Abstract
Water pollution is a pressing issue due to growing levels of industrialization and increasing amounts of domestic wastewater. The possibility of harvesting energy from waste has captured the attention of the scientists across the globe. In this regard, the membrane-less microbial fuel cell (ML-MFC) has come across as a sustainable choice of technology for energy generation along with wastewater treatment. This unit behaves like a bioreactor relying on the bacteria that act as a biocatalyst oxidizing the organic matter to produce electricity. In this chapter, the focus is laid on the influence of chemical and physical parameters of the ML-MFC unit on the productivity of the process from electricity generation and wastewater treatment aspects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anode
- Cathode
- Conductivity
- Electricity generation
- Membrane-less microbial fuel cell
- Operating temperature
- pH
- Reactor design
- Substrate pretreatment
- Wastewater treatment
8.1 Introduction
Since the last few decades, immense thrust on the sustainability factor has driven the scientific community to seek ways of addressing the issues causing harm to the ecosystem (Nastro 2014). On the other hand, concerns regarding the depletion of crude oil and the consequential rush to find alternative energy sources have also gained momentum. In view of this, microbial fuel cells (MFCs) have become of global interest as a sustainable technology with immense potential to generate electricity from organic matter in wastewaters, concurrently treating the wastewaters and contributing to environmental remediation (Feng et al. 2008). The MFC technology holds promise to fulfill at least a part of the future energy needs (Logan 2010). Moreover, it is self-sustaining, affordable, and does not require huge capital investments.
The MFCs function by converting the chemical energy derived from the organic matter present in the wastewater directly into electrical energy, with the help of electrogenic bacteria working as a biocatalyst (Min and Logan 2004). An MFC primarily consists of three parts: an electrode system with a container, a microorganism culture (anaerobic or aerobic) in a growing medium, and a substrate solution to nourish the microbes (Cheng et al. 2006). The MFCs are designed with different configurations, in general, they are classified as single-chamber or dual-chamber MFCs (Khan et al. 2018). Most single-chamber MFCs usually contain a proton-exchange membrane (PEM) to separate the anode and the cathode compartments. However, in most cases, the membrane offers a considerable internal resistance that negatively influences the electrochemical performance of the MFCs. Besides, high costs of the membrane have rendered conventional MFCs commercially unviable (Logan 2010). Recent efforts have been directed to achieve high output without a membrane, thus giving rise to the concept of membrane-less microbial fuel cell (ML-MFC), as depicted in Fig. 8.1. The presence of an additional anode layer (in the middle of the cell) in Fig. 8.1b acts as a barrier preventing the movement of organic materials towards the cathode, which holds the key to high efficiency (Kim et al. 2016). Thus, the ML-MFCs overcome the drawback of conventional MFCs since the membrane limits the functioning of the MFC by decelerating the transfer of protons through it (Jang et al. 2004).
Schematic representation of an ML-MFC (a) single-chamber unit, (b) dual-chamber unit. (Redrawn with modifications from Ref. Kim et al. (2016))
There are several major advantages offered by an ML-MFC with regard to the energy output, process economics, environmental impact, as well as the overall feasibility (Fig. 8.2). Firstly, ML-MFC does not require any energy input from an external source. Secondly, it can operate at ambient temperature and pressure conditions. Thirdly, it is eco-friendly as it does not produce any toxic by-products. It can help to curtain the environmental pollution levels, thus prevent damage to the planet (Yang et al. 2009). Fourthly, its operation without an ion-exchange membrane decreases the ohmic cell resistance, along with the manufacturing cost of the reactor (Clauwaert and Verstraete 2009). Finally, depending on the design, it can have a long operational lifespan with durability in keeping up the efficiency (Zhang and Ye 2015).
Advantages of MFCs. (Redrawn with modifications from Ref. Li et al. (2014))
The process of bio-electricity generation in most kinds of MFCs is similar, where the substrate with microorganisms is contained in the anode compartment (if the anode is partitioned from the cathode using a separator) of the MFC chamber (Lin et al. 2013). The microorganisms play the role of a catalyst in the process of energy generation, producing electrons and protons along with carbon dioxide (CO2) gas. With the passage of time, they create a biofilm on the anode surface thus catalyzing the anaerobic bacterial oxidation (Li et al. 2014; Waller and Trabold 2013). The protons or hydrogen ions (H+) move from the anode towards the cathode, while the electrons transfer through an external electrical circuit connected to the cathode (Lin et al. 2013). Oxygen, which acts as the electron acceptor, is pumped from the air through the catholyte into the cathode compartment or, it is directly received if the cathode is in contact with the air (air-cathode) (Zhang and Ye 2015). In the cathode compartment, hydrogen ions and oxygen react with the help of electrons to form water or hydrogen peroxide (Luo et al. 2017). The cathode electrode is generally coated with a catalyst such as platinum to assist in the reaction of hydrogen ions with oxygen, or in other words, to facilitate the oxygen reduction reaction (Rahimnejad and Najafpour 2011). The basic chemical reactions taking place at the electrodes with the organic materials are depicted by Eqs. (8.1)–(8.3). The specific reactions may vary in anode compartment according to the type of substrate used; however, the products remain the same with altered stoichiometric coefficients (Khan et al. 2018). At the anode, the partition reaction in the case of glucose as substrate occurs as indicated by Eq. (8.1).
At the cathode, the partition reaction occurs as indicated by Eqs. (8.2) and (8.3).
where, ΔGo = − 5792.2 kJ/mol.
The substrate, which functions as a fuel for the MFC unit, contains organic compounds that are degraded by the microorganisms during the process of oxidation (Pauline and Boopathi 2018; Luo et al. 2007). Agri-food industries produce a lot of waste that is easily broken down by bacteria and can be a good substrate for MFCs. Other common substrates may include glucose, acetate, as well as wastewaters from the domestic household, dairy, slaughter-house, refinery, and dyeing industry (Pallavi and Udayashankara 2016; Jothinathan et al. 2018; Savizi et al. 2012). The functioning of MFCs is influenced by a number of chemical, physical, and biological factors that have a significant impact on the overall efficiency of the unit. The physical and chemical factors include the reactor design (Jang et al. 2004; Luo et al. 2007; Liu and Logan 2004; Ye et al. 2018); pH (Gil et al. 2003; Jadhav and Ghangrekar 2009); temperature (Feng et al. 2008; Jadhav and Ghangrekar 2009; Ahn and Logan 2010); electrode surface area with electrode spacing (Ghangrekar and Shinde 2007; Cheng and Logan 2011); and the pretreatment of the influent (Jadhav and Ghangrekar 2009; Ghangrekar and Shinde 2008; Yang et al. 2013). The biological factors include the type of microbial culture used, and the conditioning of the substrate for the microorganisms (Malvankar et al. 2012; Hassan et al. 2014). Many varieties of exogenous bacteria have been studied for their capability to produce electrons (Cheng and Logan 2007). A major issue in ML-MFCs is the mixing of the anolyte with the catholyte. This causes a decline in the efficiency of the cathode electrode due to biofouling. The ultimate aim is to reduce the internal resistance of the unit, enhance the maximum conversion rate of the organic materials inside the substrate, and increase the efficiency of the anode and cathode electrodes (Rabaey and Verstraete 2005).
The concept of an MFC was first postulated by Michael Cressé Potter from Escherichia coli and Saccharomyces with platinum electrodes way back in 1912, but its low power intensity led to little interest in it at the time (Yang et al. 2011). In the early 1990s, MFCs regained interest as a promising technology for bioenergy and wastewater treatment after substantial advancements lead to improved output efficiency (Rahimnejad et al. 2015). In 1999, Kim et al. reported the first mediator-less MFC (Logan 2008). Several developments have been made since then leading to increased power density of MFCs from less than 1 W/m3 to over 4000 W/m3. Many microorganisms have the ability to produce electrons from the metabolism of organic matter (Cheng et al. 2006; Liu et al. 2004). There are different methods by which the bacteria transfer electrons to the anode: transfer of electrons through mediator, direct transfer of electrons, and transfer of electrons through bacterial nanowires (Khan et al. 2018). The bacterial culture needs a favorable environment to thrive; the pH and temperature greatly affect the bacterial activity, thus influencing the performance of the reactor. Several reviews have been published on MFCs, each with a different flavor or emphasis in terms of the design features, substrates, microbial metabolism, their performance and challenges (Hindatu et al. 2017; Wei et al. 2011). In this chapter, the focus lies on the major chemical and physical factors impacting the performance and efficiency of ML-MFCs for power generation as well as water treatment. It brings up the recent advances in the ML-MFC technology and is expected to contribute to the future efforts in its development.
