Abstract
Urinary heat shock protein 70 (Hsp70) is rapidly increased in patients with clinical acute kidney injury, indicating that it constitutes a component of the endogenous stress response to renal injury. Moreover, experimental models have demonstrated that Hsp70 activation is associated with the cytoprotective actions of several drugs following obstruction, including nitric oxide (NO) donors, geranylgeranylacetone, vitamin D, and rosuvastatin. Discrete and synergistic effects of the biological activities of Hsp70 may explain its cytoprotective role in obstructive nephropathy. Basic studies point to a combination of effects including inhibition of apoptosis and inflammation, repair of damaged proteins, prevention of unfolded protein aggregation, targeting of damaged protein for degradation, and cytoskeletal stabilization as primary effectors of Hsp70 action. This review summarizes our understanding of how the biological actions of Hsp70 may affect renal cytoprotection in the context of obstructive injury. The potential of Hsp70 to be of central importance to the mechanism of action of various drugs that modify the genesis of experimental obstructive nephropathy is considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper urinary tract dilatation, secondary to congenital developmental errors, is one of the most common sonographic findings diagnosed prenatally. It is usually caused by transient urine flow impairment at the level of the uretero-pelvic junction or vesico-ureteric junction (Dias et al. 2014) and is estimated to occur in up to 1 % of live births (Mazzei et al. 2012). Sustained, antenatal obstruction results in hydronephrosis which in turn results in significant injury to the developing kidney (Becker and Baum 2006). Hence, congenital hydronephrosis constitutes a major cause of renal insufficiency in neonates and infants (Ahmadzadeh et al. 2009).
A major feature of the injury sustained by the kidney during obstruction is a profound induction of apoptosis in the tubular epithelium (Docherty et al. 2006). The stimuli responsible for the induction of apoptosis are varied and include mechanical stress secondary to pulsatile retrograde pressure transfer from ureteric peristalsis (Power et al. 2004), hypoxia (Cachat et al. 2003) occurring secondary to reductions in renal blood flow in the obstructed kidney, and inflammatory reactions (Manucha 2007) caused by the influx of innate immune cells in response to chemotactic signals from the damaged renal parenchyma. Apoptotic cell death in the renal tubule is associated with reductions in Bcl-2/Bax ratios and caspase activation (Dendooven et al. 2011) triggered by both the classical mitochondrial and receptor-mediated (most notably tumor necrosis factor-α) apoptotic pathways (Manucha et al. 2005).
Sustained ureteric obstruction elicits inflammatory and fibrotic progression in the affected kidney, characterized by macrophage and lymphocyte infiltration and fibroblast activation with attendant extracellular matrix deposition in the tubulointerstitium (Manucha et al. 2007).
Thus, congenital obstructive nephropathy disrupts normal renal development and causes chronic progressive interstitial fibrosis, which contributes to renal growth arrest, ultimately leading to chronic renal failure. Therefore, renal growth and development are severely affected by obstructive injury through complex interactions between regulators of cell proliferation and apoptosis. A large number of cell types present in the early embryonic kidney are not found at later stages of development, with apoptosis as the main mechanism for cell selection. Normal kidney development requires the conversion of mesenchymal cells into polarized epithelial cells (Ekblom 1989), an exceptional process during nephrogenesis.
Although congenital obstruction is the primary cause of end-stage renal disease in children, the mechanisms underlying chronic obstructive nephropathy have not yet been completely elucidated (Mazzei et al. 2010a). Relating changes in gene expression to phenotypic patterns in human congenital obstructive nephropathy represent an advance in the identification of the genes/proteins that play important roles (Trnka et al. 2010).
Studies in neonatal rats may provide insight into the functional development of the kidney, since nephrogenesis continues at a rapid pace up to day 8 after birth and is virtually complete by days 14–19. In this regard, experimentally induced unilateral ureteral obstruction (UUO) has emerged as an interesting model for studying neonatal hydronephrosis and for the assessment of potential therapeutic approaches. This model mimics, in an accelerated manner, the different stages of human neonatal hydronephrosis leading to tubulointerstitial fibrosis, apoptosis, and tubular atrophy (Mazzei et al. 2010a). UUO in neonatal rats impairs nephrogenesis, glomerular maturation, and tubular cellular proliferation (Chevalier 1998). The earlier the UUO maneuver is performed, the more severe is the growth impairment of the ipsilateral kidney (Chevalier et al. 2002).
While pharmacological protection from tubular apoptosis is clearly likely to be of benefit to the preservation of renal structure and function in the adult kidney, the situation in neonatal obstruction in rodents and in utero obstruction in humans is somewhat more nuanced. This is based on the fact that in these cases, the kidney is still passing through the nephrogenic program, and in order for a pharmacological agent to be of true worth, it must not only be cytoprotective but also allow for preservation of nephrogenesis, in order that the developing kidney achieves its optimal quota of excretory units and hence its optimal function (Mazzei et al. 2012).
Many signals may positively or negatively affect the rat kidney after UUO by altering regulatory proteins that initiate apoptosis and inducing changes in mitochondrial function (Manucha 2007). Nitric oxide (NO) can either induce or inhibit apoptosis in different circumstances (Kim et al. 1999), with increases in factors such as heat shock protein 70 and Bcl-2 playing an important role in the former.
Immediately after UUO, heat shock protein (HSP) expression was increased to minimize cell death and preserve cell integrity by inhibiting apoptotic pathways. However, after 14 days of obstruction, decreased endogenous NO and lower inducible nitric oxide synthase (iNOS) expression at messenger RNA (mRNA) and protein levels associated with downregulation of heat shock protein 70 (Hsp70) expression were shown in apoptosis induction (Manucha et al. 2011; Mazzei and Manucha 2013); therefore, harnessing and pharmacological prolongation of the early response may be of therapeutic value (Lebherz-Eichinger et al. 2012).
Hsp70 in normal renal physiology
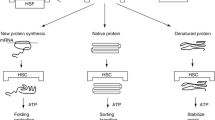
HSPs are abundant cellular proteins and are phylogenetically highly conserved. They play essential roles in mediating protein folding, assembly, transport, degradation, translocation, or refolding stress denatured proteins, preventing their irreversible aggregation with other proteins in the cell and removing irreparably damaged proteins that would otherwise accumulate and initiate cell death (Morimoto 1998; Bukau et al. 2006; Goloubinoff and De Los Rios 2007; Manucha and Vallés 2008a, b; Hartl and Hayer-Hartl 2009; Kampinga and Craig 2010; Manucha et al. 2011).
As alluded to above, the expression of HSPs can be markedly upregulated by various stressors, in a process termed heat-shock response (HSR). This confers cytoprotection from subsequent injuries and, hence, has clinical application such as preconditioning strategies in transplantation and major cardiac surgery (Jones et al. 2011).
Hsp70 consists of an N-terminal (ATPase domain) and C-terminal substrate-binding domain connected by a short flexible linker. Its principal locations within the cells are cytosol, nucleus, and mitochondria (O’Neill and Hughes 2014). Hsp70 induction is an early survival signal elaborated by stressed cells to counter cellular damage and hasten recovery (Gething and Sambrook 1992).
The main representative chaperone of the Hsp70 family, constitutively expressed, is heat shock protein 73 (Hsp73; 70-kDa heat shock cognate protein). Hsp73 have been detected in cells throughout the kidney. With the exception of podocytes, Bowman’s epithelium, and proximal tubule cells, the immunoreactivity for Hsp73 is similar in the nucleus and cytoplasm. This ubiquitous presence of Hsp73 can be attributed to the need, also of nonstressed cells, for assistance in protein folding, trafficking, and controlled degradation. Additionally, there is a strong heat shock protein 72 (Hsp72) expression, the principal inducible member of the Hsp70 family, in the normal kidney. Hsp72 was identified in the renal cortex (only in individual collecting duct cells) and medulla. All tubules were stained weakly in the outer medulla, while an intense staining was noted in the papilla collecting duct epithelium and in the urothelium lining the papilla (Beck et al. 2000). A hypothesis has emerged to explain this based on an inference that renal Hsp72 expression increases along the cortico-medullary axis as a function of increases in extracellular tonicity.
The expression of Hsp70 was closely correlated with changes in interstitial osmolality during the development of the kidney. Hsp70 plays an important role in the protection of the long ascending limb of Henle’s loop (ATL) during renal development. In adult animals, Hsp70 was expressed in the medullary thin ascending limb of Henle’s loop and inner medullary collecting duct (IMCD) (Kang et al. 2011).
