Abstract
Various molecular and cellular processes are involved in renal fibrosis, such as oxidative stress, inflammation, endothelial cell injury, and apoptosis. Heat shock proteins (HSPs) are implicated in the progression of chronic kidney disease (CKD). Our aim was to evaluate changes in urine and serum HSP levels over time and their relationships with the clinical parameters of CKD in children. In total, 117 children with CKD and 56 healthy children were examined. The CKD group was followed up prospectively for 24 months. Serum and urine HSP27, HSP40, HSP47, HSP60, HSP70, HSP72, and HSP90 levels and serum anti-HSP60 and anti-HSP70 levels were measured by ELISA at baseline, 12 months, and 24 months. The urine levels of all HSPs and the serum levels of HSP40, HSP47, HSP60, HSP70, anti-HSP60, and anti-HSP70 were higher at baseline in the CKD group than in the control group. Over the months, serum HSP47 and HSP60 levels steadily decreased, whereas HSP90 and anti-HSP60 levels steadily increased. Urine HSP levels were elevated in children with CKD; however, with the exception of HSP90, they decreased over time. In conclusion, our study demonstrates that CKD progression is a complicated process that involves HSPs, but they do not predict CKD progression. The protective role of HSPs against CKD may weaken over time, and HSP90 may have a detrimental effect on the disease course.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) in children is caused by various congenital and acquired kidney diseases, as well as systemic disorders. However, CKD progresses similarly, regardless of the underlying pathology. The main reason for progressive loss of renal function in CKD is fibrosis in the glomeruli and tubulointerstitial area. Various molecular and cellular processes affect renal fibrosis, including oxidative stress, inflammation, endothelial cell injury, and apoptosis. Progressive renal function loss leads to end-stage renal disease (ESRD), which requires dialysis or renal transplantation.
As molecular chaperones, heat shock proteins (HSPs) have a wide range of intracellular and extracellular functions. Under normal circumstances, HSPs are continuously expressed and regulate the turnover of intracellular proteins as well as cellular integrity (Barutta et al. 2008; Chebotareva et al. 2017). Under stress conditions, such as ischemia, oxidative stress, and inflammation, HSP expression increases and promotes cell integrity by facilitating protein folding, the refolding of denatured proteins, and the removal of permanently damaged proteins (Barutta et al. 2008; Chebotareva et al. 2017). HSPs can be released into the extracellular space and exert a wide range of regulatory extracellular functions (Barutta et al. 2008; Chebotareva et al. 2017). The aforementioned factors and processes are present in CKD. Therefore, HSPs are likely to be involved in the progression of the disease. There are very limited data about HSP levels in CKD. Some experimental and clinical studies showed variability in the urine and serum levels of various HSPs in CKD, based on analyses of single urine or serum samples (Musial et al. 2009, 2010; Lebherz-Eichinger et al. 2012; Musial & Zwolinska 2012).
Our aim was to evaluate changes over time in urine and serum HSP levels and their relationships with the clinical parameters of CKD in children. We also aimed to evaluate the relationships of HSPs with oxidative stress, inflammation, endothelial cell injury, and apoptotic markers as part of the “Progression of Chronic Kidney Disease in Children and Markers of Apoptosis, Inflammation, Oxidative Stress, Endothelial Dysfunction and Heat Shock Proteins: The PROGRESS STUDY.” Herein, we report the serum and urine HSP levels of children with CKD over 24 months (three sampling periods with an interval of 12 months) as the first part of the PROGRESS STUDY. This is the first study to evaluate both serum and urine HSP levels in children with CKD over time.
Materials and methods
Approval for this study was obtained from our local ethics committee (Project No: 2015/487). Informed consent was obtained from the parents of all participants.
Study population
From four pediatric nephrology centers, 117 patients (37 females and 80 males) with CKD who presented for routine follow-ups were enrolled into the CKD group. Patients aged 0–18 years diagnosed with stages 2–5 CKD according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2013) were enrolled. The exclusion criteria were a diagnosis of stage 1 CKD according to the KDIGO guidelines, previous dialysis treatment or renal transplantation, and age > 18 years.
The control group consisted of 56 healthy children (27 females and 29 males). The inclusion criteria for the control group were age 0–18 years, no chronic diseases according to the medical history, and no other disease or operation within the 3-month period before, or during, the sampling.