8.2 Electricity Generation
Bacteria are the crux of the MFC technology with the ability to produce and transfer electricity through their pili to their surroundings. Many factors play roles in the efficient harnessing of energy and accelerating the oxygen reduction reaction (ORR) such as the use of different substrates, pretreatment of the substrate, anode and cathode electrode performance, and the design of the reactor.
The design of MFC is the most significant factor influencing the electricity production and water treatment capability and overall process efficiency (Pant et al. 2010). One of the main design factors is the membrane, which has a huge impact on the productivity as well as the cost of MFC. Many studies have investigated and compared the performance of MFCs with and without membranes, under the same experimental conditions. For example, Sung and his team worked on improving the cathodic ORR without precious metals using a carbon cathode and replacing platinum as a catalyst due to its high cost. They compared a single-chamber MFC (SMFC) (air-cathode and without membrane) with a two-chamber MFC (TMFC) (consisting of PEM); and observed that the ML-MFC gave the highest power and ORR activity. This was due to the enormous internal resistance offered by the ion-exchange membrane, which is obvious from the resistance values of 45 and 80 Ω for SMFC and TMFC, respectively (Song et al. 2020). Another study found that an ML-MFC produced 140% higher power output of 520–570 μW with more stability compared to an MFC with ceramic membranes and salt-bridge connection (You et al. 2020). Similarly, MFC configurations having a metal anode with carbonaceous cathode showed that the ML-MFC dominated MFC with membrane giving superior performance in terms of maximum power density (MPD) (Yamashita et al. 2016). Thus, it is evident that the use of either salt-bridge or membrane contributes to a direct rise in the internal resistance of the reactor, which is a detrimental factor for achieving higher output power and overall efficiency (Min et al. 2005). It is not surprising that single-chamber ML-MFCs are preferable over the MFCs with membranes, and recent efforts have been directed towards the improvement of their performance to make them commercially successful (Logan 2010).
As stated, many factors influence the performance of MFCs and the final output of the reactor is a synergistic combination of each of the influencing factors, thus making the direct comparisons of the effect of individual factors among different studies difficult. Most studies on MFCs have probed the effects of pH variation, substrate type, design configuration, operating temperature, electrode materials, the oxygen amount in the anode, diffused oxygen sneaked into the system, etc. Therefore, the evaluations and comparison will be based on rational thoughts and research outcomes, and available theories and facts.
8.2.1 Anode and Cathode Electrodes
MFC has come across as a sustainable concept not only for electricity generation but also as a solution for the environmental concerns facing the globe (Palanisamy et al. 2019). The choice of electrodes is a crucial and challenging aspect in the design of MFCs for optimum performance. Efforts for the improvement of anode are directed towards the “optimal provision for bacterial attachment” and “better capability of collecting of more electrons from the bacteria and the medium” (Hindatu et al. 2017). For the cathode, its performance in terms of oxygen and hydrogen reaction (ORR) significantly affects the coulombic efficiency (CE) of the MFC and helps to boost the flow of electricity, durability, and long-term steadiness (Santoro et al. 2013; Jiménez González et al. 2020). Also, the placement of the cathode in the ML-MFC plays an important role. If the MFC is designed with an air-cathode, it faces some concerns like water loss, ORR activity, oxygen intrusion, cathode deterioration, biofilm formation from the substrate-faced side, and anode performance and capability for fostering bacterial cultures. Many studies have been published in the last two decades dealing with these issues and their solutions. Although there has been a considerable progress in the electrodes to overcome their poor performance, some issues still remain unsolved that have made MFC technology unviable for large-scale operations (Zhou et al. 2012).
Till date, various materials have been explored for the development of electrode materials for the MFCs. Carbonaceous materials (like carbon cloth, carbon felt, graphite) and metallic materials (like copper, zinc, stainless steel) are widely used for making anode and cathode electrodes (Fig. 8.3) (Santoro et al. 2017). Carbon is abundantly available in nature, and possesses exceptional and unique characteristics like interaction with the electroactive-biofilm, conductivity, and the durability in toxic environments. For these reasons, carbonaceous materials find extensive applications as electrodes for MFCs (Santoro et al. 2017). Several analytical techniques such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD) are commonly employed to characterize the structure of the electrode material. Most studies have evaluated the electrodes for their electrochemical performance (ORR) by cyclic voltammetry and electrochemical impedance spectroscopy, whereas some others have focused on oxygen diffusion through the cathode, MPD, CE, and chemical oxygen demand (COD).
Several types of carbonaceous and metallic materials used for anode and cathode electrode. (Published from Ref. Santoro et al. (2017) under CC BY 4.0 license)
8.2.1.1 Cathode Electrode
As mentioned, carbonaceous materials have unique properties such as electrical conductivity (Saba et al. 2017), corrosion resistivity (Slate et al. 2019), mechanical strength (Jia et al. 2018), high surface area (Yang et al. 2018), biocompatibility (Zhao et al. 2018), chemical stability (Cai et al. 2020a), environmental safety and low cost (Liu et al. 2020). In a study, a carbon cathode was prepared from mixed carbon powder (Vulcan XC-72) and 30 wt% polytetrafluoroethylene (PTFE) solution, coated with different numbers of diffusion layers (DLs) from the airside. The optimum performance was obtained with four DLs, which significantly improved the CE from 19.1% to 32%, while the MPD increased by 42% compared to an uncoated carbon cathode. Also, the open-circuit potential analyses revealed that the maximum potential difference between the cathode having four DLs and the uncoated cathode was 117 mV at 0.6 mA/cm2. Obviously, the oxygen permeability and water loss from cathode decreased with increasing number of DLs (Cheng et al. 2006). Any attempt for sealing the cathode further lead to higher internal resistivity like in case of the MFC with PEM. For sealing the cathode to have lower resistivity, a spun-bonded olefin sheet was used in a study instead of a PTFE coating. In the beginning (on day 5), this cathode produced an MPD of 750 mW/m2, current density of 2.0 A/m2 (32 A/m3), a CE of 55%, low resistance of about 4 Ω, and total internal resistance of 131 Ω, causing no water leakage in the cathode and a drop in resistance by 400%. After day 42, the overall internal resistance built up to 160 Ω due to the loss of platinum catalyst by 8.26% (w/w) and development of the bacterial biofilm on the catalyst, thus limiting the ORR ability. Finally, after 53 days, the cathode potential declined gradually to 280 mW/m2 and 1.4 A/m2 (Tugtas et al. 2011). In another work, different ratios of PTFE to carbon (200%, 100%, 80%, and 60%) were tested on the cathode by Guerrini et al. They found that a high PTFE ratio prevented the transfer of the protons and inhibited the ORR electrocatalysis. The optimum ratio was between 60% and 80%, which produced a current of 700 μA after 52 days. It was observed that more carbon contact enhanced the current production. Oxygen was not a limiting factor, instead, the biomass activity slightly influenced the cathode (Guerrini et al. 2015).
Despite all the attempts for improving the performance of the cathode, there is an increase in the resistance due to the coated layers and the biofilm formation over the cathode. Furthermore, the erosion of the cathode causes development of the cracks, which leads to wetting of the cathode with subsequent decrease in its ORR activity. Therefore, different materials with different properties have been explored for making cathode, producing a varying range of voltage and current. One of the reasons that make MFC non-commercial is the use of precious metals like platinum as a catalyst on cathode for increasing the ORR activity (Song et al. 2020). Many studies have been conducted on the cathode of an ML-MFC with efforts to achieve high ORR activity at a reasonable cost. Recently, in a research conducted by Song et al., nitrogen- and phosphorus-doped ordered mesoporous carbon (NPOMC) was used as a catalyst replacing platinum (Song et al. 2020). As elucidated in Fig. 8.4, the phosphate active sites on the mesoporous carbon surface are a doping center for the ORR, where the nanoporous structure of the catalyst helps to intensify the ORR activity and the nitrogen-doping sites cause a change in the charge distribution. The NPOMC in the ML-MFC gave only 30% of the ORR activity of Pt/C (154.0 mW/m2) with 30–40% lower MPD. Moreover, gradual formation of biofilm on the surface of the catalyst (biofouling) blocked the nano-structured active sites of the NPOMC and drastically affected its performance and stability. The electrochemical performance showed results gave a current of 1.33 mA and an internal resistance of 286 Ω after 30 days of operation (Song et al. 2020).