Hsp70 displays weak ATPase activity and cyclically binds and releases hydrophobic segments of unfolded and partially folded proteins in an ATP/ADP-dependent reaction cycle. The complex consisting of ADP, Hsp70, and nonnative polypeptides is relatively stable, thus preventing incorrect interaction between protein domains. The exchange of ADP for ATP results in a low-affinity complex that releases the substrate polypeptide rapidly and thus allows the folding process to advance (Beissinger and Buchner 1998; Fink 1999; Hartl 1996). The binding and release of substrate polypeptides to Hsp70 are modulated by co-factors (Hsp40, Hip, BAG) that may also regulate ADP/ATP exchange or ATP hydrolysis (Luders et al. 1998).
Hsp70 in kidney disease
Inducible Hsp70 expression has been shown to enhance the survival of mammalian cells exposed to numerous types of stimuli that induce stress and apoptosis (Jäättelä 1999). Furthermore, expression of Hsp72 is one of the major mechanisms acting against cellular injury; it protects renal epithelial cells from apoptosis by ameliorating outer mitochondrial membrane injury and inhibiting subsequent caspase activation (Li et al. 2002). A low expression of Hsp70 is correlated with several kidney diseases (Rusai et al. 2010).
Hsp72 induction ameliorates both renal tubular epithelial cell apoptosis and renal tubulointerstitial fibrosis in obstructive nephropathy (Mao et al. 2008). Furthermore, HSPs are believed to prevent injury and restore normal cellular function in the kidney following ischemia-reperfusion injury (IRI). Indeed, there is a marked change in renal HSP expression with hsp70 gene products showing a 43-fold increase and hsp27 a 12-fold increase (Zhang et al. 2008). HSPs interact with important proteins involved in apoptotic pathways, and this has crucial consequences for cell survival, proliferation, and apoptosis following IRI (Lanneau et al. 2008). For instance, in renal IRI, Hsp70 limits apoptosis by controlling the activity of the kinases Akt and glycogen synthase kinase 3β that regulate the activity of the proapoptotic protein Bax (Wang et al. 2011). As a result, renal epithelial cells might be rescued from apoptotic cell death following HSP induction (Aufricht 2005). It is therefore of interest that cortical Hsp70 levels following renal IRI inversely correlate with apoptosis, tubular injury, and renal dysfunction (Wang et al. 2011).
Hsp70−/− mice show worsened kidney function, tubular injury, and survival following renal IRI. The protective effect from renal IRI provided by the Hsp70-inducing agent, geranylgeranylacetone, is also abrogated in Hsp70 knockout mice (Wang et al. 2011). Other strategies have been used to manipulate HSP responses and protect kidneys from ischemic damage. For example, the inhibition of Hsp90 may mediate protection from ischemic damage through induction of Hsp70 or nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) deactivation, and selective renal overexpression of Hsp27 (O’Neill et al. 2012; Sonoda et al. 2010; Kim et al. 2010; Harrison et al. 2008).
Mediators and mechanisms of Hsp70-based cytoprotection
Interaction between nitric oxide and Hsp70
Both pro-apoptotic and anti-apoptotic effects of NO have been demonstrated (Cachat et al. 2003). Whereas excessive NO production induces cell death (Messmer and Brune 1996), protection against apoptosis has been shown at lower levels which correspond to those capable of inducing Hsp70 (Kim et al. 1997; Mannick et al. 1997; Manucha and Vallés 2008a, b).
Renal damage, including apoptosis and fibrosis, is significantly improved by treatment with L-arginine, suggesting that increased NO availability could be beneficial in UUO relief (Ito et al. 2005). Yoo and colleges reported that, in complete UUO, iNOS attenuates apoptosis and increases renal parenchymal thickness (Yoo et al. 2010). We have found decreased endogenous NO, in neonatal UUO (Manucha and Vallés 2008a, b). In addition, endothelial nitric oxide synthase (eNOS) knockout mice develop tubule cell apoptosis and necrosis (Forbes et al. 2007).
A novel alternative antiapoptotic mechanism for NO is the induction of heat shock protein 32 (Hsp32; heme oxygenase 1 or HO-1) and Hsp70, by means of NO-mediated modification in intracellular antioxidants levels (Mosser et al. 1997). The mechanism by which NO stimulates the expression of Hsp70 may involve the interaction of NO with thiol-containing molecules. Ample evidence exists to support the view that NO readily oxidizes low molecular weight thiols, forming S-nitrosothiols and disulfide. Among cellular low molecular weight thiols, glutathione is the most abundant as well as being one of the intracellular targets of NO. NO can oxidize intracellular reduced glutathione and thereby change the antioxidant levels within the cell, resulting in oxidative or nitrosative stress. This action stimulates the induction of Hsp32 and Hsp70, which protect cells from apoptotic cell death (Kanner et al. 1991; Harbrecht et al. 1994).
Both reactive oxygen intermediate (ROI) production and lipid peroxidation are inhibited by NO donor-induced Hsp70 expression. Furthermore, only cells overexpressing Hsp70 were found to be protected from both ROI and tumor necrosis factor alpha (TNF-α)-induced cytotoxicity. Overexpression of Hsp27 only protected from exogenous ROI exposure but not from TNF-α cytotoxicity (Jäättelä et al. 1992; Jäättelä and Wissing 1993).
Studies in our laboratory have suggested that NO can produce resistance to obstruction-induced cell death by inhibiting the intrinsic mitochondria apoptotic pathway, through the induction of Hsp70 expression (Fig. 1a). In obstructed neonatal rats, in vivo administration of L-arginine induced Hsp70 expression, which was associated with cytoprotection from apoptosis and transiently decreased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. Opposite effects were obtained after nitro L-arginine methyl ester (L-NAME) treatment (Manucha and Vallés 2008a, b).
Implication of Hsp70 as a mediator of the anti-apoptotic effects of nitric oxide. a Cellular stress is associated with protein misfolding and increases in intracellular NO. In response to such stresses, HSF trimerises in an NO-dependent manner and translocates to the nucleus to transcriptionally activate the expression of Hsp70 via interaction with HSEs on the promoter region. The net effect of this is an increase in the cellular chaperone capacity which favors cell survival. Additionally Hsp70 via its links with WT-1 stabilizes Bcl-2 limiting the potential for Cyt C release from the mitochondrion and the activation of the intrinsic apoptotic pathway. Positive feedback on this system is achieved via the activity of iNOS. b Reduced cellular NO production decreases WT-1, and Bcl-2 levels. This is associated with Cyt C release and the activation of the intrinsic pathway of apoptosis. Hsp70 expression is also reduced and sequestration with Hsf1 prevents the inhibition of APAF-1 which promotes the progression of the apoptotic pathway. HSF heat shock factor, NO nitric oxide, WT-1 Wilms tumor 1, Bcl-2 B cell lymphoma 2, APAF-1 apoptotic protease activating factor 1
Nitric oxide mediated induction of Hsp70: role of Hsf1 and beyond
Pretreatment of hepatocytes with NO has been shown to alter the redox state accompanied by oxidation of glutathione (GSH) and by formation of S-nitrosoglutathione. A GSH-oxidizing agent (diamide) and a GSH-alkylating agent (N-ethylmaleimide) both induced Hsp70 mRNA, but a GSH synthesis inhibitor (buthionine sulfoximine) did not; this suggests that NO induces Hsp70 expression through GSH oxidation (Kim et al. 1997). Such induction may occur via the activation of heat shock factor 1 (Hsf1) (Xu et al. 1997).
Accumulating evidence indicates that NO stimulates the S-nitrosylation of numerous proteins (Li et al. 1997), by interaction with a free sulfhydryl group to form a rapidly decaying S-nitrosothiol. NO may serve as an oxidizing agent to form a disulfide bond, producing a relatively persistent covalent modification (Ignarro 1990), which is essential for cell survival (Kolpakov et al. 1995). Indeed, NO-induced Hsf1 activation leading to Hsp70 expression was completely blocked by dithiothreitol, a disulfide-reducing agent. Supporting these data, induced Hsp70 could play a role in the repair of denatured proteins modified by NO and in folding of nascent polypeptide chains (Xu et al. 1997).
Hsf1 mediates stress-induced heat shock gene expression (Fawcett et al. 1994; Sorger 1991).