The CKD group was followed up prospectively for 24 months. Upon enrolling in the study, the patients and control group underwent routine physical examinations. Height and weight measurements of the patients and controls were obtained by the same auxologist, according to standard methods. The estimated glomerular filtration rate (eGFR) was calculated using the Schwartz formula (Schwartz et al. 2009). The patients were followed-up at pediatric nephrology departments implementing CKD protocols. Each patient’s diet was regulated by a dietician according to their age, weight, body mass index (BMI), and biochemical parameters. The annual hemoglobin (Hb), serum parathyroid hormone (PTH), calcium (Ca), phosphorus (P), alkaline phosphatase (ALP), and ferritin levels of the patients were obtained from their medical records. Rapid CKD progression was described as the requirement to undergo dialysis or renal transplantation, and/or a 25% reduction in eGFR from baseline. Twelve patients dropped out of the study by month 24 due either to loss to follow-up (n = 5) or dialysis or kidney transplant treatment (n = 7).
Among CKD group, patients with methylmalonic acidemia, primary hyperoxaluria, cystinosis, and cystic kidney disease were accepted as systemic disorders.
Blood and urine samples
Samples were obtained from patients at baseline, 12 months, and 24 months and were obtained from controls once at baseline. During the sampling, the patients did not have a history of infection, operation, or hospitalization in the last 3-month period. Urinary tract infection was excluded by urinalysis and urine culture.
Urine and serum levels of creatinine (Cr), HSP27, HSP40, HSP47, HSP60, HSP70, HSP72, and HSP90; serum levels of anti-HSP60 and anti-HSP70; and urine protein levels were measured. HSP27, HSP40, HSP60, HSP70, HSP72, and HSP90 levels are expressed as ng/ml; anti-HSP60 and anti-HSP70 levels are expressed as pg/ml.
Urine and blood samples were centrifuged for 10 min at 2,000 g. Aliquots of serum and urine supernatant were stored at – 80 °C until assayed. Serum and urine concentrations of HSP27, HSP40, HSP47, HSP60, HSP70, HSP72, and HSP90 were evaluated by commercially available enzyme-linked immunosorbent assay (ELISA) kits (YH Biosearch, Shanghai, China) (Cat nos. YHB1476Hu, YHB1477Hu YHB1478Hu, YHB1479Hu, YHB1480Hu, YHB1481Hu, and YHB1482Hu, respectively). The detection and quantification limits were > 1 ng/ml for HSP27, > 0.2 ng/ml for HSP40/HSP90, > 0.5 ng/ml for HSP47/HSP72, > 100 ng/L for HSP60, and > 2 ng/ml for HSP70. The detection sensitivity of HSP27, HSP40, HSP47, HSP60, HSP70, HSP72, and HSP90 was 0.52 ng/ml, 1.09 ng/ml, 0.25 ng/ml, 50.12 ng/L, 1.02 ng/ml, 0.25 ng/ml, and 0.11 ng/ml, respectively. Each sample was tested in duplicate according to the manufacturer’s instructions. The intra-assay coefficients of variation (CVs) of HSP27, HSP40, HSP47, HSP60, HSP70, HSP72, and HSP90 were < 10%, and the inter-assay CVs were < 12%.
Serum anti-HSP60 and anti-HSP70 antibody levels were measured using an ELISA kit (Abbkine, Wuhan, China) (cat nos. KTE62740 and KTE62741, respectively) according to the manufacturer’s instructions. Each sample was tested in duplicate according to the manufacturer’s instructions. The concentrations for each sample were calculated according to standard curves of optical density values; the arithmetical mean was obtained as the final result.
An Architect c16000 analyzer (Abbott Laboratories, Abbott Park, IL, USA) was used to measure serum and urine Cr and urine protein levels, which are expressed as mg/dl. The urine protein/Cr ratios are expressed as mg/mg. Fractional excretion of HSPs (FeHSPs) are calculated using the formula: 100 * (urine HSP × serum creatinine) / (serum HSP × urine creatinine).
Statistical analysis
Statistical analyses were performed using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). Descriptive data are expressed as means ± SD (min–max) or median (interquartile range). A Chi-square test was performed to evaluate the qualitative data. The normality of the parameters was tested using the Kolmogorov–Smirnov test. A Mann–Whitney U test was used to compare the CKD and control groups, the groups with and without rapid CKD progression, and the groups with and without systemic disorder. A Kruskal Wallis test was used to compare the baseline CKD stage groups. A Friedman test was conducted to test for significant changes from baseline at 12 or 24 months. A Wilcoxon test was performed to test for significant pairwise differences, with the Bonferroni correction applied to adjust for multiple comparisons. The relationships among variables were analyzed using Spearman’s correlation coefficients. p Values < 0.05 were considered statistically significant.
Results
The participants’ mean age was 11.4 ± 4.9 years (range: 0.1–18.0 years) in the CKD group and 11.6 ± 3.7 years (range: 3.6–18.0 years) in the control group (p = 0.769). There was a higher proportion of males in the CKD group than in the control group (p = 0.034). The demographic and clinical characteristics of the patients are given in Table 1. There was no difference between baseline and 24-months urine protein/Cr ratios in our patients (p = 0.577).
