Abstract
The objective of this study was to evaluate the performance of an upflow membrane-less microbial fuel cell (UFML MFC) with various types of carbon material as cathodes in power output and chemical oxygen demand (COD) reduction. The UFML MFC was designed with carbon felt as anode material, and the bioreactor was filled with 0.6-cm diameter of gravel at the anode region. Carbon flake, Pt-loaded carbon paper, and carbon felt were used as cathode electrodes. The voltage output (power density) for the carbon flake cathode and Pt-loaded carbon paper cathode was 384 ± 16 mV (44.4 ± 2.5 mW/m2) and 399 ± 9 mV (44.1 ± 3 mW/m2), respectively. The percentage of COD reduction at the anode region and effluent was above 75 and 85 %, respectively, for all cathode materials. The coulombic efficiency was 15.95, 6.09, and 15.32 % for Pt-loaded carbon paper, carbon felt, and carbon flake, respectively. The result suggests that power generation and COD reduction were influenced by the cathode material. Carbon flake can be considered as a cost-effective cathode material in UFML MFC for future application in real biological wastewater treatment process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microbial fuel cells (MFCs) have been used for organic wastewater treatment to produce renewable energy (Min et al. 2005; Lu et al. 2009; Fornero et al. 2010). However, the direct implementation of MFC for organic wastewater treatment was not easy. The MFC required a specific design for better performance of electricity generation to overcome economic obstacles (Oh et al. 2010; Wang et al. 2012).
A microbial fuel cell is an ideal fuel cell designed for wastewater treatment to degrade the organic matter by using microorganisms as biocatalyst and simultaneously generate electricity (Cui et al. 2014a, b). The mechanism of MFC is the breakdown of organic matter by anaerobic microorganisms surrounding the anode electrode to generate electrons and protons. The electrons will be transferred from the anode electrode to the cathode electrode through an external circuit. The electrons will then react with the protons transferred from the anode region and oxygen from air to form water. Therefore, the complete transfer of the electrons can generate current in a closed circuit.
The conventional design of MFC usually consists of either a single chamber or double chambers with a proton exchange membrane as separator (Mansoorian et al. 2013; Venkata Mohan et al. 2008) to avoid diffusion of oxygen from the cathode to the anode region. However, there were some MFCs designed without the usage of a proton exchange membrane (Du et al. 2008; Yang et al. 2009), and this would reduce the operation cost for the implementation in larger scale MFC in real wastewater treatment plant. There are quite a number of studies on the comparison of different types of anode electrode materials in the power output, but the comparison on the different types of carbon material as cathode is still limited (Zhou et al. 2011). Moreover, they are focused on the electricity output from the MFC rather than the feasibility of the MFC in wastewater treatment (Liu et al. 2012). The common material used as cathode by other researchers was carbon containing platinum which could enhance the oxygen reduction rate especially in a reactor with single-chamber upflow membrane-less MFC. However, low-cost carbon material could be used as cathode and the growth of biofilm on the carbon electrode may act as biocatalyst to enhance the oxygen reduction rate and subsequently improve the voltage output (He and Angenent 2006). Besides, a catholyte such as potassium ferricyanide was used to increase the mass transfer of the electrons in the reactor (Wei et al. 2012). However, the use of a metal catalyst electrode and a catholyte in the substrate solution could poison microorganisms (Wang et al. 2013). Besides membrane, catholyte, and electrode, other factors, such as type of substrate, organic loading rate, internal resistance of the reactor, operation pH, and temperature (Jana et al. 2010), could also improve the performance of power generation (Gil et al. 2003).
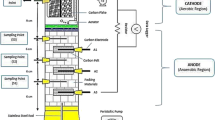
In this study, an upflow membrane-less microbial fuel cell (UFML MFC) was developed with low-cost bio-cathode to treat wastewater and generate electricity simultaneously. This design is more applicable to the real biological wastewater treatment process (Fig. 1). The power output and reduction efficiency of chemical oxygen demand (COD) were investigated with different properties of cathode materials. Carbon flake and carbon felt were used as bio-cathodes to compare the carbon paper loaded with platinum in terms of power output and reduction efficiency of COD. The result demonstrated that carbon flake has better performance in power output and degradation of organic compounds. The result of this study may contribute to the application in real wastewater treatment by using a low-cost MFC technology in the future.