The accumulation of misfolded proteins causes the mobilization of the HSPs resulting in the free pool of Hsp70, and the subsequent removal of the negative regulatory influence on Hsf1 activation, during heat shock or other stresses. The released Hsf1 is phosphorylated and assembles into trimers, acquires DNA binding activity, and leads to elevated hsp70 mRNA transcripts (Fig. 1a). The molecular mechanism underlying the antiapoptotic effects of NO-mediated HSP expression may be associated with two possibilities (Harbrecht et al. 1992). The first is the direct suppression of apoptotic signal transduction involving the inhibition of caspase family protease activation. The second involves the chaperon-mediated import of precursor proteins into mitochondria by HSPs. This action controls mitochondrial function and membrane permeability, thereby preventing the release of cytochrome c that is required for further activation of caspases. Other results indicated that Hsp70 could modulate the apoptosis cascade during renal obstruction (Dmitrieva and Burg 2005; Manucha et al. 2005; Van de Water et al. 2006). We have reported that the apoptotic effect created by lower NO was directly associated with decreased Hsp70 expression and induction of the apoptotic signal transduction involving the activation of caspase 3 by decreasing stabilization of Bcl-2 (Fig. 1b) (Manucha and Vallés 2008a, b).
Bcl-2-dependent and Bcl-2-independent effects of Hsp70 induction on the intrinsic (mitochondrial) apoptosis pathway
It is unlikely that Hsp70 acts directly as a mitochondrial antioxidant. Hsp70 may instead block signal transduction to the mitochondria, resulting in the inhibition of mitochondrial ROI production either by inhibiting second lipid messenger(s) to the mitochondria ( Jacquier-Sarlin et al. 1994) or by preventing the interaction between the death domain of TNF-α receptor and signal molecule(s) (Hsu et al. 1995). Alternatively, it is possible that Hsp70 may enhance the chaperon-mediated import of precursor proteins into the mitochondria which control mitochondrial function and lead to decreased reactive oxygen species (ROS) formation (Harkness et al. 1994). As previously mentioned, our laboratory suggested that the effect of the interaction of NO with Hsp70 is a result of their capacity to prevent the activation of the mitochondrial apoptotic pathway in neonatal early kidney obstruction. Induction of Hsp70 protects cells not only from damage due to apoptosis induction but also from damage due to oxidative injury.
The apoptotic cascade initiation is in part regulated by protein-protein interactions between death-promoting (Bax, Bad, and Bcl-xs) and death-inhibiting (Bcl-2, Bcl-xL, and Mcl-1) members of the Bcl-2 family (Nuñez and Clarke 1994). Bcl-2-expressing cells resist apoptosis initiated by a number of physiological and stressful conditions (Tsujimoto 1989). In prior heat stress in ATP-depleted renal tubular cells, the interaction between Hsp70 and Bcl-2 may be responsible, at least in part, for the protection afforded by Hsp70 against ATP depletion injury (Wang et al. 1999). Binding of Bcl-2 and Hsp70 increased after L-arginine administration (Fig. 1a) (Manucha and Vallés 2008a, b).
Given its localization within mitochondria and its role in preventing cytochrome c release, preservation of Bcl-2 by Hsp70 could account for the protection of epithelial cells (Borkan et al. 1993).
It has also been proposed that HSPs act by means of a mechanism independent of Bcl-2, intervening at several points to halt progression of the apoptotic cascade (Strasser and Anderson 1995). Previous studies have indicated that at least some of the antiapoptotic activity of Hsp70 can be attributed to its ability to suppress the activity of JUN-kinase (Kumar and Tatu 2003; Gabai et al. 1997) (Fig. 1a). Activation of stress-activated protein kinase SAPK/c-Jun N-terminal kinase (JNK) has been strongly inhibited in cells in which Hsp70 was induced to a high level, indicating that Hsp70 blocks apoptosis by inhibiting signaling events upstream of SAPK/JNK activation. Hsp70 also inhibits apoptosis events at some point downstream of SAPK/JNK activation (caspase 3-mediated). Alternatively, Hsp70 may prevent cell death by interfering with the ability of cytochrome c and Apaf-1 to recruit pro-caspase 9. In this case, Hsp70 suppresses apoptosis by directly associating with Apaf-1 and blocking the assembly of a functional apoptosome (Beere et al. 2000) (Fig. 1a).
Effect of Hsp70 on pro-inflammatory activation of epithelial cells
Inflammation is one of the most critical pathophysiological processes involved in the propagation of renal disease. Hsp70 and its receptors protect against inflammation through multiple mechanisms (Jones et al. 2011; Chen et al. 2007).
Typical pro-inflammatory agonists modify the heat shock-induced transcriptional program and expression of hsp genes following exposure to heat shock, suggesting a complex reciprocal regulation between the inflammatory pathway and the HSR pathway (Singh and Hasday 2013).
Hsp70 has both anti-inflammatory and pro-inflammatory effects depending on the nature of the stimulus, cell type, context, and intracellular or extracellular location (Kim and Yenari 2013). Intracellular effects are often anti-inflammatory. A decreased expression of renal intracellular Hsp72 may contribute to activation of the Toll-like receptor 4 (TLR4) signaling pathway (Yan et al. 2007). Toll-like receptors (TLRs) were established as moderators of renal inflammation (Valles et al. 2012). They are expressed in various cell types, including renal epithelial cells (Eleftheriadis and Lawson 2009; Eleftheriadis et al. 2012). TLR4 is a further regulator of NF-κB (O'Neill et al. 2014). Intracellular Hsp70 in sub-lethally stressed cells can limit pro-inflammatory NF-κB signaling by stabilizing IκB, inhibiting NF-κB p65 translocation to the nucleus or marking pro-inflammatory Hsp90 client proteins for degradation (O’Neill et al. 2012). Hsp90 inhibition upregulates protective HSPs (especially Hsp70) and potentially downregulates NF-κB by disruption of the IκB kinase complex (Fig. 2). NF-κB modulates several genes that participate in the inflammation during kidney injury (Sanz et al. 2010).
Opposing actions of extracellular versus intracellular Hsp70 on NF-κB pathway activation. Extracellular Hsp70 contributes to the inflammatory response through acting as a ligand for TLR4 via which it results in activation of the NF-κB pathway (1). Intracellular Hsp70 blocks NF-κB activation and p50/p65 nuclear translocation through inhibition of IKK mediated IκB phosphorylation. Hsp70 heat shock protein 70, TLR4 Toll-like receptor 4, NF-κB nuclear factor kappaB, IKK IκB kinase
Extracellular effects, on the other hand, can lead to inflammatory cytokine production or induction of regulatory immune cells and reduced inflammation. In the extracellular environment, HSPs appear to act as ligands or co-factors. Extracellular Hsp70 acts as a ligand for TLR4, and together they could participate in regulating innate immunity via NF-κB activation dependent pathways.
Extracellular Hsp72- induced cytokine release was found to be mediated through Toll-like receptor 2 (TLR2), TLR4, and downstream activation of NF-κB. This contrasts with the inhibition of NF-κB activation due to intracellular effects.
TLR4 initiates the signaling cascade triggered by lipopolysaccharide from Gram-negative bacteria. A role for Hsp70 in the response to lipopolysaccharide has been identified. Hsp70 and Hsp 90 can be immobilized in the plasma membrane and colocalize with lipopolysaccharide and TLR4, following an initial transient interaction of lipopolysaccharide with cluster of differentiation 14 (CD14). Lipopolysaccharide signaling is mediated by a large complex which can include Hsp70. The composition of the complex determines whether signaling results in induction or inhibition of the immune response.
On the other hand, and of particular interest, experimental data suggest that angiotensin II receptor, type 2 (AT2) through activation of NF-κB participates in the recruitment of renal inflammatory cells (Ruiz-Ortega et al. 2006). Previously, Ishizaka et al. (2002) demonstrated that angiotensin II (Ang II) infusion induces renal Hsp70. The increasing levels of Ang II induce pro-inflammatory cytokines, NF-κB activation, adhesion molecules, chemokines, growth factors, and oxidative stress (Grande et al. 2010; Klahr 2001; Manucha 2007). Interestingly, Hsp70 is involved in the regulation of Ang II-induced NF-κB. In agreement, HSPs were related to the pro-inflammatory transcription factor NF-κB (Voegeli et al. 2008).
Another fundamental step in the pro-inflammatory mechanism (Ferder et al. 2006) is the activation of the NADPH oxidase. It is believed that Hsp70 in proximal tubule membranes would exert cellular protection by modulation of the NADPH oxidase catalytic subunit Nox4 (Bocanegra et al. 2010). The NADPH oxidase family of enzymes has a major role catalyzing the production of superoxides and other ROS. In turn, they play an important role for cellular signal transduction; however, an excess may cause oxidative stress, currently known as a major cause of renal inflammation and subsequent damage (Joshi et al. 2013). The pro-inflammatory state would be amplified by changes in renal antioxidants and ROS levels (Rinaldi Tosi et al. 2011). In this context, both the oxidative stress and the induction of the mitochondrial apoptosis could be prevented by Hsp70 expression (Manucha et al. 2011).