Comparison of HSPs between the CKD and control groups
The HSP levels of the CKD and control groups in serum and urine are given in Tables 2 and 3, respectively. Serum HSP40, HSP47, HSP60, HSP70, anti-HSP60, and anti-HSP70 levels at baseline were higher in the CKD group than in the control group (Table 2). Also, HSP40, anti-HSP60, and anti-HSP70 levels at 12 and 24 months were higher in the CKD group than in the control group (Table 2).
All urine HSP levels were higher in the CKD group than in the control group at baseline, 12 months, and 24 months (Table 3).
HSP levels of the CKD group over time
Serum HSPs
Serum HSP47 and HSP60 levels steadily decreased, whereas HSP90 and anti-HSP60 levels steadily increased, over time (Table 2). In contrast, serum HSP27 and anti-HSP70 levels increased over time, although the differences among time points were not statistically significant (Table 2).
At 24 months, serum HSP70, HSP90, and anti-HSP60 levels had significantly increased, whereas HSP47 and HSP60 levels had significantly decreased, relative to baseline (Table 2). There were no differences between the baseline and 24-month serum HSP27, HSP40, HSP72, and anti-HSP70 levels (Table 2).
Urine HSPs
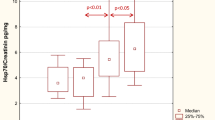
Urine HSP levels steadily decreased over time, except for HSP70 and HSP90 (Table 3). Comparing the baseline and 24-month results, urine HSP90 levels increased, whereas those of all other HSPs significantly decreased (Table 3). The course of eGFR, urine protein/Cr, and urine HSPs over time are given in Fig. 1.
The course of eGFR, urine protein/creatinine and urine HSPs over time. eGFR: estimated glomerular filtration rate. uHSP: urine heat shock protein. eGFR, uHSP27, uHSP70, uHSP72, and uHSP47 were given on the primary axis (left side); uHSP40, uHSP60, uHSP90, and urine protein/creatinine data were given on the secondary axis (right side)
Urine HSPs/creatinine
Almost all urine HSPs/Cr levels demonstrated similar patterns of urine HSPs over time except urine HSP90/Cr (Table 4).
Fractional excretion of HSPs
Although all fractional excretion of HSPs was higher in the CKD group than in controls, they did not change over time in the CKD group (Table 5).
Comparison of HSPs according to CKD stages at baseline and their changes over time with respect to the baseline stages
When we evaluated the HSPs levels at the baseline CKD stage, there were no differences between patients at different CKD stages in terms of serum HSPs and of most of the urine HSPs except HSP70 and HSP72 (Table 6). Therefore, we evaluated the course of HSPs over the years separately for each CKD stage group at baseline (Tables 7 and 8). Serum HSP60 levels gradually decreased over time among the CKD stages 2, 3a, and 3b groups. Serum HSP47 levels decreased in the CKD stage 3a and 3b groups. Serum HSP70 levels demonstrated a similar pattern in the whole CKD group as in the CKD stage 2 and 3a groups over time. Serum HSP90 levels increased over time in the CKD stage 3b group (Table 7). Urine HSP47 and HSP60 demonstrated similar patterns over time in all CKD stage groups. The decreasing pattern of urine HSP27 and HSP72 was more noticeable in the CKD stage 3a and 3b groups. Urine HSP40 decreased over time in all but the CKD stages 4–5 groups. Changes of urine HSP70 and HSP90 were significant over time in the CKD stages 3a and 4–5 groups (Table 8).
Comparison of the groups with and without rapid CKD progression
We evaluated whether baseline HSPs predicted rapid CKD progression. Baseline serum and urine HSP levels did not differ between the participants with and without rapid CKD progression (Table 9), although patients with rapid CKD progression had higher baseline urine protein/Cr ratio and lower baseline eGFR levels than patients without rapid CKD progression (p = 0.013 and p = 0.005, respectively). These results demonstrated that baseline HSPs did not predict rapid CKD progression.
Comparison of the HSPs between groups with and without systemic disorders
Due to the possible interference of HSPs by other non-renal organ involvement or systemic disorders in the CKD group, we compared HSP levels between the patients with and without systemic disorders. Only urine HSP27 levels were higher in patients without systemic disorders (Table 10).
Correlations of clinical parameters with baseline HSP levels
There was no correlation between serum HSP levels and age, or serum Ca, P, ALP, PTH, and Hb levels (p > 0.05), except that serum HSP90 levels correlated positively with Hb levels (r = 0.208, p = 0.025). Serum HSP27, HSP60, and HSP72 levels correlated negatively with urine protein/Cr ratios (r = − 0.263 p = 0.005, r = − 0.255 p = 0.006, and r = − 0.235, p = 0.012, respectively).