Surface morphology of nitrogen- and phosphorus-doped ordered mesoporous carbon (NOPMC) illustrating the mechanism of oxygen reduction reaction. (Reproduced with permission from Ref. Song et al. (2020) © 2020 Elsevier publisher)
In another work, canvas cloth was used for the fabrication of electrodes. The non-conductive material was made to conduct electricity by coating it with one of nickel or graphite along with manganese dioxide (MnO2) as catalyst. The nickel-coated canvas produced a better result than the graphite-coated canvas with an MPD of 86.03 mW/m2, COD reduction of 95%, and CE of 30.2% (Zhuang et al. 2009). In yet another study, bamboo charcoal coated with platinum was used as a cathode in an ML-MFC giving maximum power and voltage of 1.16 mW and 0.50 V, respectively (Yang et al. 2009). Feng et al. employed stainless steel coated with polypyrrole/anthraquinone-2-sulfonate film as a cathode. It produced an MPD of 575 mW/m2, and the cathode showed a great ability to reduce oxygen and inhibit water leakage (Feng et al. 2011). Iron-based catalysts such as ricobendazole and niclosamide could be a possible alternative for platinum on the cathode electrode, as they have shown 20–25% higher efficiency than that of platinum (Santoro et al. 2016). Similarly, the ability pristine graphene to enhance extracellular electron transfer has been exploited for platinum-free electrodes, producing a volumetric power of 3.51 W/m3 (Call et al. 2017). Thus, it can be concluded that sealing of the air-cathode electrode, choice of electrode material and cathode coating catalyst are crucial considerations to achieve a high ORR activity. Still, there is immense scope for improvement of the MFC with lots of undiscovered possibilities for the cathode—the main challenges being long lifetime, stable performance, and cost of the electrodes.
8.2.1.2 Anode Electrode
The anode material and its arrangement play a vital role in attachment of the bacteria, enrichment of the biofilm, oxidation of the substrate, as well as transfer of electrons between the bacteria and the electrode, which in turn affect the final output. Several methods of modification such as surface treatment (ammonia gas treatment (Cheng and Logan 2007), heat treatment (Feng et al. 2010), acid treatment, electrochemical oxidation), surface modification with nanomaterials, and surface coating with conductive polymers (Hindatu et al. 2017) have been used to improve the overall performance of an ML-MFC. All main parameters used for assessing the cathode electrode also hold true for the anode electrode; in addition, the anode should also be bio-compatible with the bacteria (Kumar et al. 2013).
A study used an anode made of carbon cloth treated with ammonia, while the cathode was carbon cloth with platinum catalyst. This combination led to a substantial improvement in CE by 20% compared to the untreated anode, and an MPD increment from 1640 to 1970 mW/m2. The power attained was 7.5 times higher than that from the untreated electrode, and the start-up time was reduced by 50% (Cheng and Logan 2007). In another study, carbon fiber brush treated with acid and heat was used as the anode produced an MPD of 1370 mW/m2, which was greater by 34% and 7% than that achieved by using untreated electrode and only heat treated electrode, respectively (Feng et al. 2010). Likewise, modification of the anode was done using nitric acid (CM-N) and ammonium nitrate (CM-A). CM-N performed better than CM-A giving an MPD of 792 mW/m2 and CE of 24%, which were greater than that achieved using an untreated anode by 43% and 71%, respectively. Also, the modified anodes showed deep cracks and rough surface that aided in improved electron transferring property (Zhou et al. 2012). An MPD of 1788 mW/m3 was achieved in a similar study by treating the anode surface with nitric acid and ammonia (Yang et al. 2014).
Lin et al. worked with different materials (stainless steel, copper, gold, and graphite carbon cloth) as electrodes. Copper anode showed erosion, while hindered electron transfer was observed in case of stainless steel. On the other hand, the gold electrode showed a very high performance. The best result in terms of open-circuit voltage (0.49 V) was achieved with the gold anode and carbon cloth cathode (Lin et al. 2013). Another study used an anode of graphite felt coated with iron oxide (Fe2O3) and ferric oxyhydroxide (FeOOH) giving an MPD of 18 W/m3 (Wang et al. 2013). A voltage of 573 mV and an MPD of 884 mW/m2 were obtained when polyaniline was used with a graphene-modified carbon cloth as an anode (Huang et al. 2016). A flame-oxidized stainless steel anode resulted in an MPD of 1063 mW/m2 that was 24% higher compared to the untreated anode. Furthermore, it was also appreciably higher by 323% than the same MFC configuration with membrane (Yamashita et al. 2016).
Peng et al. added 5% of nickel-iron oxide (NiFe2O4) to the anode to achieve an MPD of 806.4 mW/m2, reducing the internal resistance by 39% compared to the untreated anode (Peng et al. 2017). Using indium tin oxide coated glass as an anode produced the voltage and power output of 471 mV and 418.8 mW/m2, respectively. However, its COD removal efficiency was lower than the carbon brush (Jiang et al. 2018). Another study found that the use of carbonized cotton textile modified with molybdenum carbide nanoparticles as an anode delivered an MPD of 1.12 W/m2. This material offered a super performance in conductivity, high biocompatibility, strong electrochemical activity, and cost-effectiveness (Zeng et al. 2018). Graphene coated with iron sulfide (FeS2) nanoparticles was employed as an anode on different substrates, achieving an MPD of 3220 mW/m2 and an outstanding current density of 3.06 A/m2 with COD removal of 1319 mg/l (Wang et al. 2018). Many studies have shown tremendous progress by improving the anode with different materials, nanoparticles and composites to give superior electrochemical performance. It is of prime significance to check the compatibility of the treated anode with bacteria, its conductivity and durability to ensure optimum efficiency.
8.2.2 Effect of Operating Temperature
Literature has proven that temperature has a great impact on the performance of ML-MFC by affecting the growth and survival of microbial communities, the conductivity of the substrate solution, internal resistance, and start-up time (Gadkari et al. 2020). A study on a single-chamber, air-cathode ML-MFC compared two operating temperatures (20 and 30 °C) and found a stable power density of 187 ± 8 mW/m2 and CE of 10% at 30 °C. When the reactor was adjusted to 20 °C, MPD and CE decreased to 155 mW/m2 and 8.9%, respectively. Moreover, as the temperature decreased from 30 to 20 °C, the cathode potential showed a massive drop by 315%; while the anode electrode potential lessened by 21% (Feng et al. 2008). Another work examined ML-MFC under different ranges of temperature, i.e. 20–35 and 8–22 °C. The higher working temperature range resulted in higher COD removal of 90%, lower current of 0.7 mA, and CE 1.5%. On the contrary, at lower temperature range, the COD removal reduced to 59%, current rose to 1.4 mA, and CE increased to 5%. Thus, the lower temperature range favored the current and coulombic efficiency, while higher temperature worked better for COD removal (Jadhav and Ghangrekar 2009). However, a different trend was observed in a continuous flow ML-MFC operating at 30 °C, which showed a power density of 422 mW/m2 (12.8 W/m3), COD removal of 26%, and CE of 1.7%. At ambient temperature (23 °C), the power density reduced to 345 mW/m2 (10.5 W/m3), COD removal reduced to 19%, while CE dropped to a mere 0.7% (Ahn and Logan 2010). Another study analyzed the effects of varying temperature in the range 15–35 °C, and found the best results at 35 °C showing MPD, current and CE of 74 mW/m3, 2.51 mA, and 10%, respectively (Tee et al. 2018).
The start-up temperature must be taken into consideration for maintaining longer stability of power in batch and continuous mode ML-MFCs. Generally, the microbial community’s sensitivity towards temperature decides on the maximum operating temperature, beyond which the bacterial activity degenerates. In most studies, the observed optimum temperature range from 20 to 32 °C favored the growth of methanogenic bacteria and demonstrated a linear rise in the power output. The highest limit of operating temperature is usually 35 °C, above which the reactor efficiency begins to decline.
8.2.3 Effect of pH
The bioactivity of the microbial community is considerably dependent on the pH of the substrate (Marashi and Kariminia 2015). In a study, an increase of the pH value above 8.5 led to the precipitation of carbonates on the cathode surface in batch mode of ML-MFC reactors. The deposition of the carbonate layer acted as a barrier and decreased the electrochemically-active area, similar to the PTFE effect on cathode activity (Guerrini et al. 2015). Another work tested the effect of pH in the range 5.5–7.5, and found that the internal resistance decreased as the pH difference between the anode and cathode solutions increased. The highest current was generated within an optimum pH range of 6.0–7.0 indicating the lower microbial activity at sub-optimal pH compared to the optimal pH (Jadhav and Ghangrekar 2009). Similar observations were found in yet another study that concluded the optimal pH of an ML-MFC as 7 (Gil et al. 2003). The effect of three different pH conditions (5.5, 7.0, and 8.5) was assessed on an ML-MFC. The highest power density was observed at pH 8.5 that was greater by 40% and 66% than the power densities observed at pH values of 7.0 and 5.4, respectively. This was evident as even though acidogenic and methanogenic bacteria are inactive in alkaline conditions, electrogenic bacteria are active in that environment (Marashi and Kariminia 2015). When the pH of the anodic solution increased from 5.4 to 9.0 due to the hydrolysis of the urea, it caused a decrease in the anodic performance, implying that the anode can take a limited pH working range (Santoro et al. 2013). The acidogenic bacteria have been shown to be active at pH 5.5, where the hydrogen production dominates overcoming the degradation of the pollutants and decreasing COD removal, as compared to neutral or alkaline conditions (Marashi and Kariminia 2015). In contrast, alkaline environment is favorable for the electrogenic bacteria leading to a higher power generation (Yuan et al. 2011).