Finally, Hsp70 interacts with the vitamin D receptor (VDR) and plays a role in controlling concentrations of the VDR within cells (Lutz et al. 2001). Vitamin D has also been demonstrated to be a nontoxic inducer of Hsp70 in the rat kidney (Kim et al. 2005). NADPH oxidase activity was reverted in mitochondrial fractions from vitamin D inducer–treated animals (García et al. 2012a, b). Furthermore, VDR-modulated Hsp70/AT1 expression may protect the kidneys of spontaneously hypertensive rats (SHR) at the structural and functional levels (García et al. 2014). Previously, vitamin D treatment increased Hsp70 expression which was localized to renal tubular cells in the outer medulla (Kim et al. 2005). Meanwhile, Adams et al. (2003) suggested that Hsp70-related intracellular vitamin D-binding proteins act as regulators of vitamin D metabolism. Hsp70 may interact with VDR prior to the activation of the latter by vitamin D (Swamy et al. 1999). Consistently, experimental and clinical evidence indicates that vitamin D deficiency (characterized by dysfunction of tubular epithelial cells and/or loss of renal parenchyma) and Ang II upregulation play a pivotal role in the progression of renal disease associated with obstructive nephropathy (Klahr 2001; Zhang et al. 2010).
Hsp70/NO renal cytoprotective action as consequence of statin treatment
Other strategies performed on slowing the progression of chronic kidney disease (CKD) have included the beneficial, lipid-independent effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) (Zhou et al. 2008). Statins exert beneficial effects upon CKD, including restoration/normalization of endothelial function, upregulation of NO, reduction in oxidative stress, vascular inflammation, and fibrosis (Zhou et al. 2004; Mizuguchi et al. 2004; Tian et al. 2006; Vieira et al. 2005).
Statins have been demonstrated to restore NO levels by several mechanisms. Previously, it has been demonstrated that NO generated from several compounds induces Hsp70, and this effect relies on the Hsf1 activation (Xu et al. 1997) through its nuclear translocation (Uchiyama et al. 2007).
Rosuvastatin, a member of statins drug class, prevents apoptosis induction and oxidative stress generation in association with increased eNOS and Hsp70 expression.
We have suggested that one mechanism by which rosuvastatin protects cortex tubular cells from obstruction-induced oxidative stress and apoptosis is through eNOS/Hsp70 interaction (Manucha et al. 2011).
Our group found increased hsf1 and hsp70 mRNA transcripts and enhanced Hsp70 protein levels in cortex membrane fractions from obstructed rosuvastatin-treated rats. Furthermore, after rosuvastatin administration, increased Hsp70 and eNOS expression was associated with reductions in tubular apoptosis.
These results suggest that after rosuvastatin treatment, the presence of eNOS associated with Hsp70 protein expression in cortex membrane fractions may serve to modulate oxidative stress and the apoptotic process in obstructed kidney (Manucha et al. 2011).
In relation to the mechanism by which increased Hsp70 expression in membrane fraction by rosuvastatin protects from apoptosis, it has been reported that selective overexpression of Bcl-2 ameliorates membrane damage (Saikumar et al. 1998). Previously, we showed that the interaction between Hsp72 and Bcl-2 could afford cytoprotection in obstruction (Manucha and Vallés 2008a, b). In addition to preventing cytochrome c-dependent caspase activation, Hsp70 can inhibit apoptosis by antagonizing the apoptosis-inducing factor, a proapoptotic protein that exhibits an NADPH oxidase activity, which contributes to DNA injury (Ruchalski et al. 2003). Furthermore, a physical interaction between Hsp70 and eNOS proteins by co-immunoprecipitation was demonstrated by our group.
We studied the effect of rosuvastatin treatment on animals subjected to UUO, since previous studies had shown rosuvastatin protection against podocyte apoptosis in vitro (Cormack-Aboud et al. 2009), despite that NO was thought to participate in tissue-specific cell maturation (Vasil’eva et al. 1997). However, there is a paucity of information concerning possible interactions between NO and expression of genes linked to kidney development and function, like wt-1 (Mazzei et al. 2010a). Our results, though, suggested that treatment with rosuvastatin might slow or even reverse the process of apoptosis during neonatal UUO by modulating WT-1 mRNA expression through renal NO bioavailability associated with Hsp70 interaction (Mazzei et al. 2010a).
Cellular stress response linked to Nrf2-related vitagene system in the pathogenesis of obstructive kidney disease: emerging role of mitochondrial dysfunction
Apoptosis and renal fibrosis are processes inherent to the CKD, and consequently a clear deregulation of the mitochondrial respiratory mechanism has been described in patients with CKD associated with an increase of the oxidative stress and inflammation (Manucha 2014). In this context, many strategies have been evaluated as new anti-inflammatory tools to regulate mitochondrial oxidative stress, which directly affects the inflammatory process and apoptosis. Thus, epigallocatechin-3-gallate (EGCG), a catechin polyphenol, has been proven to have many bioactivities, and the renoprotective effect of EGCG has been recently demonstrated in UUO (Zhou et al. 2013; Wang et al. 2015a). Interestingly, these studies demonstrated that EGCG restores kidney weight loss, renal dysfunction, oxidative stress, and inflammatory responses during UUO. In addition, EGCG could induce both NF-κB and nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2) nuclear translocation in the UUO kidney, thereby promoting HO-1 production (Wang et al. 2015b). These results indicate that the renoprotective effect of EGCG might be through its NF-κB and Nrf2 signaling pathway regulations. This is consistent with previous reports about the emerging relationship between cellular stress response and the Nrf2 (Calabrese et al. 2011). HO-1 appears to be a new anti-inflammatory, anti-oxidant, and anti-proliferative factor. However, humans differ quantitatively in their ability to mount an HO-1 response, and the ability of a patient with certain genotypes to respond may be an important endogenous protective factor (Exner et al. 2004).
On the other hand, cyclooxygenase type 2 (COX-2) is known to play a predominant role in the progression of kidney injury in obstructive nephropathy (Zuo et al. 2002; Manucha et al. 2004). Closely related to this, the efficacy of chitosan/small interfering RNA (siRNA) nanoparticles to knockdown COX-2 specifically in macrophages to prevent kidney injury induced by UUO has been developed as therapeutical strategy. Specifically, chitosan/siRNA nanoparticles were demonstrated to accumulate in macrophages in the obstructed kidney. Consistent with the imaging data, the obstructed kidney contained a higher amount of siRNA and macrophages. Chitosan-formulated siRNA against COX-2 was evaluated on RAW macrophages, which demonstrated reduced COX-2 expression and activity after lipopolysaccharide (LPS) stimulation. Injection of COX-2 chitosan/siRNA nanoparticles in mice subjected to UUO diminished the UUO-induced COX-2 expression. Likewise, macrophages in the obstructed kidney had reduced COX-2 immunoreactivity, and histological examination showed lesser tubular damage in COX-2 siRNA-treated UUO mice. Parenchymal inflammation, assessed by TNF-α and interleukin 6 mRNA expression, was attenuated by COX-2 siRNA. Furthermore, treatment with COX-2 siRNA reduced HO-1 and cleaved caspase-3 in UUO mice, indicating lesser oxidative stress and apoptosis. These results thus show a novel strategy to prevent UUO-induced kidney damage by using chitosan/siRNA nanoparticles to knockdown COX-2 specifically in macrophages (Yang et al. 2015). Moreover, mitochondrial abnormality has been shown in many kidney disease models (Manucha et al. 2015; Diez et al. 2015; Manucha 2014; García et al. 2014, 2014). However, its role in the pathogenesis of CKD is still uncertain. Nevertheless, in a current study, a mitochondrial complex I inhibitor rotenone was applied to the mice subjected to UUO and, as consequence, a remarkable attenuation of tubular injury was detected. In line with the improvement of kidney morphology, rotenone significantly blunted fibrotic response as shown by downregulation of fibronectin (FN), plasminogen activator inhibitor-1 (PAI-1), collagen I, collagen II,I and α-SMA, paralleled with a substantial decrease of TGF-β1. Meanwhile, the oxidative stress markers thiobarbituric-acid-reactive substances (TBARS) and HO-1 and inflammatory markers TNF-α, IL-1β, and ICAM-1 were markedly decreased. More importantly, rotenone moderately but significantly restored the reduction of mitochondrial DNA copy number and mitochondrial NADH dehydrogenase subunit 1 (mtND1) expression in obstructed kidneys, suggesting an amelioration of mitochondrial injury (Sun et al. 2014). Originally, and of particular interest to the present review, we evaluated that the pro-inflammatory state would be amplified by changes in renal antioxidants and ROS levels (Rinaldi Tosi et al. 2011). In this context, both the oxidative stress and the induction of mitochondrial apoptosis could be prevented by Hsp70 expression (Manucha et al. 2011). The emerging relationship between cellular stress response and the Nrf2-related vitagene system has been discussed (Calabrese et al. 2011). In this regard, enhanced lipid peroxidation through higher TBARS levels and increased oxidative stress resulted in reduced total antioxidant activity and enhanced NADPH oxidase activity, as demonstrated by Rinaldi Tosi et al. (2011). This was accompanied by decreased inducible Hsp70 expression and a progressive reduction of Nrf2 and its target gene products glutathione S-transferase A2 (GSTA2) and NADPH/quinone oxidoreductase 1 (NQO1), whereas the Nrf2 repressor Kelch-like ECH-associated protein-1 (Keap1) was upregulated.