Hb levels were negatively correlated with urine HSP60 and HSP70 levels (r = − 0.210 and p = 0.023 and r = − 0.208 and p = 0.025, respectively). Serum ferritin levels were negatively correlated with urine HSP27, HSP40, and HSP47 levels (r = − 0.243, p = 0.018; r = − 0.245, p = 0.017; and r = − 0.260, p = 0.012, respectively) and positively correlated with urine HSP60 levels (r = 0.269, p = 0.009). Urine HSP70 levels were positively correlated with urine protein/Cr ratios (r = 0.216 and p = 0.022). There was no correlation between urine HSP levels and age, or serum Ca, P, ALP, and PTH levels (p > 0.05).
Correlations among HSPs
Serum HSPs
Correlations among serum HSPs are given in Table S1. All serum HSPs were positively correlated with each other, except that anti-HSP60 was positively correlated only with serum HSP40, HSP72, HSP90, and anti-HSP70.
Urine HSPs
Urine HSP27 was positively correlated with urine HSP72. Urine HSP40, HSP47, and HSP70 were positively correlated with each other. Additionally, urine HSP47 was positively correlated with urine HSP90. Urine HSP60 was positively correlated with urine HSP70 and HSP72 and negatively correlated with urine HSP47 and HSP90. Also, there was a negative correlation between urine HSP90 and HSP72. The correlations among urine HSPs are given in Table S2.
Serum and urine HSPs
Serum HSP40, HSP47, and HSP90 were correlated with the corresponding urine HSPs (r = 0.259, p = 0.005, r = 0.405, p < 0.001, and r = 0.502, p < 0.001, respectively).
Discussion
Here, we report HSP dysregulation in children with CKD. This is the first study to evaluate HSPs over time in children with CKD and their relationships with disease progression. Serum and urine levels of almost all of the HSPs changed during the 2-year study period. Serum levels of HSPs may not only be affected by kidney disease, and the urine data may reflect the severity of kidney disease much more than the serum data. Thus, the results for urine HSPs were more remarkable than those for serum HSPs. All urine HSP levels were higher in the CKD patients than in the controls. Additionally, all of the urine HSPs decreased over time, except HSP70 and HSP90. Higher levels of urine HSPs reflect the response to various stress factors, such as oxidative stress, inflammation, endothelial dysfunction, and apoptosis, all of which are characteristic of CKD. However, the decrease in urine HSP levels in children with CKD over time may be due to a decrease in the upregulation of HSP expression in the kidney, as viable and functional cell expression diminishes over time in CKD. It may also be due to the reduced extracellular secretions associated with structural alterations of HSPs in the uremic milieu. Reduced urine HSP expression appears to be associated with CKD progression.
HSP90 was the only HSP to increase in urine over time. Serum HPS90 levels also increased over time. Musial et al. reported higher serum HSP90-alpha levels in pre-dialyzed and dialyzed CKD patients than in controls (Musial et al. 2009, 2010). They observed higher serum HSP90-alpha levels in children on automated peritoneal dialysis than in pre-dialyzed CKD patients (Musial et al. 2010). HSP90 plays a role in both apoptosis and cell survival (Lebherz-Eichinger et al. 2013). HSP90 inactivates apoptosis signal-regulating kinase 1, thus inhibiting JNK-mediated cell death and promoting cell survival (Lebherz-Eichinger et al. 2013). HSP90 is also involved in JNK-mediated cell death, promoting apoptosis (Lebherz-Eichinger et al. 2013). Inhibition of HSP90 prevents fibrosis in various animal models of renal injury (Harrison et al. 2008; Noh et al. 2012; Barrera-Chimal et al. 2014; O'Neill et al. 2014). Heat shock transcription factor 1 (HSF1) promotes the production of HSPs (Chebotareva et al. 2017). HSP90 suppresses the expression of HSPs by repressing HSF1. Geldanamycin, an HSP90 inhibitor, cleaves HSF1 from the HSP90/HSF1 complex and consequently promotes the production of HSP70 and HSP40 (Chebotareva et al. 2017). In our study, increased HSP90 expression appeared to inhibit further increases in HSP70 and HSP40, and attenuated their protective effects in CKD by inhibiting HSF1.
Ghosh et al. (2018) demonstrated that HSP90 is an important chaperone for Hb maturation in erythroid and non-erythroid cells, enabling heme insertion into Hb-β and Hb-α (Ghosh et al. 2018). HSP90 inhibitors prevent Hb maturation by blocking heme insertion (Ghosh et al. 2018). Additionally, some clinical trials on cancer treatments showed anemia to be a side effect of most HSP inhibitors (Pillai & Ramalingam 2012). We found a positive correlation between Hb and serum HSP90 levels. This implies that increased serum HSP90 expression has a role in anemia in CKD.