2 Methods and Materials
2.1 Inoculum and Substrates
Synthetic wastewater was used as fuel for this membrane-less upflow MFC. Sodium acetate (1.569 g/L) was the main organic matter in the nutrient solution (pH 6.55) which contains (per liter) the following: NH4Cl 0.31 g, KCl 0.13 g, K2HPO4 3.4 g, KH2PO4 4.4 g, MgCl2 ⋅ 6H2O 0.1 g, and NaCl 0.116 g. Mixed cultures activated sludge was used to inoculate the anode material in a closed, tight container for anaerobic microorganism cultivation. The activated sludge was collected from a wastewater treatment plant of a rubber glove industry (Shorubber (Malaysia) Sdn. Bhd).
2.2 Fabrication of UFML MFC Reactor and Operation
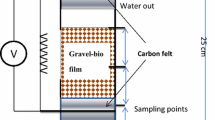
UFML MFC was developed by using an acrylic column. The UFML MFC was a hollow tube cylinder with 4.5-cm diameter and 25-cm height. The UFML MFC was filled with 6 mm of gravel between the anode and cathode regions. The gravel functions as a medium for biofilm growth and the void volume of the reactor was 0.11 L. The gravel and the anode electrode were immersed in anaerobic sludge for a month before setting up the UFML MFC to immobilize the microorganisms on the surface of the gravel and anode electrode. The gravel was used as separator to divide the cathode and anode compartment. There was a total of three anode electrodes placed at different distances from the cathode electrode. Carbon felt was used as the anode electrode with the dimension of 3 cm × 2.5 cm × 2 cm (L × W × H). Three different materials were used as cathode electrode for each UFML MFC in this study to compare the performance in power generation. The cathode materials were carbon felt dimensioned 3 cm × 2.5 cm × 2 cm (L × W × H), Pt-loaded carbon paper 2 cm × 5 cm (L × W), and carbon flake 4.4 cm × 5 cm (D × H).
The synthetic wastewater was pumped into the UFML MFC continuously by using a peristaltic pump (BT100-100M, Longer Precision Pump) at a flow rate of 0.152 mL/min for 1-h intervals on and off per day. The synthetic wastewater flowed upward from the anode region to the cathode region. The anode and cathode electrodes were connected with a copper wire through 1000 Ω resistance. Aeration was provided at the cathode region and the reactors were operated at room temperature.
2.3 Analytical Methods
The voltage of MFC was monitored continuously at 2-min intervals by using a data logger (Graphtec GL820). Various resistors (50–20,000 Ω) were used for polarization test. The half-cell potential of the anode and cathode was measured by using a Ag/AgCl reference electrode in the reactor. The internal resistance was calculated from the slope of the half-cell potential of the anode and cathode.
All samples collected from the anode region and cathode region were centrifuged (CENCE L500) before analysis. A spectrophotometer (DR 2800, Hach) was used to measure the COD. Ohm’s law (V = IR) was used for current (I) calculation where V and R represent the voltage and resistance, respectively. Current density (A/m2) and power density (W/m2) were calculated by the geometrical surface area of the anode electrode. The columbic efficiency (CE) was calculated by the ratio of experimental coulombs to theoretical coulombs transferred. CE = MI (F b q ΔCOD)− 1 where M is the molecular weight of the substrate, I is the stable current, F is the Faraday constant (96,485 C/mol), b is the number of moles of electrons produced per mole of substrate, q is the volumetric influent flow rate, and ΔCOD is the difference in the influent and effluent COD (Logan et al. 2006).
3 Result and Discussion
3.1 Performance of Electricity Generation by Various Cathode Materials
The performance of voltage output in different cathode materials with external resistor 1000 Ω is shown in Fig. 2. The voltage was stable for more than 3 months with continuous flow process. Pt-loaded carbon paper showed the highest voltage output among the cathode materials which was 399 ± 9 mV. Surprisingly, the carbon flake as cathode material also produced a high voltage output, 384 ± 16 mV, which was comparable to Pt-loaded carbon paper cathode. The voltage output of the carbon flake was slightly lower which could be ascribed to the overvoltage caused by the mass transfer loss of fuel in the anode region (Min and Angelidaki 2008). The ability of the mass transfer from the anode region to the cathode region was due to diffusion, migration, and convection, and its resistance can be a limiting factor in electricity generation. The voltage generation was the lowest in carbon felt cathode which was 150 ± 2 mV but showed better stability of the voltage output compared to the Pt-loaded carbon paper cathode and carbon flake cathode. It was observed that the pH was slightly increased from 7.3 (anode region) to 7.5 (cathode region), and this indicated sufficient proton availability to support the oxygen reduction rate. The higher voltage output in the Pt-loaded carbon paper and carbon flake cathodes could be due to the higher oxygen reduction rate on these materials. Meanwhile, insufficient protons in the cathode region may cause slight instability of the voltage output. The pH value at the cathode region increased from 7.3 (anode region) to 9.6 which indicated the loss of protons (Wang et al. 2013).