Collectively, mitochondrial complex I inhibitor rotenone protected kidneys against obstructive injury possibly via inhibition of mitochondrial oxidative stress, inflammation, and fibrosis, suggesting an important role of mitochondrial dysfunction in the pathogenesis of obstructive kidney disease. In addition, the magnitude of cytoprotection in obstruction depends on the combined contribution of induced activation of Nrf2 upregulating its downstream gene products and Hsp70 response. Finally, the Keap1/Nrf2/ARE pathway and the HSR are inducible cytoprotective systems regulated by transcription factors Nrf2 and Hsf1, respectively. Distinct small-molecule Nrf2 activators, which react with sulfhydryl groups, upregulate Hsp70, a prototypic Hsf1-dependent gene. Hsp70 upregulation requires Hsf1 but is Nrf2 independent. The differential concentration dependence of the two responses suggests that activation of Nrf2 precedes that of Hsf1: The Keap1/Nrf2/ARE pathway is at the forefront of cellular defense, protecting against instant danger; the HSR closely follows to resolve subsequent potentially devastating damage, saving the proteome. This concept could have positive consequences for the treatment of renal inflammatory pathologies and related diseases (Zhang et al. 2011).
WT-1 and p53 as potential Co-factors for Hsp70 cytoprotective effects
Wilms tumor gene identified as missing or mutated in embryonic kidney cancer cells (Buckler et al. 1991) appears to be the main determinant for initiating epithelial mesenchymal transformation. In situ hybridization studies have shown that wt-1 is selectively expressed in the metanephric blastema and glomerular epithelium during embryonic and fetal development (Pritchard-Jones et al. 1990), suggesting that wt-1 is involved in regulating proliferation and cell differentiation.
During congenital obstructive nephropathy, major regulators of mesenchymal-epithelial transformation and renal tubular development, such as wt-1 and sall1, are decreased (Liapis 2003).
Johannesen et al. (2003) have shown functional interactions between the gene promoter of iNOS and WT-1. Also, a modulatory role of NO in the proliferation of T cells expressing WT-1 has been suggested (Marcet-Palacios et al. 2007). In fact, decreased NO and iNOS/Hsp70 expressions were associated with WT-1 low expression in obstructed kidneys (Mazzei et al. 2010b).
The effects of NO availability and wt-1 mRNA expression were also studied in vitro in MDCK cells. Low NO availability was associated with low expression of Hsp70 and WT-1. However, wt-1 and hsp70 mRNA expressions were increased when MDCK cells were incubated for 72 h with NO donors (Mazzei et al. 2010a).
Hsp70 is involved in the activity modulation of tumor suppressor proteins, including p53 and WT-1 (Cheng et al. 2001). Moreover, WT-1 and Hsp70 are physically associated in embryonic rat kidney cells, where the amino-terminal transactivation domain of WT-1 is required for binding to Hsp70, and domain expression itself is sufficient to induce Hsp70 expression (Maheswaran et al. 1998). In addition, NO stimulates the expression of enzymes and transcription factors involved in DNA repair and modulation of apoptosis, such as the tumor suppressor p53. In turn, p53 interacts with WT-1 and modulates its ability to regulate the transcription of its respective target genes (Scharnhorst et al. 2000). Consequently, it was suggested that increased NO availability induces p53 and WT-1 mRNA expression (Mazzei et al. 2010a). Moreover, WT-1 can stabilize p53, adjust its trans-activational properties, and inhibit its ability to induce apoptosis without affecting cellular arrest (Maheswaran et al. 1995). This effect may explain the elevated p53 levels observed by other authors during obstructive nephropathy apoptosis induction (Cummings 1996; Morrissey and Klahr 1999; Miyajima et al. 2000; Topcu et al. 2008). Furthermore, NO treatment preserves vascular smooth muscle cells from mitochondrial-dependent apoptosis and drives cells to quiescence through an increase in p53 (Duran et al. 2009). Therefore, it appears that NO /p53/WT-1 interaction can modulate the WT-1 expression and/or function. Of special interest, p53 protein interacts with members of the Hsp70 chaperone family that can regulate its function (Lane et al. 1993; Takenaka et al. 1995). In this regard, neonatal UUO shows low p53 and Hsp70 expressions, which are increased in association with higher NO levels under rosuvastatin treatment. Conversely, MDCK cells with NO deprivation expressed low Hsp70 and p53 mRNA levels. These observations suggest a potential role for NO bioavailability and Hsp70 interaction during kidney differentiation.
Conclusions and perspectives
The gathered data suggest that relevant levels of NO may contribute to apoptotic pathway suppression by the upregulation of Hsp70 and that interaction is an early line of defense for protecting cells from death. The induction of Hsp70 expression precedes conventional markers of renal injury, protecting cells not only from damage due to apoptosis induction but also from damage due to oxidative injury, fibrosis, and inflammation (Fig. 3). Despite this potential for cellular protection strategies involving HSPs, it is often unclear which of the particular functions of the multifunctional HSP molecule are key to reducing the injury, and it has been suggested that there may be a combination of effects including: repair of damaged proteins, prevention of unfolded protein aggregation, targeting of damaged protein for degradation, inhibition of apoptosis, cytoskeleton stabilization, and immunological effects upon leukocytes (Kelly 2005). There is an additional possibility that HSP expression could have an important influence over the behavior of mononuclear phagocytes and lymphocytes, key cells involved in renal IRI which are capable of both initiating and resolving tissue injury (Kim et al. 2014; O’Neill and Hughes 2014).
Intracellular Hsp70 exerts pleiotropic direct and indirect effects on renal inflammation. Hsp70 can indirectly reduce the pro-inflammatory activation of renal epithelia via prevention of oxidative stress through its inhibitory effects on NADPH oxidase activity and ROS activation. Hsp70 also negatively regulates NF-kB activation downstream of signals originating at the plasma membrane such as TLR4. Hsp70 effects on the VDR may also accentuate repressive anti-inflammatory signaling. Factors such as CD4+ CD25+ Foxp3 + regulatory T cells partially mediate the Hsp70-induced renoprotective effect. ROS reactive oxygen species, TLR4 Toll-like receptor 4, VDR vitamin D receptor, CD4 helper T cell (cluster of differentiation 4), CD25 helper T cell (cluster of differentiation 25), Foxp3 Forkhead box P3
Further studies will continue to elucidate the regulatory events of these processes in obstructive nephropathy and provide further insight into the role of HSPs in the mechanisms of action of reno-protective drugs.