HSP70 and HSP72 are the most abundant HSPs in the cell. Urine HSP72 levels increase gradually in rats as renal ischemia progresses (Barrera-Chimal et al. 2011). HSP70 and HSP72 are involved in many intracellular pathways. HSP70 inhibits apoptosis in various ways and exerts anti-inflammatory effects, especially by inhibiting the NF-kB pathway (Maddock and Westenfelder 1996; Neuhofer et al. 2004). A protective role of HSP70 has been demonstrated in animal models and cell-culture studies, in which urea levels were elevated (Mao et al. 2008; Barisic et al 2002; Kim et al. 2014). Inhibition of HSP70 suppressed Treg cells and abolished their protective effects (Kim et al. 2014). Induction of HSP70 attenuated fibrosis progression in models of unilateral ureteral obstruction (Mazzei et al. 2015). Clinical studies evaluating the role of HSP70/72 in CKD are limited. Musial et al. (2010) found that anti-HSP70 was elevated in children with CKD, although serum HSP70 levels did not differ from those of controls (Musial et al. 2010). Urine HSP70 levels were increased approximately fourfold in adults with stage 4–5 CKD compared to healthy controls (Lebherz-Eichinger et al. 2012). In our study, serum HSP70 levels were higher in CKD patients than in controls, although serum HSP72 levels at baseline did not differ between the groups. However, serum HSP70 levels were higher at 24 months compared to baseline. Urine HSP70 and HSP72 levels were higher in patients than in controls during the study period, although they decreased over time. Anti-HSP70 levels were also higher in patients than in controls and increased over time. Serum HSP70 and HSP72 levels may be dynamically controlled and affected by various factors. Progressive decreases in urine HSP72 levels in children with CKD could provide insights into renal injury. Our results imply that the renal stress response mediated by HSP72 decreases as CKD progresses.
HSP40 acts as a co-chaperone of HSP70. HSP40 recognizes unfolding or misfolding proteins and transports them to HSP70 for refolding (Alderson et al. 2016). HSP47 is an important and specific chaperone for collagen biosynthesis (Beck et al. 2000). Overexpression of HSP47 involves fibrosis and glomerulosclerosis, which proceed in accordance with increasing collagen production (Chebotareva et al. 2017). HSP47 expression is increased during fibrosis in mouse models of unilateral ureteral obstruction (Moriyama et al. 1998; Xiao et al. 2012). However, there are no clear data regarding the roles of HSP40 and HSP47 in CKD. Urine and serum HSP40 and HSP47 levels were higher in our patients at baseline, and gradually decreased over time. HSP40 and HSP47 expression during fibrotic processes may decrease once fibrosis is completed in some parts of the kidney. Although urinary HSP40 and HSP47 levels decreased over time, their elevated levels at month 24 indicate that the overproduction of HSP40 and HSP47 occurs only in those parts of the kidney in which a fibrotic process continues. A counteracting mechanism may inhibit increases in HSP47.
In our study, the median serum HSP27 level did not differ between the patients and the controls and also showed no increasing or decreasing trend over time. Urine HSP27 levels were higher in patients than controls, and urine HSP27 levels decreased over time. Musial and Zwolinska (2012, 2013) evaluated serum HSP27 in children with CKD and observed elevated levels in dialysis CKD patients versus pre-dialysis CKD patients. They concluded that serum HSP27 levels increase as CKD progresses, except between CKD stages 2 and 3 (Musial and Zwolinska 2018). Lebherz-Eichenger et al. (2012) demonstrated elevated serum HSP27 levels in adult CKD stage 3–5 patients, but not in stage 1–2 patients. Also, although urine HSP27 was elevated in CKD stage 2 and 5 patients, this was not the case in stages 1, 3, and 4 patients (Lebherz-Eichenger et al. 2012). Musial and Zwolinska (2018) demonstrated differences in urine HSP27/Cr levels between CKD patients and controls from stage 4 CKD onward. However, comparison with our study is problematic because of differences in the study designs. The aforementioned studies evaluated a single urine or serum sample in CKD patients, whereas we measured HSPs on multiple occasions in the same patients. Nevertheless, obvious dysregulation of HSP27 occurs in children with CKD; urine HSP27 levels remained higher in our patient group at month 24. HSP27 plays a role in both extrinsic and intrinsic apoptotic pathways. HSP27 inhibits apoptotic pathways by blocking the interaction between Fas and its receptor (Fas-L) in an extrinsic way and by inhibiting cytochrome c release in an intrinsic way (Vidyasagar et al. 2012). HSP27 inhibited apoptosis and promoted autophagy in ischemia/reperfusion-induced experimental models of acute kidney injury (Matsumoto et al. 2015). The increase in urine HSP27 expression seen in CKD may reflect a mechanism designed to suppress apoptosis. HSP27 overexpression has a beneficial effect in ischemia, toxic tubular injury, and renal fibrogenesis (Kim et al. 2010; Vidyasagar et al. 2012; Chebotareva et al. 2017). Additionally, overexpression of HSP27 reduces oxidative stress by increasing levels of glutathione. Multiple stressors are involved in CKD progression, such as reactive oxygen species, uremic toxins, inflammation, and apoptosis. Increased urine HSP27 levels reflect a protective effect of these stressors in CKD. Also, HSP27 has a protective effect against renal fibrogenesis and decreases oxidative stress. However, this protective effect appears to wane with decreases in urine HSP27 levels as CKD progresses.