In this study, DO level in the cathode region varied between 5.5 and 6.3 mg/L, whereas that in the anode region varied between 0.3 and 0.5 mg/L. Gravel was used as a physical barrier between the anode and the cathode regions in UFML MFC. Therefore, oxygen diffusion from the cathode to the anode region may be reduced. However, a slight increase in dissolved oxygen during MFC operation was not found to adversely affect power generation over subsequent cycles if sufficient substrate (acetate) was provided. The performance of MFC was not permanently affected by oxygen diffusion since anaerobic microorganisms can continue to function once oxygen was eliminated from the anodic chamber at any time and MFC power will be restored (Oh et al. 2009).
The polarization curves were obtained when the voltage generation was stable after 3 months of operation by varying the external resistance from 50 to 20,000 Ω. A comparison on power output generation from these three different cathodes is shown in Fig. 3. The highest power density output among the three different cathodes was carbon flake, which was 44.4 ± 2.5 mW/m2 at a current density of 141.41 mA/m2. The power density was 0.3 mW/m2 higher than the carbon paper with Pt-loaded cathode, which was 44.1 ± 3 mW/m2 at a current density of 140.84 mA/m2. Carbon felt cathode generated the lowest power density among all the cathode materials, which was 6.5 ± 0.15 mW/m2 at a current density of 54.26 mA/m2. Based on the polarization curve, the internal resistance was equal to the external resistance at maximum power output. Lower internal resistance can improve the performance of electricity generation (Deng et al. 2010). However, based on Fig. 3, all of the cathode materials have the same internal resistance which was 600 Ω.
The highest power output of carbon flake could be ascribed to the large specific surface area (1100 m2/g) compared to carbon felt (660.5 m2/g) and Pt-loaded carbon paper (0.3 m2/g) (Wang et al. 2013). Carbon flake provides greater contact at the three-phase interface resulting in greater performance in power generation (Cheng and Wu 2013). Therefore, power output of carbon flake was comparable to the small specific surface area but high redox potential of Pt-loaded carbon paper (Zhou et al. 2011). Besides, the larger surface area of carbon flake may enhance the attachment and rapid growth of microorganisms which could act as biocatalyst for electricity generation. However, the surface area of bio-cathode was not normalized for comparison of current and power density (Wei et al. 2011). Carbon felt has a larger specific surface area than Pt-loaded carbon paper but yet low power output.
The half-cell potential of the anode and cathode was measured with a reference electrode (Ag/AgCl). External resistance was varied from 50 to 20,000 Ω. Figure 4 shows that the half-cell potential of the anode for different cathode materials was comparable while it showed difference in cathode potential. The carbon flake cathode showed higher potential than the Pt-loaded carbon paper cathode at any current density. The performance of the carbon felt was much lower than that of the carbon flake cathode and Pt-loaded carbon paper and resulted in lower voltage generation (Pu et al. 2014).
The cathode potential of carbon flake decreased gradually from 326 to 305 mV at 240 mA/m2 with a slope of −0.1075 Ωm2, while the anode potential of carbon flake increased from −375 to 260.6 mV with a slope of 2.8311 Ωm2. Limitation of mass transport could occur due to the increase of the anode potential at higher current density (Min and Angelidaki 2008). The internal resistance of carbon flake can be calculated (2.8311 − (−0.1075)) / 0.0037 and the value was 794 Ω, based on the surface area of the anode electrode (Min et al. 2012). The internal resistance of the Pt-loaded carbon paper was 753 Ω, while carbon felt has higher internal resistance of 803 Ω.
3.2 Performance of COD Reduction
The UFML MFC designed in this study was able to continuously generate electricity and remove organic compounds. The COD reduction efficiency was analyzed in the anode and cathode regions. The COD reduction efficiency for all different cathode materials was almost the same in the anode region and cathode region. The three setup of UFML MFC was different in cathode materials, and the concentration of influent COD for all bioreactors was 1600 mg COD/L at HRT 1 day.