References
Adams JS, Chen H, Chun RF, Nguyen L, Wu S, Ren SY, Barsony J, Gacad MA (2003) Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles zoo. J Cell Biochem 88(2):308–314
Ahmadzadeh A, Tahmasebi M, Gharibvand MM (2009) Causes and outcome of prenatally diagnosed hydronephrosis. Saudi J Kidney Dis Transpl 20:246–250
Aufricht C (2005) Heat-shock protein 70: molecular supertool? Pediatr Nephrol 20:707–713
Beck FX, Neuhofer W, Muller E (2000) Molecular chaperones in the kidney: distribution, putative roles, and regulation. Am J Physiol Ren Physiol 279(2):F203–F215
Becker A, Baum M (2006) Obstructive uropathy. Early Hum Dev 82:15–22
Beere HM, Wolf BB, Cain K, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475
Beissinger M, Buchner J (1998) How chaperones fold proteins. Biol Chem Mar 379(3):245–259
Bocanegra V, Manucha W, Pena MR, Cacciamani V, Valles PG (2010) Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens 28(1):143–155
Borkan SC, Emami A, Schwartz JH (1993) Heat stress protein associated cytoprotection in inner medullary collecting duct cells from rat kidney. Am J Physiol 265:F333–F341
Buckler AJ, Pelletier J, Haber DA, Glaser T, Housman DE (1991) Isolation, characterization, and expression of the murine Wilms’ tumor gene (WT-1) during kidney development. Mol Cell Biol 11:1707–1712
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451
Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, Chevalier RL (2003) Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int 63:564–575
Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ (2011) Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Asp Med 32(4-6):279–230
Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW (2007) Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets 6(2):91–100
Cheng H, Cenciarelli C, Shao Z, Vidal M, Parks WP, Pagano M, Cheng-Mayer C (2001) Human T cell leucemia virus type 1 Tax associates with a molecular chaperone complex containing hTid-1 and Hsp70. Curr Biol 11:1771–1775
Chevalier RL (1998) Pathophysiology of obstructive nephropathy in the newborn. Semin Nephrol 18:585–593
Chevalier RL, Thornhill BA, Chang AY, Cachat F, Lackey A (2002) Recovery from release of ureteral obstruction in the rat: relationship to nephrogenesis. Kidney Int 61:2033–2043
Cormack-Aboud FC, Brinkkoetter PT, Pippin JW, Shankland SJ, Durvasula RV (2009) Rosuvastatin protects against podocyte apoptosis in vitro. Nephrol Dial Transplant 24:404–412
Cummings MC (1996) Increased p53mRNAexpression in liver and kidney apoptosis. Biochim Biophys Acta 1315(2):100–104
Dendooven A, Ishola DA Jr, Nguyen TQ, Van der Giezen DM, Kok RJ, Goldschmeding R, Joles JA (2011) Oxidative stress in obstructive nephropathy. Int J Exp Pathol 92:202–210
Dias T, Sairam S, Kumarasiri S (2014) Ultrasound diagnosis of fetal renal abnormalities. Best Pract Res Clin Obstet Gynaecol 28(3):403–415
Diez ER, Altamirano LB, García IM, Mazzei L, Prado NJ, Fornes MW, Carrión FD, Zumino AZ, Ferder L, Manucha W (2015) Heart remodeling and ischemia-reperfusion arrhythmias linked to myocardial vitamin d receptors deficiency in obstructive nephropathy are reversed by paricalcitol. J Cardiovasc Pharmacol Ther 20(2):211–220
Dmitrieva NI, Burg MB (2005) Hypertonic stress response. Mutat Res 569(1-2):65–74
Docherty NG, O’Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW (2006) Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Ren Physiol 290:F4–F13
Duran X, Vilahur G, Badimon L (2009) Exogenous in vivo NO donor treatment preserves p53 levels and protects vascular cells from apoptosis. Atherosclerosis 205:101–106
Ekblom P (1989) Developmentally regulated conversion of mesenchyme to epithelium. FASEB J 3:2141–2160
Eleftheriadis T, Lawson BR (2009) Toll-like receptors and kidney diseases. Inflamm Allergy Drug Targets 8(3):191–201
Eleftheriadis T, Pissas G, Liakopoulos V, Stefanidis I, Lawson BR (2012) Toll-like receptors and their role in renal pathologies. Inflamm Allergy Drug Targets 11(6):464–477
Exner M, Minar E, Wagner O, Schillinger M (2004) The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med 37(8):1097–1104
Fawcett TW, Sylvester SL, Sarge KD, Morimoto RI, Holbrook NJ (1994) Effects of neurohormonal stress and aging on the activation of mammalian heat shock factor 1. J Biol Chem 269:32272–32278
Ferder L, Inserra F, Martinez-Maldonado M (2006) Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep 8(3):191–198
Fink AL (1999) Chaperone-mediated protein folding. Physiol Rev 79(2):425–49
Forbes MS, Thornhill BA, Park MH, Chevalier RL (2007) Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol 170(1):87–99
Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY (1997) Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem 272(29):18033–18037
García IM, Altamirano L, Mazzei L, Fornes M, Molina MN, Ferder L, Manucha W (2012a) Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Ren Physiol 302(12):F1595–F1605
García IM, Mazzei L, Benardón ME, Oliveros L, Cuello-Carrión FD, Gil Lorenzo A, Manucha W, Vallés PG (2012b) Caveolin-1-eNOS/Hsp70 interactions mediate rosuvastatin antifibrotic effects in neonatal obstructive nephropathy. Nitric Oxide 27(2):95–105
García IM, Altamirano L, Mazzei L, Fornés M, Cuello-Carrión FD, Ferder L, Manucha W (2014) Vitamin D receptor-modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones 19(4):479–491
Gething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355:33–45
Goloubinoff P, De Los RP (2007) The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem Sci 32:372–380
Grande MT, Pérez-Barriocanal F, López-Novoa JM (2010) Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm (Lond) 7:19. doi:10.1186/1476-9255-7-19
Harbrecht BG, Billiar TR, Stadler J, Demetris AJ, Ochoa J, Curran RD, Simmons RL (1992) Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J Leukoc Biol 52:390–394
Harbrecht BG, Stadler J, Demetris AJ, Simmons RL, Billiar TR (1994) Nitric oxide and prostaglandins interact to prevent hepatic damage during murine endotoxemia. Am J Physiol 266:G1004–G1010
Harkness TAA, Nargang FE, van der Klei I, Neupert W, Lill R (1994) A crucial role of the mitochondrial protein import receptor MOM19 for the biogenesis of mitochondria. J Cell Biol 124:637–648
Harrison EM, Sharpe E, Bellamy CO, McNally SJ, Devey L, Garden OJ, Ross JA, Wigmore SJ (2008) Heat-shock protein 90-binding agents protect renal cells from oxidative stress and reduce kidney ischemia-reperfusion injury. Am J Physiol Ren Physiol 295:F397–F405
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381(6583):571–579
Hartl FU, Hayer-Hartl M (2009) Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16:574–581
Hsu H, Xiong J, Goeddel DV (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495–504
Ignarro LJ (1990) Biological actions and properties of endothelium derived nitric oxide. Ann Rev Pharmacol Toxicol 30:535–560
Ishizaka N, Aizawa T, Ohno M, Usui Si S, Mori I, Tang SS, Ingelfinger JR, Kimura S, Nagai R (2002) Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension 39(1):122–128
Ito K, Chen J, Seshan SV, Khodadadian JJ, Gallagher R, El Chaar M, Vaughan ED Jr, Poppas DP, Felsen D (2005) Dietary arginine supplementation attenuates renal damage after relief of unilateral ureteral obstruction in rats. Kidney Int 68:515–528
Jäättelä M (1999) Escaping cell death: survival proteins in cancer. Exp Cell Res 248:30–43
Jäättelä M, Wissing D (1993) Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med 177(1):231–236
Jäättelä M, Wissing D, Bauer PA, Li GC (1992) Major heat shock protein Hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J 11:3507–3512
Jacquier-Sarlin MR, Fuller K, Dinh-Xuan AT, Richard MJ, Polla BS (1994) Protective effects of Hsp70 in inflammation. Experientia (Basel) 50:1031–1038
Johannesen J, Karlsen AE, Pociot F, Roenn SG, Nerup J (2003) Strain dependent rat iNOS promoter activity-correlation to identified WT-1 transcription factor binding site. Autoimmunity 36:167–175
Jones Q, Voegeli TS, Li G, Chen Y, Currie RW (2011) Heat-shock proteins protect against ischemia and inflammation through multiple mechanisms. Inflamm Allergy Drug Targets 10:247–259