Musial et al. (2009, 2010) demonstrated that serum HSP60 levels were lower in CKD and dialyzed patients compared to controls; however, anti-HSP60 levels were higher than in the control group. Our pre-dialysis patient group had higher serum and urine HSP60 and anti-HSP60 levels than the controls. However, serum HSP60 and urine HSP60 levels were decreased, and anti-HSP60 levels increased, at month 24. Thus, HSP60 levels decreased with CKD progression. Considering this finding together with those of Musial et al. (2009, 2010), HSP60 levels may be lowest when patients reach the ESRD stage. Our results indicate that anti-HSP60 has opposite effects to HSP60 on CKD progression, which implies that anti-HSP60 impedes the beneficial effects of HSP60 and also reduces the levels thereof.
Despite the fact that FeHSPs were higher in patients than in controls, they did not change over time. The urine HSPs always remained at higher levels than in the controls, although some serum HSPs did not differ between the controls at some points of the study (Tables 2 and 3). According to these results, we consider that urine HSPs may originate from the kidney itself. The decrease of urine HSPs over time without any change in FeHSPs may arise from the deterioration of the renal stress response rather than from a decrease in renal excretion of HSPs. Since there was no difference between baseline and 24-month urine protein/Cr ratios in our patients, we assume that there was no clinically significant increase in the permeability of the glomerular filtration barrier in our patient group during the study period. Therefore, we consider that the HSP changes with time may not be related to changes of membrane porosity.
There were no differences in patients at different CKD stages at baseline in terms of most HSPs except urine HSP70 and HSP72. Some studies reported different HSP70 and 27 levels among the different CKD stages (Musial and Zwolinska 2018; Lebherz-Eichinger et al. 2012). Because of the different concept of our study, we evaluated the course of HSP levels over time in patients who were classified separately according to the baseline CKD stage group. We observed that the changes of urine HSPs over time were more prominent in patients with CKD stage 3 at baseline, although most HSPs showed the similar pattern that of the whole CKD group. These results are reasonable considering that progression and complication rates of CKD increase in stage 3.
In addition to their effects and their role as molecular chaperones, HSPs cooperate with each other and with other chaperones in intracellular and extracellular environments. HSP60 and HSP70 have anti-inflammatory properties and act synergistically to regulate the immune response (Chebotareva et al. 2017). In our study, a positive correlation was found between HSP60 and HSP70 levels in both serum and urine, indicating that they work together to reduce inflammation in CKD. Some experimental studies reported that HSP27, HSP70, HSP60, and HSP90 were co-expressed in the kidney, under both normal and pathological conditions (Dihazi et al. 2011; Donnelly et al. 2013; O'Neill et al. 2013; Chebotareva et al. 2017). HSP40 acts a co-chaperone of HSP70 in an ATP-dependent manner, and HSP40/70 complexes recruit HSP90. Together, they regulate the integrity of proteins by forming HSP40/70 and HSP40/70/90 complexes (Kampinga and Bergink 2016; Chebotareva et al. 2017). We demonstrated that serum HSP40, HSP70, and HSP90 levels were correlated, as were urine HSP40 and HSP70 levels. Sreedharan et al. (2011) demonstrated that blockade of HSP70 induction in cultured proximal tubule cells attenuated the cytoprotective effects of HSP27 overproduction. There was a correlation between serum HSP27 and HSP70 levels, but this was not seen in urine samples. However, both HSP27 and HSP70 levels were decreased at month 24 relative to baseline. Although there were some correlations among HSPs, their synergistic cell-protecting effects are probably diminished in the uremic milieu.
Despite its relatively small sample size, our study has important implications. We demonstrated elevated HSP urine levels in children with CKD, with a tendency to decrease over time. These results imply that the protective roles of HSPs in CKD diminish over time. It has been suggested that HSP90 has a detrimental effect on the course of CKD, which is supported by our finding that serum and urine HSP90 levels increased over time. CKD progression is a complicated process; this study demonstrated that HSPs are involved in, but do not predict, CKD progression.