8.2.4 Effect of Substrate Pretreatment
The quality of the substrate fed to the reactor is a crucial factor influencing the performance of the ML-MFC. The organic molecules need to be decomposed and dissolved in the substrate for the bacteria to be able to consume them. In a study, a sludge was kept in the anode chamber for 15 days, followed by heat treatment at 100 °C and cooling to room temperature. It was then re-inoculated, while no inoculation was done for the cathode. As a result, the COD removal efficiency reached 91.4% at an organic loading rate (OLR) of 2.65 kg COD/m3.d, giving a maximum power density of 6.73 mW/m2, and a current density of 70.74 mA/m2 (Ghangrekar and Shinde 2008). In a similar way, a power density of 0.32 ± 0.01 W/m2 was obtained with a sludge fermented for 9 days at 30 °C prior to dilution with the primary effluent, whereas the untreated primary effluent gave a power density of 0.24 ± 0.03 W/m2. The fermentation caused a reduction in the total suspended solids from 26.1 to 16.5 g/l, and the pH from 5.7 to 4.5. Additionally, it increased the conductivity from 2.4 mS/cm to 4.7 mS/cm (Yang et al. 2013).
8.2.5 Effect of Reactor Design
The design of ML-MFC reactor is the cornerstone for deciding the type of cathode material and assembly suitable for the reactor. The cathode can be either submerged completely in the substrate or it can be placed as an air-cathode electrode, where one half has contact with the substrate and the other half is exposed to the air. For the air-cathode, the ORR reaction occurs with the oxygen in the air; while for the submerged cathode, air is supplied through a compressor. The anode electrode is placed inside the substrate.
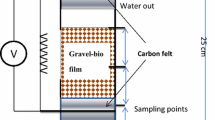
In a study, an ML-MFC was designed with a cylindrical shape having a diameter of 10 cm and a height of 100 cm. Graphite felt was used as the anode (surface area of 465 cm2) and placed at the bottom of the reactor; with glass wool (4 cm depth) and glass bead (4 cm depth) placed above the anode, as shown in Fig. 8.5. The cathode, made of graphite felt (surface area of 89 cm2), was placed at the top of the reactor and the compartment was aerated. The electrode spacing was varied between 10 and 30 cm. The inlet of wastewater was from the bottom and after passing through the layers, it was discharged from the top. The set-up yielded a power density of 1.3 mW/m2 at a current density of 6–9 mA/m2 (Jang et al. 2004). A similar design by Ghangrekar and Shinde gave the maximum voltage of 358 mV, power density of 10.9 mW/m2, COD removal of 88%, and BOD removal of 87% (Ghangrekar and Shinde 2007). A two-chambered cylindrical ML-MFC of Plexiglas was designed with a diameter of 75 mm and a height of 100 mm. The two chambers were separated by a carbon paper, and the electrodes too were made of carbon paper. The cathode electrode was coated with platinum and dipped into the cathode chamber. After 400 h long run, the maximum voltage output of 551 mV and power density of 121 mW/m2 were attained (Luo et al. 2007). In another work, the cathode and anode compartments were placed at different levels, the cathode chamber was located above from the anode chamber so that the outlet of anode was connected to the inlet of cathode through a valve. The substrate was driven by gravity into the anode from the storage container that was placed at a higher level than the cathode. The air was pumped into cathode chamber, the influent entered from the anode and went through the connection to the cathode. The reactor assembly resulted in a maximum voltage of 160.7 mV, an MPD of 24.33 mW/m3, COD removal efficiency of 90.45% (Du et al. 2011).
Cylindrical ML-MFC with anode and cathode placed inside the reactor. (Redrawn with modifications from Ref. Jang et al. (2004))
The cylindrical reactors have the advantage of capturing maximum number of protons escaping from the anode, where the influent enters in from the bottom of the reactor and leaves from the top. The influent comes in contact with the cathode as it emerges out of the reactor, which accelerates the movement of hydrogen from anode to cathode. Moreover, the speed of the fluid flow affects the electricity production and the treatment of influent. Despite many advantages of the cylindrical reactor, there are some drawbacks associated with these types of designs. Firstly, the spacing between the two electrodes affects the reactor output and needs to be optimized. Secondly, there is a requirement for external air pumping into the cathode compartment. Thirdly, when the cathode is completely immersed in the substrate, it leads to the rapid formation of the biofilm resulting in biofouling, thus lowering the performance of the cathode. Finally, the cathode area may not be enough for the reactor; the literature shows that the optimal ratio of the cathode size to the anode size must be 2:1 or higher (Cheng and Logan 2011).
Another variation in the reactor design could be wherein the cathode is placed outside the reactor. In a study, a cylinder-shaped reactor made of Plexiglas was designed having 3 cm diameter, 13 cm height, and evenly drilled holes on the wall, as shown in Fig. 8.6. The cathode was a cylinder wrapped with a flexible carbon cloth coated with C/Pt, as an air-cathode. Carbon granules, with a surface area of 31 cm2, were used as the anode. The inlet was from the bottom, while the effluent exited from the top. The reactor displayed a voltage of 0.384 V with a maximum volumetric power of 50.2 W/m3 at a current density of 216 A/m3 (with an internal resistance of 27 Ω) (You et al. 2007). Furthermore, this design helped to have a longer lifespan and higher performance of the cathode, simultaneously decreasing the overall internal resistance by minimizing the distance between the cathode and the anode.
Cylindrical ML-MFC with anode inside and cathode placed outside the reactor. (Reproduced with permission from Ref. You et al. (2007) © 2007 Elsevier publisher)
A modified ML-MFC configuration was developed to solve the issue of distance between cathode and anode. The design consisted of a twin cylindrical compartment with a volume of 1.85 l, an air-cathode made of carbon cloth coated with platinum from the air-facing side, and a brush-type anode made of carbon cloth connected with titanium wires. The anode and cathode were placed on each end of the compartment, with the cathode being at a distance of 1 cm from the anode. The design resulted in an MPD of 39–53 mW/m2 from the cattle manure solid waste (Lee and Nirmalakhandan 2011). Liu and Logan used a Plexiglas cylindrical container open from both the sides with 4 cm length and 3 cm diameter, as shown in Fig. 8.7. The anode and cathode electrodes were placed on opposite sides of the cylinder. The anode was made of carbon paper and the cathode was made of carbon cloth coated with platinum on the air-facing side. The inner side of the cathode was examined with and without PEM. The MFC in the absence of PEM could achieve an MPD of 146 mW/m2 with 20% CE using domestic wastewater as the substrate; while in the presence of PEM, it produced an MPD of 28 mW/m2 at 28% CE (Liu and Logan 2004). A similar reactor was built with a glass tube placed on top of the reactor (4 cm long and 1.4 cm inner diameter) with a perforated cap to help the aerobic bacteria access air. The anode was made of carbon fiber brush, and the cathode was made of carbon cloth coated with platinum. The results showed an MPD of 268.5 mW/m2, COD removal of 67%, phosphorus removal of 97%, and ammonia removal of 99% (Jiang 2017). It implies that the productivity of small-sized reactors is generally higher than that of big-sized reactors due to the lower internal resistance of the former. However, a disadvantage of these designs is that the mixing of cathodic and anodic compartments is inevitable, thus creating biofouling on the inner side of cathode.
Design of a lab-scale single-chamber ML-MFC. (Reproduced with permission from Ref. Liu and Logan (2004) © 2004 ACS publisher)
In a different work, a micro-sized ML-MFC was designed with dimensions of 15 × 5 mm, thickness of 0.37 mm with a volume of 83 μl, with the anode and cathode made of carbon cloth (Fig. 8.8). Glass fiber was placed between the electrodes to alleviate the mixing of the fluids from the two compartments and for assisting in hydrogen transfer. The electrons were transferred through the titanium foils connected to each electrode. The electrodes were held with the help of acrylic cover plates. The cell was sealed with a silicone pad stacked vertically, resulting in an MPD of 3.2 mW/cm3 (Ye et al. 2018).