Joshi S, Peck AB, Khan SR (2013) NADPH oxidase as a therapeutic target for oxalate induced injury in kidneys. Oxidative Med Cell Longev 2013:462361
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592
Kang SS, Song JH, Lee MY, Kang YH, Lim SS, Ryu SY, Jung JY (2011) Developmental immunolocalization of heat shock protein 70 (HSP70) in epithelial cell of rat kidney. Histol Histopathol 26(11):1363–73
Kanner J, Harel S, Granit R (1991) Nitric oxide as an antioxidant. Arch Biochem Biophys 289:130–136
Kelly KJ (2005) Heat-shock (stress response) proteins and renal ischemia/reperfusion injury. Contrib Nephrol Basel Karger 148:86–106
Kim JY, Yenari MA (2013) The immune modulating properties of the heat shock proteins after brain injury. Anat Cell Biol 46(1):1–7
Kim YM, de Vera ME, Watkins SC, Billiar TR (1997) Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem 272:1402–1411
Kim YM, Bombeck CA, Billiar TR (1999) Nitric oxide as a bifunctional regulator of apoptosis. Circ Res 84:253–256
Kim YO, Li C, Sun BK, Kim JS, Lim SW, Choi BS, Kim YS, Kim J, Bang BK, Yang CW (2005) Preconditioning with 1,25-dihydroxyvitamin D3 protects against subsequent ischemia reperfusion injury in the rat kidney. Nephron Exp Nephrol 100(2):e85–94
Kim M, Park SW, Chen SW, Gerthoffer WT, D’Agati VD, Lee HT (2010) Selective renal overexpression of human heat-shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Ren Physiol 299:F347–F358
Kim MG, Jung Cho E, Won Lee J, Sook Ko Y, Young Lee H, Jo SK, Cho WY, Kim HK (2014) The heat-shock protein-70- induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int 85(1):62–71
Klahr S (2001) Urinary tract obstruction. Semin Nephrol 21:133–145
Kolpakov V, Gordon D, Kulik TJ (1995) Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res 76:305–309
Kumar Y, Tatu U (2003) Stress protein flux during recovery from simulated ischemia: induced heat shock protein 70 confers cytoprotection by suppressing JNK activation and inhibiting apoptotic cell death. Proteomics 3:513–526
Lane DP, Midgley C, Hupp T (1993) Tumour suppressor genes and molecular chaperones. Philos Trans R Soc Lond B Biol Sci 339(1289):369–372, discussion 372-383
Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C (2008) Heat-shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med 12:743–761
Lebherz-Eichinger D, Ankersmit HJ, Hacker S, Hetz H, Kimberger O, Schmidt EM, Reiter T, Hörl WH, Haas M, Krenn CG, Roth GA (2012) HSP27 and HSP70 serum and urine levels in patients suffering from chronic kidney disease. Clin Chim Acta 413(1-2):282–286
Li J, Billiar TR, Talanian RV, Kim YM (1997) Nitric oxide reversibly inhibits seven members of the caspasa family via S-nitrosylation. Biochem Biophys Res Commun 240:419–424
Li F, Mao HP, Ruchalski KL, Wang YH, Choy W, Schwartz JH, Borkan SC (2002) Heat stress prevents mitochondrial injury in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 283:917–926
Liapis H (2003) Biology of congenital obstructive nephropathy. Nephron Exp Nephrol 93:87–91
Luders J, Demand J, Schonfelder S, Frien M, Zimmerman R, Hohfeld J (1998) Cofactorinduced modulation of the functional specificity of the molecular chaperone Hsc70. Biol Chem 379(10):1217–1226
Lutz W, Kohno K, Kumar R (2001) The role of heat shock protein 70 in vitamin D receptor function. Biochem Biophys Res Commun 282(5):1211–1219
Maheswaran S, Englert C, Bennett P, Heinrich G, Haber DA (1995) The WT1 gene product stabilizes p53 and inhibits p53-mediated apoptosis. Genes Dev 9:2143–2156
Maheswaran S, Englert C, Zheng G, Lee SB, Wong J, Harkin DP, Bean J, Ezzell R, Garvin AJ, McCluskey RT, DeCaprio JA, Haber DA (1998) Inhibition of cellular proliferation by the Wilms tumor suppressor WT-1 requires association with the inducible chaperone Hsp70. Genes Dev 12:1108–1120
Mannick JB, Miao XQ, Stamler JS (1997) Nitric oxide inhibits Fas-induced apoptosis. J Biol Chem 272:24125–24128
Manucha W (2007) Biochemical-molecular markers in unilateral ureteral obstruction. Biocell 31:1–12
Manucha W (2014) Mitochondria and oxidative stress participation in renal inflammatory process. Medicina (B Aires) 74(3):254–258
Manucha W, Vallés P (2008a) Hsp70/nitric oxide relationship in apoptotic modulation during obstructive nephropathy. Cell Stress Chaperones 13(4):413–420
Manucha W, Vallés PG (2008b) Cytoprotective role of nitric oxide associated with Hsp70 expression in neonatal obstructive nephropathy. Nitric Oxide 18(3):204–215
Manucha W, Oliveros L, Carrizo L, Seltzer A, Vallés P (2004) Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney Int 65(6):2091–2107
Manucha W, Carrizo L, Ruete C, Molina H, Vallés P (2005) Angiotensin II type I antagonist on oxidative stress and heat shock protein 70 (HSP-70) expression in obstructive nephropathy. Cell Mol Biol (Noisy-le-grand) 51:547–555
Manucha W, Carrizo L, Ruete C, Vallés PG (2007) Apoptosis induction is associated with decreased NHE1 expression in neonatal unilateral ureteric obstruction. BJU Int 100:191–198
Manucha W, Kurbán F, Mazzei L, Benardón ME, Bocanegra V, Tosi MR, Vallés P (2011) eNOS / Hsp70 interaction on rosuvastatin cytoprotective effect in neonatal obstructive nephropathy. Eur J Pharmacol 650(2-3):487–495
Manucha W, Ritchie B, Ferder L (2015) Hypertension and insulin resistance: implications of mitochondrial dysfunction. Curr Hypertens Rep 17(1):504. doi:10.1007/s11906-014-0504-2
Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X (2008) HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Ren Physiol 295(1):F202–F214
Marcet-Palacios M, Davoine F, Adamko DJ, Moqbel R, Befus AD (2007) Human lymphocytes express the transcriptional regulator, Wilms tumor 1: the role of WT-1 in mediating nitric oxide-dependent repression of lymphocyte proliferation. Biochem Biophys Res Commun 363:283–287
Mazzei L, Manucha W (2013) Wt-1 expression linked to nitric oxide availability during neonatal obstructive nephropathy. Adv Uro. doi:10.1155/2013/401750
Mazzei L, Garcia IM, Manucha W (2010a) Moduladores de fibrosis y apoptosis asociados a la disponibilidad de ON. Efecto de rosuvastatina en nefropatía obstructiva neonatal. Bioanalisis 34(6):20–26
Mazzei L, García IM, Cacciamani V, Benardón ME, Manucha W (2010b) WT-1 mRNA expression is modulated by nitric oxide availability and Hsp70 interaction after neonatal unilateral ureteral obstruction. Biocell 34(3):121–32
Mazzei LJ, García IM, Altamirano L, Docherty NG, Manucha W (2012) Rosuvastatin preserves renal structure following unilateral ureteric obstruction in the neonatal rat. Am J Nephrol 35(2):103–13
Messmer UK, Brune B (1996) Nitric oxide-induced apoptosis: p53-dependent and p53-independent signaling pathways. Biochem J 319:299–305
Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D (2000) Antibody to transforming growth factor-β ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58(6):2301–2313
Mizuguchi Y, Miyajima A, Kosaka T, Asano T, Hayakawa M (2004) Atorvastatin ameliorates renal tissue damage in unilateral ureteral obstruction. J Urol 172:2456–2459
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3896
Morrissey JJ, Klahr S (1999) Effect of AT2 receptor blockade on the pathogenesis of renal fibrosis. Am J Physiol 276(1):F39–F45
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B (1997) Role of the human heat shock protein Hsp70 in protection against stress-induced apoptosis. Mol Cell Biol 17:5317–5327
Nuñez G, Clarke MF (1994) The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol 4:399–403
O’Neill S, Hughes J (2014) Heat-shock protein-70 and regulatory T-cell-mediated protection from ischemic injury. Kidney Int 85:5–7
O’Neill S, Ross JA, Wigmore SJ, Harrison EM (2012) The role of heat-shock protein 90 in modulating ischemia-reperfusion injury in the kidney. Expert Opin Investig Drugs 21:1535–1548
O'Neill S, Harrison EM, Ross JA, Wigmore SJ, Hughes J (2014) Heat-shock proteins and acute ischaemic kidney injury. Nephron Exp Nephrol 126:167–174
Power RE, Doyle BT, Higgins D, Brady HR, Fitzpatrick JM, Watson RW (2004) Mechanical deformation induced apoptosis in human proximal renal tubular epithelial cells is caspase dependent. J Urol 171:457–461
