Han H, Shen J (2012) Study on electricity-generation characteristic of two-chambered microbial fuel cell in continuous flow mode. Int J Hydrog Energy 37:1067–1073
Yoshizawa T, Miyahara M, Kouzuma A, Watanabe K (2014) Conversion of activated-sludge reactors to microbial fuel cells for wastewater treatment coupled to electricity generation. J Biosci Bioeng 118(5):533–539
Zhang F, Ahn Y, Logan BE (2014) Treating refinery wastewaters in microbial fuel cells using separator electrode assembly or spaced electrode configurations. Biores Technol 152:46–52
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ong, SA., Yamagiwa, K. Evaluation on the electricity generation using membrane-less fixed-bed upflow microbial fuel cell. Rend. Fis. Acc. Lincei 29, 103–107 (2018). https://doi.org/10.1007/s12210-018-0665-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-018-0665-z