Design of a millimeter scale ML-MFC. (Published from Ref. Ye et al. (2018) under CC BY-NC 3.0 license)
Many efforts have been made for using biochar as electrode to develop economical and environmentally-friendly MFCs without compromising on their performance. A study fabricated a cathode using a bamboo tube, by carbonizing it at 900 °C in nitrogen atmosphere followed by heat treatment at 350 °C to increase porosity (Fig. 8.9). The cathode was also brushed with polytetrafluoroethylene solution on the external side to make it water proof. A carbon fiber brush was used as the anode. The cell produced an MPD of 40.4 W/m3 and CE of 55% (Yang et al. 2017). The biomass materials can be a great replacement for expensive electrode materials for ML-MFCs due to their renewability, wide availability, and low cost. One of the approaches to better design with low cost is preventing mixing of anolyte with catholyte, minimizing oxygen intrusion, avoiding biofilm formation, and alleviating cathode deterioration. This can be achieved by putting a separator or an additional anode in the container to create a two-chamber ML-MFC (Kim et al. 2016). Recently, Nawaz and his team assembled a conical dual-chambered ML-MFC fabricated from graphite-based materials, as shown in Fig. 8.10. The cathode chamber was concentrically placed inside the anode chamber with 4 mm space in between them. The anode was sealed from the top with an acrylic lid, and air was pumped into the cathode. This design accomplished a treatment efficiency of 84.4% and MPD of 15.03 W/m3 (Nawaz et al. 2020).
(a) Fabrication process of cathode from bamboo tube; (b) MFC with cathode made from bamboo tube. (Published from Ref. Yang et al. (2017) under CC BY 3.0 license)
Schematic representation of an ML-MFC with two containers concentrically placed inside one another. (Reproduced with permission from Ref. Nawaz et al. (2020) © 2020 Elsevier publisher)
8.2.6 Effect of Electrode Surface Area and Electrode Spacing
The surface area of electrodes and the spacing between them are crucial factors to be taken into account for optimum performance of the ML-MFCs. A study investigated the effects of varying the electrode spacing (20, 24, and 28 cm) and anode area. It was found that more MPD could be achieved when the electrodes were placed close to each other. The maximum voltage of 358 mV was obtained at a distance of 20 cm. The power densities of 4.66, 6.45, and 10.13 mW/m2 were achieved at anode surface area of 210.64, 140.43, and 70.21 cm2 respectively. It shows that the power density decreases with increasing surface area of the anode (Ghangrekar and Shinde 2007). The trials for scale-up of ML-MFC showed that doubling the cathode surface area increased the power output by 62% and doubling the anode surface area increased the power output by 12% (Cheng and Logan 2011). In an experiment with a three-column MFC connected in series, an enhancement in the anode surface area from 360 to 1080 cm2 in each column increased the maximum power output by 264% for column 1, 118% for column 2, and 151% for column 3. Also, the COD and BOD removal efficiencies were increased by 137% for column 1, 279% for column 2, 182% for column 3, and 63% for column 1, 161% for column 2 and 159% for column 3, respectively (Gálvez et al. 2009).
8.2.7 Effect of Substrate Conductivity
The conductivity of the substrate is another factor having a profound influence on the output performance of the ML-MFCs. One of the ways of enhancing the conductivity is by adding metal ions to the substrate. However, the addition of metal ions creates a toxic environment for the microorganisms. So MFCs are limited by the requirement to compromise between the toxicity issue and conductivity of the substrate (Dong et al. 2015). It has been found that when the ions are added, the MPD initially increased linearly with rising electrolyte conductivity, the MPD escalated from 0.11 W/m2 at a conductivity of 1e−4 Sm−1 to 1.02 W/m2 at a conductivity of 1e−2 Sm−1. Further increase in conductivity beyond 2e−1 Sm−1 showed no more improvement in MPD (Gadkari et al. 2020).
In a trial, phosphate buffer was added to the substrate solution to increase its conductivity, which increased the power density from 1330 to 1640 mW/m2 (Cheng and Logan 2007). In a similar test, increasing the concentration of phosphate buffer from 50 to 200 mM caused the MPD to rise from 438 to 528 mW/m2 (Feng et al. 2008). In another study, addition of sodium acetate solution to the substrate was found to enhance the overall power density to 400 mW/m2 (Jiang and Li 2009). Likewise, putting as little as 0.5% of NaCl and Na2SO4 (1:1 ratio) changed the power density from 34 to 43 mW/m2 and augmented the phenol removal rate by 4%. Increasing the amount of salt to 1% raised the MFC power density to 45 mW/m2, whereas 2% salt resulted in an inhibitory effect on power generation (Du et al. 2015; Mousavi et al. 2016). Thus, inorganic salts can greatly improve the conductivity and decrease the resistance in the solution, thus boosting the efficiency of ML-MFCs.
Most studies are in agreement with each other, where the output power increases with increasing ionic strength of the substrate. Higher conductivity implies better ionic conduction and ohmic loss reduction, which results in the improvement of power output from the ML-MFCs. Having said that, there is a limit to the degree of salinity that the substrate solution can take. It is important to ensure that the bacterial cultures do not get negatively impacted by the high salinity of the solution, since high content of salt may be intolerable for bacteria (Mousavi et al. 2016; Aaron et al. 2010). Thus, the bacterial response to external stimuli as regards the substances added to improve the ionic strength of the substrate should be carefully evaluated.
8.3 Water Treatment (Substrate)
The substrate is of paramount importance in any biological process, serving as the nutrient and energy source, and has a tremendous impact on the energy production in ML-MFCs (Yang et al. 2009). A countless variety of substrates comprised of organic matter in pure forms or diverse mixtures from wastewaters or lignocellulosic biomass are widely used in MFCs (Pant et al. 2010). Several ways are available for treating the wastewaters, the most common method being the aerobic activated sludge process, which requires pumping large amounts of air or oxygen into the reserve tanks (Capodaglio and Olsson 2020). The oxygen supplying expenses are estimated to be between 50% and 70% of the entire energy demand of a conventional facility (Capodaglio and Olsson 2020). Here, we focus on the ability of ML-MFC in wastewater treatment in respect of its organic, inorganic, and heavy metal content. Different researchers have connoted the ML-MFC performance with different measurable parameters such as current (or current density in mA/cm2 or mA/m3), voltage (open circuit or close circuit), COD/BOD reduction, MPD, CE, inorganic or heavy metal reduction to evaluate the efficiency of various substrate.
Many studies have attempted to maximize the efficiency of ML-MFCs for treating wastewater substrates containing organic materials as well as producing power. According to a study, glucose and wastewater produced an MPD of 494 and 146 mW/m2 with CE of 9–12% and 20%, respectively. The glucose-fed unit could achieve glucose removal efficiency of 98%. Using a PEM, the same study gave an MPD of 262 and 28 mW/m2 with CE of 40–55% and 28%, respectively (Liu and Logan 2004). It indicated that in the absence of PEM, the MPD increased and CE decreased due to considerable oxygen diffusion into the anode. Moreover, the concentration of glucose also affected the power output, the MPD improved with increasing glucose concentration. Similar observations were made in another research using acetate and butyrate as substrates, resulting in an MPD of 506 and 305 mW/m2, respectively (Liu et al. 2005). The power density was found to be 54% higher for acetate, and 57% higher for butyrate compared to that obtained using a PEM (Liu et al. 2005). Feng et al. reported MPD values for beer brewery wastewater, glucose (0.6 g/l), and domestic wastewater as 205, 494, and 146 mW/m2, respectively (Feng et al. 2008). The COD removal efficiency increased from 54% to 98% when the strength of brewery wastewater rose from 84 to 1600 mg/l.
Coal-tar refinery wastewater as substrate was reported to produce an MPD of 4.5 mW/m2 at a voltage of 543 mV, along with a COD reduction of 88%, 57% of sulfate elimination, and 41% of sulfur removal. Furthermore, the ML-MFC could remove over 90% of phenol and 2-methyl phenol (Park et al. 2012). Passage of human urine through an ML-MFC achieved a current of 0.18–0.23 mA (Santoro et al. 2013). The initial COD of 10.9 g/l was reduced to 3.6 g/l after 4-day retention in the batch mode MFC unit. A study analyzed the effect of varying substrate concentration using phenol in the range of 25–200 mg/l. As the concentration of phenol increased from 25 to 100 mg/l, an increase in the removal of phenol from 80% to 97% was observed. It could attain an MPD of 49.8 mW/m2 and a current density of 292.8 mA/m2 (Buitrón and Moreno-Andrade 2014). An ML-MFC unit used rice straw pretreated with acid (to degrade cellulose) to generate an MPD of 137.6 mW/m2, and COD removal efficiency of 79% at an initial COD of 400 mg/l (Wang et al. 2014). Similarly, purified terephthalic acid wastewater produced an MPD of 65.6 mW/m2 at COD of 8000 mg/l (Marashi and Kariminia 2015).