The overall COD reduction efficiency at the anode region was about 75 % whereas the further reduction efficiency of COD at the cathode region was up to 85 %. It was observed that the COD was further reduced by 11, 16.28, and 13.12 % at the cathode for MFC with Pt-loaded carbon paper, carbon felt, and carbon flake, respectively. The surface morphology of carbon felt could be one of the reasons for better COD reduction (Cui et al. 2014a) as the roughness of surface and specific surface area in carbon felt may enhance the attachment and rapid growth of microorganisms (Zhou et al. 2011).
The further reduction of COD at the cathode region could be due to the unutilized organic compound that transferred from the anode region oxidized by the microorganisms that grew at the carbon cathode materials or bio-cathode (Jang et al. 2004). This may inhibit the cathode reaction which could cause the instability of electricity generation or failure to the MFC because aerobic microorganisms have higher affinity for oxygen than the cathode (Moon et al. 2005). Therefore, sufficient supply of oxygen in the cathode region was very important and it could avoid instability of electricity generation. Thus, the further reduction of COD at the cathode region may cause lower CE. As shown in Fig. 5, the COD reduction efficiency was 75 % at the anode region which resulted in the CE performance of 15.95, 6.09, and 15.32 % for Pt-loaded carbon paper, carbon felt, and carbon flake, respectively. The CE performance indicated that electricity generation was not associated with the consumption of organic matter. The low CE may be due to other processes such as biomass production and fermentation in the anode region (He et al. 2005).
4 Conclusions
Electricity generation and wastewater treatment were accomplished simultaneously in this innovative UFML MFC with different cathode materials. The properties of cathode material could influence the power output and COD reduction. However, stable voltage output and high COD reduction efficiency were monitored over a long period of operation. Carbon flake cathode is more cost-effective compared to the costly Pt-loaded carbon paper cathode. The power generation and CE for carbon flake and Pt-loaded carbon paper were comparable. The COD reduction efficiency at the anode region and effluent did not show a significant difference. Therefore, it was found that carbon flake is the best cathode material in UPML MFC. This could be considered as a step towards application of the MFC in real wastewater treatment.
References
Cheng, S., & Wu, J. (2013). Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells. Bioelectrochemistry, 92, 22–26.
Cui, D., Wang, Y. Q., Xing, L. D., & Li, W. S. (2014a). Which determines power generation of microbial fuel cell based on carbon anode, surface morphology or oxygen-containing group? International Journal of Hydrogen Energy, 39, 15081–15087.
Cui, Y., Rashid, N., Hu, N., Rehman, M. S. U., & Han, J.-I. (2014b). Electricity generation and microalgae cultivation in microbial fuel cell using microalgae-enriched anode and bio-cathode. Energy Conversion and Management, 79, 674–680.
Deng, Q., Li, X., Zuo, J., Ling, A., & Logan, B. E. (2010). Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. Journal of Power Sources, 195, 1130–1135.
Du, Z., Li, Q., Tong, M., Li, S., & Li, H. (2008). Electricity generation using membrane-less microbial fuel cell during wastewater treatment. Chinese Journal of Chemical Engineering, 16, 772–777.
Fornero, J. J., Rosenbaum, M., & Angenent, L. T. (2010). Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanalysis, 22, 832–843.
Gil, G.-C., Chang, I.-S., Kim, B. H., Kim, M., Jang, J.-K., Park, H. S., & Kim, H. J. (2003). Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosensors and Bioelectronics, 18, 327–334.
He, Z., & Angenent, L. T. (2006). Application of bacterial biocathodes in microbial fuel cells. Electroanalysis, 18, 2009–2015.
He, Z., Minteer, S. D., & Angenent, L. T. (2005). Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environmental Science & Technology, 39, 5262–5267.
Jana, P. S., Behera, M., & Ghangrekar, M. M. (2010). Performance comparison of up-flow microbial fuel cells fabricated using proton exchange membrane and earthen cylinder. International Journal of Hydrogen Energy, 35, 5681–5686.
Jang, J. K., Pham, T. H., Chang, I. S., Kang, K. H., Moon, H., Cho, K. S., & Kim, B. H. (2004). Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process Biochemistry, 39, 1007–1012.
Liu, J., Qiao, Y., Guo, C. X., Lim, S., Song, H., & Li, C. M. (2012). Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresource Technology, 114, 275–80.