Pritchard-Jones K, Fleming S, Davidson D, Bickmore W, Porteous D, Gosden C, Bard J, Buckler A, Pelletier J, Housman D (1990) The candidate Wilms tumor gene is involved in genitourinary development. Nature 346:194–197
Rinaldi Tosi ME, Bocanegra V, Manucha W, Gil Lorenzo A, Valles PG (2011) The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) responses. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO). Cell Stress Chaperones 16(1):57–68
Ruchalski K, Mao H, Singh SK, Wang Y, Mosser DD, Li F, Schwartz JH, Borkan SC (2003) HSP72 inhibits apoptosis-inducing factor release in ATP-depleted renal epithelial cells. Am J Physiol Cell Physiol 285:1483–1493
Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J (2006) Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 21(1):16–20
Rusai K, Banki NF, Prokai A, Podracka L, Szebeni B, Tulassay T, Reusz GS, Sallay P, Körmendy R, Szabo AJ, Fekete A (2010) Heat shock protein polymorphism predisposes to urinary tract malformations and renal transplantation in children. Transplant Proc 42(6):2309–2311
Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA (1998) Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17:3401–3415
Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A (2010) NF-kappaB in renal inflammation. J Am Soc Nephrol 21(8):1254–1262
Scharnhorst V, Dekker P, van der Eb AJ, Jochemsen AG (2000) Physical interaction between Wilms tumor 1 and p53 proteins modulates their functions. J Biol Chem 275(14):10202–10211
Singh IS, Hasday JD (2013) Fever, hyperthermia and the heat shock response. Int J Hyperth 29(5):423–435
Sonoda H, Prachasilchai W, Kondo H, Yokota-Ikeda N, Oshikawa S, Ito K, Ikeda M (2010) The protective effect of radicicol against renal ischemia- reperfusion injury in mice. J Pharmacol Sci 112:242–246
Sorger PK (1991) Heat shock factor and the heat shock response. Cell 65:363–366
Strasser A, Anderson RL (1995) Bcl-2 and thermotolerance cooperate in cell survival. Cell Growth Differ 6:799–805
Sun Y, Zhang Y, Zhao D, Ding G, Huang S, Zhang A, Jia Z (2014) Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediat Inflamm. doi:10.1155/2014/670106
Swamy N, Mohr SC, Xu W, Ray R (1999) Vitamin D receptor interacts with DnaK/heat shock protein 70: identification of DnaK interaction site on vitamin D receptor. Arch Biochem Biophys 363(2):219–26
Takenaka IM, Leung SM, McAndrew SJ, Brown JP, Hightower LE (1995) Hsc70-binding peptides selected from a phage display peptide library that resemble organellar targeting sequences. J Biol Chem 270(34):19839–19844
Tian S, Ding G, Jia R, Chu G (2006) Tubulointerstitial macrophage accumulation is regulated by sequentially expressed osteopontin and macrophage colony-stimulating factor: implication for the role of atorvastatin. Mediat Inflamm 2:12919
Topcu SO, Celik S, Erturhan S, Erbagci A, Yagci F, Ucak R (2008) Verapamil prevents the apoptotic and hemodynamic changes in response to unilateral ureteral obstruction. Int J Urol 15(4):350–355
Trnka P, Hiatt MJ, Ivanova L, Tarantal AF, Matsell DG (2010) Phenotypic transition of the collecting duct epithelium in congenital urinary tract obstruction. J Biomed Biotechnol 696034
Tsujimoto Y (1989) Stress-resistance conferred by high level of Bcl-2 protein in human B lymphoblastoid cell. Oncogene 4:1331–1336
Uchiyama T, Atsuta H, Utsugi T, Oguri M, Hasegawa A, Nakamura T, Nakai A, Nakata M, Maruyama I, Tomura H, Okajima F, Tomono S, Kawazu S, Nagai R, Kurabayashi M (2007) HSF1 and constitutively active HSF1 improve vascular endothelial function (heat shock proteins improve vascular endothelial function. Atherosclerosis 190:321–329
Valles PG, Melechuck S, Gonzalez A, Manucha W, Bocanegra V, Valles R (2012) Toll-like receptor 4 expression on circulating leucocytes in hemolytic uremic syndrome. Pediatr Nephrol 27(3):407–415
Van de Water B, de Graauw M, Le Dévédec S, Alderliesten M (2006) Cellular stress responses and molecular mechanisms of nephrotoxicity. Toxicol Lett 162(1):83–93
Vasil’eva TV, Michurina TV, Radionova IV, Kuzin BA, Enikolopov GN, Khrushchov NG (1997) NO synthetase in the cells of different tissues and organs of the mouse. Ontogenez 28:458–462
Vieira JM Jr, Mantovani E, Rodrigues LT, Dellê H, Noronha IL, Fujihara CK, Zatz R (2005) Simvastatin attenuates renal inflammation, tubular transdifferentiation and interstitial fibrosis in rats with unilateral ureteral obstruction. Nephrol Dial Transplant 20:1582–1591
Voegeli TS, Wintink AJ, Chen Y, Currie RW (2008) Heat shock proteins 27 and 70 regulating angiotensin II-induced NF-kappaB: a possible connection to blood pressure control? Appl Physiol Nutr Metab 33(5):1042–1049
Wang Y, Knowiton AA, Christensen TG (1999) Prior heat stress inhibits apoptosis in adenosine triphosphate-depleted renal tubular cells. Kidney Int 55:2224–2235
Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC (2011) Induction of heat-shock protein 70 inhibits ischemic renal injury. Kidney Int 79:861–870
Wang Y, Wang B, Du F, Su X, Sun G, Zhou G, Bian X, Liu N (2015a) Epigallocatechin-3-gallate attenuates unilateral ureteral obstruction-induced renal interstitial fibrosis in mice. J Histochem Cytochem 63(4):270–279, A
Wang Y, Wang B, Du F, Su X, Sun G, Zhou G, Bian X, Liu N (2015b) Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin Pharmacol Toxicol. doi:10.1111/bcpt.12383. B
Xu Q, Hu Y, Kleindienst R, Wick G (1997) Nitric oxide induces heat shock protein 70 expression in vascular smooth muscle cells via activation of heat shock factor 1. J Clin Invest 100:1089–1097
Yan CG, Zhu DF, Wang F (2007) Study on the expressions and roles of renal heat shock protein 72 and Toll-like receptor 4 in hepatorenal syndrome in rat. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 19(12):731–734
Yang C, Nilsson L, Cheema MU, Wang Y, Frøkiær J, Gao S, Kjems J, Nørregaard R (2015) Chitosan/siRNA nanoparticles targeting cyclooxygenase type 2 attenuate unilateral ureteral obstruction-induced kidney injury in mice. Theranostics 5(2):110–123
Yoo KH, Thornhill BA, Forbes MS, Chevalier RL (2010) Inducible nitric oxide synthase modulates hydronephrosis following partial or complete unilateral ureteral obstruction in the neonatal mouse. Am J Physiol Ren Physiol 298(1):F62–F71
Zhang PL, Lun M, Schworer CM, Blasick TM, Masker KK, Jones JB, Carey DJ (2008) Heat-shock protein expression is highly sensitive to ischemia-reperfusion injury in rat kidneys. Ann Clin Lab Sci 38:57–64
Zhang Y, Kong J, Deb DK, Chang A, Li YC (2010) Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol 21:966–973
Zhang Y, Ahn YH, Benjamin IJ, Honda T, Hicks RJ, Calabrese V, Cole PA, Dinkova-Kostova AT (2011) HSF1-dependent upregulation of Hsp70 by sulfhydryl-reactive inducers of the Keap1/Nrf2/ARE pathway. Chem Biol 18(11):1355–1361
Zhou MS, Jaimes EA, Raij L (2004) Atorvastatin prevents end-organ injury in salt-sensitive hypertension role of eNOS and oxidant stress. Hypertension 44:186–190
Zhou MS, Hernandez Schuman I, Jaimes EA, Raij L (2008) Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-β, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Ren Physiol 295:F53–F59
Zhou P, Yu JF, Zhao CG, Sui FX, Teng X, Wu YB (2013) Therapeutic potential of EGCG on acute renal damage in a rat model of obstructive nephropathy. Mol Med Rep 7(4):1096–1102
Zuo Y, Ma J, Gu Y, Yang H, Lin S (2002) The renal protective effect of selective cyclooxygenase-2 inhibitor on obstructive nephropathy. Zhonghua Nei Ke Za Zhi 41(12):825–828
Acknowledgments
Our thanks to Claudia Bottero for improving the style and grammar of the text.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Research and Technology Council of Cuyo University (SECyT), Mendoza, Argentina, and from the National Council of Scientific and Technical Research (CONICET) PIP 2010-2012, both of which were awarded to Walter Manucha. Grant no. PICT 2012-0234 Préstamo BID 2777 OC/AR.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazzei, L., Docherty, N.G. & Manucha, W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress and Chaperones 20, 893–906 (2015). https://doi.org/10.1007/s12192-015-0622-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0622-z