Data availability
The original data are available after contact with the corresponding author.
Code availability
Not applicable.
References
Alderson TR, Kim JH, Markley JL (2016) Dynamical structures of Hsp70 and Hsp70-Hsp40 Complexes. Structure 24(7):1014–1030
Barrera-Chimal J, Pérez-Villalva R, Cortés-González C, Ojeda-Cervantes M, Gamba G, Morales-Buenrostro LE, Bobadilla NA (2011) Hsp72 is an early and sensitive biomarker to detect acute kidney injury. EMBO Mol Med 3(1):5–20
Barrera-Chimal J, Perez-Villalva R, Ortega JA, Uribe N, Gamba G, Cortes-Gonzalez C, Bobadilla NA (2014) Intra-renal transfection of heat shock protein 90 alpha or beta (Hsp90alpha or Hsp90beta) protects against ischemia/reperfusion injury. Nephrol Dial Transplant 29(2):301–312
Barisic K, Petrik J, Rumora L, Cepelak I, Grubisic TZ (2002) Expression of Hsp70 in kidney cells exposed to ochratoxin A. Arch Toxicol 76(4):218–226
Barutta F, Pinach S, Giunti S, Vittone F, Forbes JM, Chiarle R, Arnstein M, Perin PC, Camussi G, Cooper ME, Gruden G (2008) Heat shock protein expression in diabetic nephropathy. Am J Physiol Renal Physiol 295(6):F1817-1824
Beck FX, Neuhofer W, Muller E (2000) Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Renal Physiol 279(2):F203-215
Chebotareva N, Bobkova I, Shilov E (2017) Heat shock proteins and kidney disease: perspectives of HSP therapy. Cell Stress Chaperones 22(3):319–343
Dihazi H, Dihazi GH, Mueller C, Lahrichi L, Asif AR, Bibi A, Eltoweissy M, Vasko R, Mueller GA (2011) Proteomics characterization of cell model with renal fibrosis phenotype: osmotic stress as fibrosis triggering factor. J Proteomics 74(3):304–318
Donnelly BF, Needham PG, Snyder AC, Roy A, Khadem S, Brodsky JL, Subramanya AR (2013) Hsp70 and Hsp90 multichaperone complexes sequentially regulate thiazide-sensitive cotransporter endoplasmic reticulum-associated degradation and biogenesis. J Biol Chem 288(18):13124–13135
Ghosh A, Garee G, Sweeny EA, Nakamura Y, Stuehr DJ (2018) Hsp90 chaperones hemoglobin maturation in erythroid and nonerythroid cells. Proc Natl Acad Sci U S A 115(6):E1117-e1126
Harrison EM, Sharpe E, Bellamy CO, McNally SJ, Devey L, Garden OJ, Ross JA, Wigmore SJ (2008) Heat shock protein 90-binding agents protect renal cells from oxidative stress and reduce kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 295(2):F397-405
Kampinga HH, Bergink S (2016) Heat shock proteins as potential targets for protective strategies in neurodegeneration. Lancet Neurol 15(7):748–759
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:1–150
Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, D’Agati VD, Lee HT (2010) Selective renal overexpression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 299(2):F347-358
Kim MG, Jung Cho E, Won Lee J, Sook Ko Y, Young Lee H, Jo SK, Cho WY, Kim HK (2014) The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int 85(1):62–71
Lebherz-Eichinger D, Ankersmit HJ, Hacker S, Hetz H, Kimberger O, Schmidt EM, Reiter T, Horl WH, Haas M, Krenn CG, Roth GA (2012) HSP27 and HSP70 serum and urine levels in patients suffering from chronic kidney disease. Clin Chim Acta 413(1–2):282–286
Lebherz-Eichinger D, Krenn CG, Roth GA (2013) Keratin 18 and heat-shock protein in chronic kidney disease. Adv Clin Chem 62:123–149
Maddock AL, Westenfelder C (1996) Urea induces the heat shock response in human neuroblastoma cells. J Am Soc Nephrol 7(2):275–282
Matsumoto T, Urushido M, Ide H, Ishihara M, Hamada-Ode K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Taguchi T, Horino T, Fujimoto S, Terada Y (2015) Small Heat Shock Protein Beta-1 (HSPB1) Is upregulated and regulates autophagy and apoptosis of renal tubular cells in acute kidney injury. PLoS One 10(5):e0126229
Mazzei L, Docherty NG, Manucha W (2015) Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones 20(6):893–906
Moriyama T, Kawada N, Ando A, Yamauchi A, Horio M, Nagata K, Imai E, Hori M (1998) Up-regulation of HSP47 in the mouse kidneys with unilateral ureteral obstruction. Kidney Int 54(1):110–119