In another study, dairy, leather, and sewage wastewaters generated maximum power levels of 1.98, 1.95, and 1.28 mW, along with COD reduction levels of 85.4%, 80%, and 65%, respectively (Aswin et al. 2017). Similarly, other lignocellulose materials used as substrate have resulted in an MPD of 29 mW/m3 (Adekunle et al. 2016). Dye processing wastewater tested at several organic loadings resulted in an MPD of 515 mW/m2, CE of 56%, and COD reduction of 85% at an organic loading of 1.0 g/l COD (Karuppiah et al. 2018). In a different work, petroleum refinery wastewater demonstrated treatment ratio and power density of 45.06% and 28.27 W/m3, while the corresponding values for whey wastewater were found to be 72.76% and 23.23 W/m3, respectively (Mohanakrishna et al. 2018). Wood, being rich in organic materials, also has potential as fuel for MFCs; for example, an MPD of 8555 mW/m2 was reported using poplar wood (Erensoy and Çek 2018). Yet another investigation used tomato waste as the substrate producing an MPD of 60.041 mW/m2, current density of 99.174 mA/m2, and voltage of 0.701 V (Kamau et al. 2018).
Lately, many studies have attempted the heavy metal (sulfur, copper, mercury) removal using ML-MFCs due to their extreme toxicity and carcinogenicity. Various heavy metals are released in the effluents from tanning, cement, electroplating, and dye industries. In a study, a hydrolyzed heavy metal-containing wheat grain (HMWG) used as a substrate produced an MPD of 381 mW/m2, a CE of 15.7%, and COD reduction of 83.4% (Yuan et al. 2018). Increasing the concentration of the HMWG hydrolysate slowed down the electricity production in the reactor. In another work, Cu (II) was used as an electron acceptor to explore the mechanism of metal treatment in an ML-MFC. A low ratio of Cu (II) resulted in heavy metal reduction efficiency of 87.56%, whereas the heavy metal reduction efficiency dropped to 36.98% with increasing ratio of Cu (II) in the substrate. Furthermore, it also caused the voltage to drop from 71 to 11.1 mV (Chan et al. 2020).
In a similar way, the treatment of inorganics has been achieved in ML-MFCs. In a study on domestic wastewater treatment, the results showed maximum power generation of 200 mW, COD reduction of 75%, 68% removal of ammonical nitrogen, and 90% reduction in suspended solids (Ge and He 2016). Likewise, yogurt wastewater tested as substrate produced an MPD of 1043 mW/m2, with COD and NH4-N reduction efficiencies of 87% and 74%, respectively (Luo et al. 2017). A supercapacitor-MFC showed high pollutant removal rates; 59.4% of COD, 78.2% of NH4-N, 77.8% of nitrogen, and achieved an MPD of 298 mW/m2 (Cai et al. 2020b).
8.4 Conclusion
The MFC technology has a huge potential for clean, safe, and sustainable production of bioenergy using industrial or domestic wastewater. The ML-MFCs have outperformed the conventional MFCs with a membrane with far better efficiencies for electricity generation as well as water treatment. The presence of a membrane restricts the speed of hydrogen ion movement, thus negatively influencing the power output. Despite the several advantages of ML-MFCs and progress achieved, many challenges still need to be addressed. One of the challenges is the decline in electricity generation over time due to the biofouling of the cathode, which adds to the operational cost for replacing the cathode once it loses efficiency. Also, the oxygen intrusion from the cathode to the anode compartment leads to a decline in the performance of the anode. Furthermore, cathode catalyst degradation is still a major issue affecting the efficiency of the cathode and the CE of the MFC.
The design of ML-MFC is a crucial aspect as it can help save energy losses by decreasing the internal resistance and enhance cathode efficiency. Having a well-sealed air-cathode in a single-chamber ML-MFC could provide high output, and stable performance in the long run with easy maintenance. As for the substrate, the concentration of the metals and organics have a significant impact on the power generation and pollutant reduction. High toxicity of the substrate may result in decline of the bacteria activity. Also, excessive salinity of the substrate may prove detrimental for the bacteria, thus it is important to achieve a balanced solution conductivity for optimal bacterial activity.
References
Aaron D, Tsouris C, Hamilton CY, Borole AP (2010) Assessment of the effects of flow rate and ionic strength on the performance of an air-cathode microbial fuel cell using electrochemical impedance spectroscopy. Energies 3:592–606. https://doi.org/10.3390/en3040592
Adekunle A, Gariepy Y, Lyew D, Raghavan V (2016) Energy recovery from cassava peels in a single-chamber microbial fuel cell. Energy Sources Part A Recover Util Environ Eff 38:2495–2502. https://doi.org/10.1080/15567036.2015.1086909
Ahn Y, Logan BE (2010) Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol 101:469–475. https://doi.org/10.1016/j.biortech.2009.07.039
Aswin T, Sabarunishabegum S, Sikkandar MY (2017) Optimization of microbial fuel cell for treating industrial wastewater and simultaneous power generation. Int J Chem Sci 15:132–143
Buitrón G, Moreno-Andrade I (2014) Performance of a single-chamber microbial fuel cell degrading phenol: effect of phenol concentration and external resistance. Appl Biochem Biotechnol 174:2471–2481. https://doi.org/10.1007/s12010-014-1195-5
Cai T, Meng L, Chen G et al (2020a) Application of advanced anodes in microbial fuel cells for power generation: a review. Chemosphere 248:125985. https://doi.org/10.1016/j.chemosphere.2020.125985
Cai T, Jiang N, Zhen G et al (2020b) Simultaneous energy harvest and nitrogen removal using a supercapacitor microbial fuel cell. Environ Pollut 266:115154. https://doi.org/10.1016/j.envpol.2020.115154
Call TP, Carey T, Bombelli P et al (2017) Platinum-free, graphene based anodes and air cathodes for single chamber microbial fuel cells. J Mater Chem A 5:23872–23886. https://doi.org/10.1039/c7ta06895f
Capodaglio AG, Olsson G (2020) Energy issues in sustainable urban wastewater management: use, demand reduction and recovery in the urban water cycle. Sustainability 12:226. https://doi.org/10.3390/su12010266
Chan KK, Thung WE, Ong SA et al (2020) Simultaneous heavy metal reduction and voltage generation with synergy membrane-less microbial fuel cell. IOP Conf Ser Earth Environ Sci 463. https://doi.org/10.1088/1755-1315/463/1/012067
Cheng S, Logan BE (2007) Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem Commun 9:492–496. https://doi.org/10.1016/j.elecom.2006.10.023
Cheng S, Logan BE (2011) Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour Technol 102:4468–4473. https://doi.org/10.1016/j.biortech.2010.12.104
Cheng S, Liu H, Logan BE (2006) Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem Commun 8:489–494. https://doi.org/10.1016/j.elecom.2006.01.010
Clauwaert P, Verstraete W (2009) Methanogenesis in membraneless microbial electrolysis cells. Appl Microbiol Biotechnol 82:829–836. https://doi.org/10.1007/s00253-008-1796-4
Dong Y, Qu Y, He W et al (2015) A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour Technol 195:66–72. https://doi.org/10.1016/j.biortech.2015.06.026
Du F, Xie B, Dong W et al (2011) Continuous flowing membraneless microbial fuel cells with separated electrode chambers. Bioresour Technol 102:8914–8920. https://doi.org/10.1016/j.biortech.2011.07.056
Du Y, Feng Y, Teng Q, Li H (2015) Effect of inorganic salt in the culture on microbial fuel cells performance. Int J Electrochem Sci 10:1316–1325
Erensoy A, Çek N (2018) Alternative biofuel materials for microbial fuel cells from poplar wood. ChemistrySelect 3:11251–11257. https://doi.org/10.1002/slct.201802171
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880. https://doi.org/10.1007/s00253-008-1360-2
Feng Y, Yang Q, Wang X, Logan BE (2010) Treatment of carbon fiber brush anodes for improving power generation in air-cathode microbial fuel cells. J Power Sources 195:1841–1844. https://doi.org/10.1016/j.jpowsour.2009.10.030
Feng C, Wan Q, Lv Z et al (2011) One-step fabrication of membraneless microbial fuel cell cathode by electropolymerization of polypyrrole onto stainless steel mesh. Biosens Bioelectron 26:3953–3957. https://doi.org/10.1016/j.bios.2011.02.046