Logan, B. E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J., Freguia, S., … Rabaey, K. (2006). Microbial fuel cells: methodology and technology. Environmental Science & Technology, 40, 5181–5192.
Lu, N., Zhou, S., Zhuang, L., Zhang, J., & Ni, J. (2009). Electricity generation from starch processing wastewater using microbial fuel cell technology. Biochemical Engineering Journal, 43, 246–251.
Mansoorian, H. J., Mahvi, A. H., Jafari, A. J., Amin, M. M., Rajabizadeh, A., & Khanjani, N. (2013). Bioelectricity generation using two chamber microbial fuel cell treating wastewater from food processing. Enzyme and Microbial Technology, 52, 352–7.
Min, B., & Angelidaki, I. (2008). Innovative microbial fuel cell for electricity production from anaerobic reactors. Journal of Power Sources, 180, 641–647.
Min, B., Kim, J., Oh, S., Regan, J. M., & Logan, B. E. (2005). Electricity generation from swine wastewater using microbial fuel cells. Water Research, 39, 4961–8.
Min, B., Poulsen, F. W., Thygesen, A., & Angelidaki, I. (2012). Electric power generation by a submersible microbial fuel cell equipped with a membrane electrode assembly. Bioresource Technology, 118, 412–7.
Moon, H., Chang, I. S., Jang, J. K., & Kim, B. H. (2005). Residence time distribution in microbial fuel cell and its influence on COD removal with electricity generation. Biochemical Engineering Journal, 27, 59–65.
Oh, S. E., Kim, J. R., Joo, J. H., & Logan, B. E. (2009). Effects of applied voltages and dissolved oxygen on sustained power generation by microbial fuel cells. Water Science and Technology, 60, 1311–1317.
Oh, S. T., Kim, J. R., Premier, G. C., Lee, T. H., Kim, C., & Sloan, W. T. (2010). Sustainable wastewater treatment: how might microbial fuel cells contribute. Biotechnology Advances, 28, 871–81.
Pu, L., Li, K., Chen, Z., Zhang, P., Zhang, X., & Fu, Z. (2014). Silver electrodeposition on the activated carbon air cathode for performance improvement in microbial fuel cells. Journal of Power Sources, 268, 476–481.
Venkata Mohan, S., Veer Raghavulu, S., & Sarma, P. N. (2008). Biochemical evaluation of bioelectricity production process from anaerobic wastewater treatment in a single chambered microbial fuel cell (MFC) employing glass wool membrane. Biosensors & Bioelectronics, 23, 1326–32.
Wang, Y.-P., Liu, X.-W., Li, W.-W., Li, F., Wang, Y.-K., Sheng, G.-P., … Yu, H.-Q. (2012). A microbial fuel cell-membrane bioreactor integrated system for cost-effective wastewater treatment. Applied Energy, 98, 230–235.
Wang, H., Jiang, S. C., Wang, Y., & Xiao, B. (2013). Substrate removal and electricity generation in a membrane-less microbial fuel cell for biological treatment of wastewater. Bioresource Technology, 138, 109–16.
Wei, J., Liang, P., & Huang, X. (2011). Recent progress in electrodes for microbial fuel cells. Bioresource Technology, 102, 9335–44.
Wei, L., Han, H., & Shen, J. (2012). Effects of cathodic electron acceptors and potassium ferricyanide concentrations on the performance of microbial fuel cell. International Journal of Hydrogen Energy , 37, 12980–12986.
Yang, S., Jia, B., & Liu, H. (2009). Effects of the Pt loading side and cathode-biofilm on the performance of a membrane-less and single-chamber microbial fuel cell. Bioresource Technology, 100, 1197–202.
Zhou, M., Chi, M., Luo, J., He, H., & Jin, T. (2011). An overview of electrode materials in microbial fuel cells. Journal of Power Sources, 196, 4427–4435.
Acknowledgments
The authors would like to acknowledge the financial support of eScience Fund (Grant No. 02-01-15SF0201) provided by the Ministry of Science, Technology and Innovation (MOSTI), Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thung, WE., Ong, SA., Ho, LN. et al. Simultaneous Wastewater Treatment and Power Generation with Innovative Design of an Upflow Membrane-Less Microbial Fuel Cell. Water Air Soil Pollut 226, 165 (2015). https://doi.org/10.1007/s11270-015-2410-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2410-x