Mao H, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X (2008) HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 295(1):F202–F214
Musial K, Zwolinska D (2012) Hsp27 as a marker of cell damage in children on chronic dialysis. Cell Stress Chaperones 17(6):675–682
Musial K, Zwolinska D (2013) New markers of apoptosis in children on chronic dialysis. Apoptosis 18(1):77–84
Musial K, Zwolinska D (2018) Fractional excretion as a new marker of tubular damage in children with chronic kidney disease. Clin Chim Acta 480:99–106
Musial K, Szprynger K, Szczepanska M, Zwolinska D (2009) Heat shock proteins in children and young adults on chronic hemodialysis. Pediatr Nephrol 24(10):2029–2034
Musial K, Szprynger K, Szczepanska M, Zwolinska D (2010) "The heat shock protein profile in children with chronic kidney disease. Perit Dial Int 30(2):227–232
Neuhofer W, Holzapfel K, Fraek ML, Ouyang N, Lutz J, Beck FX (2004) Chronic COX-2 inhibition reduces medullary HSP70 expression and induces papillary apoptosis in dehydrated rats. Kidney Int 65(2):431–441
Noh H, Kim HJ, Yu MR, Kim WY, Kim J, Ryu JH, Kwon SH, Jeon JS, Han DC, Ziyadeh F (2012) Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-beta type II receptor. Lab Invest 92(11):1583–1596
O’Neill S, Ingman TG, Wigmore SJ, Harrison EM, Bellamy CO (2013) Differential expression of heat shock proteins in healthy and diseased human renal allografts. Ann Transplant 18:550–557
O’Neill S, Harrison EM, Ross JA, Wigmore SJ, Hughes J (2014) Heat-shock proteins and acute ischaemic kidney injury. Nephron Exp Nephrol 126(4):167–174
Pillai RN, Ramalingam SS (2012) Hsp90 inhibitors. J Thorac Oncol 7(16 Suppl 5):S407-408
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20(3):629–637
Sreedharan R, Riordan M, Thullin G, Van Why S, Siegel NJ, Kashgarian M (2011) The maximal cytoprotective function of the heat shock protein 27 is dependent on heat shock protein 70. Biochim Biophys Acta 1813(1):129–135
Vidyasagar A, Wilson NA, Djamali A (2012) Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 5(1):7
Xiao HB, Liu RH, Ling GH, Xiao L, Xia YC, Liu FY, Li J, Liu YH, Chen QK, Lv JL, Zhan M, Yang SK, Kanwar YS, Sun L (2012) HSP47 regulates ECM accumulation in renal proximal tubular cells induced by TGF-beta1 through ERK1/2 and JNK MAPK pathways. Am J Physiol Renal Physiol 303(5):F757-765
Acknowledgements
We gratefully acknowledge the support of The Scientific and Technological Research Council of Turkey (TUBITAK). We are also very thankful to the children who participated in this study, and to our dedicated chemist Orhan Tepeli.
Funding
This study was financially supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) within 1001-Scientific and Technological Research Projects Funding Program (project number:115S365).
Author information
Authors and Affiliations
Contributions
Conceptualization, design, and methodology: Zeynep Nagehan Yuruk Yildirim, Alev Yilmaz, Fatma Savran Oguz, Sevinc Emre, Sebahat Usta Akgul. Material preparation and data collection; Zeynep Nagehan Yuruk Yildirim, Harika Alpay, Aysel Kiyak, Nurver Akinci, Sevgi Yavuz, Gul Ozcelik, Ibrahim Gokce, Nese Ozkayin, Nurdan Yildiz, Cemile Pehlivanoglu, Nilufer Goknar, Seha Saygili, Sebahat Tulpar, Nuran Kucuk, Ilmay Bilge, Mehmet Tasdemir, Ayse Agbas, Bagdagul Aksu, Ahmet Nayir. Formal analysis and investigation: Fatma Savran Oguz, Sebahat Usta Akgul, Asuman Gedikbasi. Statistical analysis: Bagdagul Aksu, Ahmet Dirican. The first draft of the manuscript was written by Zeynep Nagehan Yuruk Yildirim, Alev Yilmaz, and Bagdagul Aksu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval for this study was obtained from the Local Ethics Committee (Project No: 2015/487).
Consent to participate
Informed consent was obtained from the parents of all participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuruk Yildirim, Z.N., Usta Akgul, S., Alpay, H. et al. PROGRESS STUDY: Progression of chronic kidney disease in children and heat shock proteins. Cell Stress and Chaperones 26, 973–987 (2021). https://doi.org/10.1007/s12192-021-01239-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-021-01239-9