Gadkari S, Fontmorin JM, Yu E, Sadhukhan J (2020) Influence of temperature and other system parameters on microbial fuel cell performance: numerical and experimental investigation. Chem Eng J 388:124176. https://doi.org/10.1016/j.cej.2020.124176
Gálvez A, Greenman J, Ieropoulos I (2009) Landfill leachate treatment with microbial fuel cells; scale-up through plurality. Bioresour Technol 100:5085–5091. https://doi.org/10.1016/j.biortech.2009.05.061
Ge Z, He Z (2016) Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: treatment, energy, and cost. Environ Sci Water Res Technol 2:274–281. https://doi.org/10.1039/c6ew00020g
Ghangrekar MM, Shinde VB (2007) Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour Technol 98:2879–2885. https://doi.org/10.1016/j.biortech.2006.09.050
Ghangrekar MM, Shinde VB (2008) Simultaneous sewage treatment and electricity generation in membrane-less microbial fuel cell. Water Sci Technol 58:37–43. https://doi.org/10.2166/wst.2008.339
Gil GC, Chang IS, Kim BH et al (2003) Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens Bioelectron 18:327–334. https://doi.org/10.1016/S0956-5663(02)00110-0
Guerrini E, Grattieri M, Faggianelli A et al (2015) PTFE effect on the electrocatalysis of the oxygen reduction reaction in membraneless microbial fuel cells. Bioelectrochemistry 106:240–247. https://doi.org/10.1016/j.bioelechem.2015.05.008
Hassan SHA, Gad El-Rab SMF, Rahimnejad M et al (2014) Electricity generation from rice straw using a microbial fuel cell. Int J Hydrog Energy 39:9490–9496. https://doi.org/10.1016/j.ijhydene.2014.03.259
Hindatu Y, Annuar MSM, Gumel AM (2017) Mini-review: anode modification for improved performance of microbial fuel cell. Renew Sust Energ Rev 73:236–248. https://doi.org/10.1016/j.rser.2017.01.138
Huang L, Li X, Ren Y, Wang X (2016) In-situ modified carbon cloth with polyaniline/graphene as anode to enhance performance of microbial fuel cell. Int J Hydrog Energy 41:11369–11379. https://doi.org/10.1016/j.ijhydene.2016.05.048
Jadhav GS, Ghangrekar MM (2009) Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour Technol 100:717–723. https://doi.org/10.1016/j.biortech.2008.07.041
Jang JK, Pham TH, Chang IS et al (2004) Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process Biochem 39:1007–1012. https://doi.org/10.1016/S0032-9592(03)00203-6
Jia Y, Feng H, Shen D et al (2018) High-performance microbial fuel cell anodes obtained from sewage sludge mixed with fly ash. J Hazard Mater 354:27–32. https://doi.org/10.1016/j.jhazmat.2018.04.008
Jiang HM (2017) Combination of microbial fuel cells with microalgae cultivation for bioelectricity generation and domestic wastewater treatment. Environ Eng Sci 34:489–495. https://doi.org/10.1089/ees.2016.0279
Jiang D, Li B (2009) Novel electrode materials to enhance the bacterial adhesion and increase the power generation in microbial fuel cells (MFCs). Water Sci Technol 59:557–563. https://doi.org/10.2166/wst.2009.007
Jiang Q, Xing D, Zhang L et al (2018) Interaction of bacteria and archaea in a microbial fuel cell with ITO anode. RSC Adv 8:28487–28495. https://doi.org/10.1039/c8ra01207e
Jiménez González ML, Benítez CH, Juarez ZA et al (2020) Study of the effect of activated carbon cathode configuration on the performance of a membrane-less microbial fuel cell. Catalysts 10:619. https://doi.org/10.3390/catal10060619
Jothinathan D, Nasrin Fathima AH, Mylsamy P et al (2018) Microbial fuel cell research using animal waste: a feebly-explored area to others. In: Microbial fuel cell technology for bioelectricity. Springer, Cham, pp 151–168
Kamau JM, Mbui DN, Mwaniki JM, Mwaura FB (2018) Utilization of rumen fluid in production of bio–energy from market waste using microbial fuel cells technology. J Appl Biotechnol Bioeng 5:227–231. https://doi.org/10.15406/jabb.2018.05.00142
Karuppiah T, Pugazhendi A, Subramanian S et al (2018) Deriving electricity from dye processing wastewater using single chamber microbial fuel cell with carbon brush anode and platinum nano coated air cathode. 3 Biotech 8. https://doi.org/10.1007/s13205-018-1462-1
Khan MD, Khan N, Sultana S et al (2018) Microbial fuel cell: waste minimization and energy generation. In: Khan OM, Mohammad Z, Iqbal I (eds) Modern age environmental problems and their remediation. Springer, Cham, pp 129–146
Kim J, Kim B, An J et al (2016) Development of anode zone using dual-anode system to reduce organic matter crossover in membraneless microbial fuel cells. Bioresour Technol 213:140–145. https://doi.org/10.1016/j.biortech.2016.03.012
Kumar GG, Sarathi VGS, Nahm KS (2013) Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens Bioelectron 43:461–475. https://doi.org/10.1016/j.bios.2012.12.048
Lee Y, Nirmalakhandan N (2011) Electricity production in membrane-less microbial fuel cell fed with livestock organic solid waste. Bioresour Technol 102:5831–5835. https://doi.org/10.1016/j.biortech.2011.02.090
Li WW, Yu HQ, He Z (2014) Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ Sci 7:911–924. https://doi.org/10.1039/c3ee43106a
Lin CC, Wei CH, Chen CI et al (2013) Characteristics of the photosynthesis microbial fuel cell with a Spirulina platensis biofilm. Bioresour Technol 135:640–643. https://doi.org/10.1016/j.biortech.2012.09.138
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046. https://doi.org/10.1021/es0499344
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285. https://doi.org/10.1021/es034923g
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39:658–662. https://doi.org/10.1021/es048927c
Liu SH, Lai CY, Chang PH et al (2020) Enhancing copper recovery and electricity generation from wastewater using low-cost membrane-less microbial fuel cell with a carbonized clay cup as cathode. J Clean Prod 247:119118. https://doi.org/10.1016/j.jclepro.2019.119118
Logan BE (2008) Microbial fuel cells. John Wiley & Sons, Hoboken, NJ
Logan BE (2010) Scaling up microbial fuel cells and other bioelectrochemical systems. Appl Microbiol Biotechnol 85:1665–1671. https://doi.org/10.1007/s00253-009-2378-9
Luo H, Liu G, Zhang R, Jin S (2007) Characteristics of generating electricity with microbial fuel cell by different organics as fuel. In: Proceedings of ISES world congress 2007, vol I–V. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 2449–2452
Luo H, Xu G, Lu Y et al (2017) Electricity generation in a microbial fuel cell using yogurt wastewater under alkaline conditions. RSC Adv 7:32826–32832. https://doi.org/10.1039/c7ra06131e
Malvankar NS, Tuominen MT, Lovley DR (2012) Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energy Environ Sci 5:5790–5797. https://doi.org/10.1039/c2ee03388g
Marashi SKF, Kariminia HR (2015) Performance of a single chamber microbial fuel cell at different organic loads and pH values using purified terephthalic acid wastewater. J Environ Heal Sci Eng 13:27. https://doi.org/10.1186/s40201-015-0179-x
Min B, Logan B (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38:5809–5814
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686. https://doi.org/10.1016/j.watres.2005.02.002
Mohanakrishna G, Abu-Reesh IM, Al-Raoush RI, He Z (2018) Cylindrical graphite based microbial fuel cell for the treatment of industrial wastewaters and bioenergy generation. Bioresour Technol 247:753–758. https://doi.org/10.1016/j.biortech.2017.09.174
Mousavi SMS, Ayati B, Ganjidoust H (2016) Phenol removal and bio-electricity generation using a single-chamber microbial fuel cell in saline and increased-temperature condition. Energy Sources Part A Recover Util Environ Eff 38:3300–3307. https://doi.org/10.1080/15567036.2016.1156196
Nastro RA (2014) Microbial fuel cells in waste treatment: recent advances. Int J Performability Eng 10:367–376
Nawaz A, Raza W, Gul H et al (2020) Upscaling feasibility of a graphite-based truncated conical microbial fuel cell for bioelectrogenesis through organic wastewater treatment. J Colloid Interface Sci 570:99–108. https://doi.org/10.1016/j.jcis.2020.02.099
Palanisamy G, Jung HY, Sadhasivam T et al (2019) A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J Clean Prod 221:598–621. https://doi.org/10.1016/j.jclepro.2019.02.172
Pallavi CK, Udayashankara TH (2016) A review on microbial fuel cells employing wastewaters as substrates for sustainable energy recovery and wastewater treatment. IOSR J Environ Sci Toxicol Food Technol 10:31–36. https://doi.org/10.9790/2402-1012023136
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543. https://doi.org/10.1016/j.biortech.2009.10.017
Park HI, Wu C, Lin LS (2012) Coal tar wastewater treatment and electricity production using a membrane-less tubular microbial fuel cell. Biotechnol Bioprocess Eng 17:654–660. https://doi.org/10.1007/s12257-011-0374-2
Pauline S, Boopathi A (2018) Treatment of slaughterhouse wastewater using microbial fuel cell. Int J Adv Res Ideas Innov Technol 4:228–230
Peng X, Chu X, Wang S et al (2017) Bio-power performance enhancement in microbial fuel cell using Ni-ferrite decorated anode. RSC Adv 7:16027–16032. https://doi.org/10.1039/C7RA01253E
Rabaey K, Verstraete W (2005) Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol 23:291–298. https://doi.org/10.1016/j.tibtech.2005.04.008
Rahimnejad M, Najafpour GD (2011) Microbial fuel cells: a new source of power. In: Biochemical engineering and biotechnology, 2nd edn. Elsevier B.V., Amsterdam, pp 1–31
Rahimnejad M, Adhami A, Darvari S et al (2015) Microbial fuel cell as new technology for bioelectricity generation: a review. Alexandria Eng J 54:745–756. https://doi.org/10.1016/j.aej.2015.03.031
Saba B, Christy AD, Yu Z, Co AC (2017) Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): an overview. Renew Sust Energ Rev 73:75–84. https://doi.org/10.1016/j.rser.2017.01.115
Santoro C, Ieropoulos I, Greenman J et al (2013) Current generation in membraneless single chamber microbial fuel cells (MFCs) treating urine. J Power Sources 238:190–196. https://doi.org/10.1016/j.jpowsour.2013.03.095
Santoro C, Serov A, Stariha L et al (2016) Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ Sci 9:2346–2353. https://doi.org/10.1039/c6ee01145d
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbial fuel cells: from fundamentals to applications. A review. J Power Sources 356:225–244. https://doi.org/10.1016/j.jpowsour.2017.03.109
Savizi ISP, Kariminia HR, Bakhshian S (2012) Simultaneous decolorization and bioelectricity generation in a dual chamber microbial fuel cell using electropolymerized-enzymatic cathode. Environ Sci Technol 46:6584–6593. https://doi.org/10.1021/es300367h
Slate AJ, Whitehead KA, Brownson DAC, Banks CE (2019) Microbial fuel cells: an overview of current technology. Renew Sust Energ Rev 101:60–81. https://doi.org/10.1016/j.rser.2018.09.044
Song YE, Lee S, Kim M et al (2020) Metal-free cathodic catalyst with nitrogen- and phosphorus-doped ordered mesoporous carbon (NPOMC) for microbial fuel cells. J Power Sources 451:227816. https://doi.org/10.1016/j.jpowsour.2020.227816
Tee PF, Abdullah MO, Tan IAW et al (2018) Bio-energy generation in an affordable, single-chamber microbial fuel cell integrated with adsorption hybrid system: effects of temperature and comparison study. Environ Technol (UK) 39:1081–1088. https://doi.org/10.1080/09593330.2017.1320433
Tugtas AE, Cavdar P, Calli B (2011) Continuous flow membrane-less air cathode microbial fuel cell with spunbonded olefin diffusion layer. Bioresour Technol 102:10425–10430. https://doi.org/10.1016/j.biortech.2011.08.082
Waller MG, Trabold TA (2013) Review of microbial fuel cells for wastewater treatment: large-scale applications, future needs and current research gaps. In: International conference on fuel cell science, engineering and technology. American Society of Mechanical Engineers, New York, p V001T01A011
Wang P, Li H, Du Z (2013) Deposition of iron on graphite felts by thermal decomposition of Fe(CO)5 for anodic modification of microbial fuel cells. Int J Electrochem Sci 8:4712–4722
Wang Z, Lee T, Lim B et al (2014) Microbial community structures differentiated in a single-chamber air-cathode microbial fuel cell fueled with rice straw hydrolysate. Biotechnol Biofuels 7:1–10. https://doi.org/10.1186/1754-6834-7-9
Wang R, Yan M, Li H et al (2018) FeS2 nanoparticles decorated graphene as microbial-fuel-cell anode achieving high power density. Adv Mater 30:1800618. https://doi.org/10.1002/adma.201800618
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102:9335–9344. https://doi.org/10.1016/j.biortech.2011.07.019
Yamashita T, Ishida M, Asakawa S et al (2016) Enhanced electrical power generation using flame-oxidized stainless steel anode in microbial fuel cells and the anodic community structure. Biotechnol Biofuels 9:1–10. https://doi.org/10.1186/s13068-016-0480-7
Yang S, Jia B, Liu H (2009) Effects of the Pt loading side and cathode-biofilm on the performance of a membrane-less and single-chamber microbial fuel cell. Bioresour Technol 100:1197–1202. https://doi.org/10.1016/j.biortech.2008.08.005
Yang Y, Sun G, Xu M (2011) Microbial fuel cells come of age. J Chem Technol Biotechnol 86:625–632. https://doi.org/10.1002/jctb.2570
Yang F, Ren L, Pu Y, Logan BE (2013) Electricity generation from fermented primary sludge using single-chamber air-cathode microbial fuel cells. Bioresour Technol 128:784–787. https://doi.org/10.1016/j.biortech.2012.10.021
Yang G, Sun Y, Yuan Z et al (2014) Application of surface-modified carbon powder in microbial fuel cells. Cuihua Xuebao/Chinese J Catal 35:770–775. https://doi.org/10.1016/s1872-2067(14)60023-1
Yang W, Li J, Zhang L et al (2017) A monolithic air cathode derived from bamboo for microbial fuel cells. RSC Adv 7:28469–28475. https://doi.org/10.1039/c7ra04571a
Yang Y, Yan L, Song J, Xu M (2018) Optimizing the electrode surface area of sediment microbial fuel cells. RSC Adv 8:25319–25324. https://doi.org/10.1039/c8ra05069d
Ye D, Zhang P, Zhu X et al (2018) Electricity generation of a laminar-flow microbial fuel cell without any additional power supply. RSC Adv 8:33637–33641. https://doi.org/10.1039/C8RA07340F
You S, Zhao Q, Zhang J et al (2007) A graphite-granule membrane-less tubular air-cathode microbial fuel cell for power generation under continuously operational conditions. J Power Sources 173:172–177. https://doi.org/10.1016/j.jpowsour.2007.07.063
You J, Fan H, Winfiel J, Ieropoulos IA (2020) Complete microbial fuel cell fabrication using additive layer manufacturing. Molecules 25:3051
Yuan Y, Zhao B, Zhou S et al (2011) Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour Technol 102:6887–6891. https://doi.org/10.1016/j.biortech.2011.04.008
Yuan GE, Deng H, Ru X, Zhang X (2018) Electricity generation from heavy metal-containing wheat grain hydrolysate using single-chamber microbial fuel cells: performance and long-term stability. Int J Electrochem Sci 13:8589–8601. https://doi.org/10.20964/2018.09.05
Zeng L, Zhao S, Zhang L, He M (2018) A facile synthesis of molybdenum carbide nanoparticles-modified carbonized cotton textile as an anode material for high-performance microbial fuel cells. RSC Adv 8:40490–40497. https://doi.org/10.1039/C8RA07502F
Zhang Y, Ye JS (2015) Graphene-based microbial fuel cells. In: Yusoff AR b M (ed) Graphene-based energy devices. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim, Germany, pp 339–354
Zhao Y, Ma Y, Li T et al (2018) Modification of carbon felt anodes using double-oxidant HNO3/H2O2 for application in microbial fuel cells. RSC Adv 8:2059–2064. https://doi.org/10.1039/c7ra12923h
Zhou M, Chi M, Wang H, Jin T (2012) Anode modification by electrochemical oxidation: a new practical method to improve the performance of microbial fuel cells. Biochem Eng J 60:151–155. https://doi.org/10.1016/j.bej.2011.10.014
Zhuang L, Zhou S, Wang Y et al (2009) Membrane-less cloth cathode assembly (CCA) for scalable microbial fuel cells. Biosens Bioelectron 24:3652–3656. https://doi.org/10.1016/j.bios.2009.05.032
Acknowledgments
This work was carried out as part of the Sustainable Raw Material Management Thematic Network—RING 2017, EFOP-3.6.2-16-2017-00010 project in the framework of the Széchenyi 2020 Program. The realization of this project is supported by the European Union, co-financed by the European Social Fund. CAT also thanks the Tempus Public Foundation for providing financial assistance under the Stipendium Hungaricum Programme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tahir, C.A., Pásztory, Z., Agarwal, C., Csóka, L. (2022). Electricity Generation and Wastewater Treatment with Membrane-Less Microbial Fuel Cell. In: Inamuddin, Ahamed, M.I., Prasad, R. (eds) Application of Microbes in Environmental and Microbial Biotechnology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-2225-0_8
Download citation
DOI: https://doi.org/10.1007/978-981-16-2225-0_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2224-3
Online ISBN: 978-981-16-2225-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)