Abstract
Selenium (Se), an antioxidant agent, provides significant protection from reactive oxygen species (ROS)-induced cell damage in vivo and in vitro. However, it is unclear whether Se can protect against zearalenone (ZEN)-induced apoptosis in chicken spleen lymphocyte. In this study, we investigated the underlying mechanism of the apoptosis induced by ZEN in chicken spleen lymphocyte and further evaluated the protective mechanism of Se on ZEN-induced apoptosis. The results show that ZEN induced an increase in ROS generation and lipid peroxidation, and a decrease in levels of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione (GSH). The results of apoptosis morphologically from acridine orange/ethidium bromide (AO/EB) fluorescent staining and flow cytometry analysis show apparent apoptosis in the ZEN-treated group, and was confirmed by the upregulation of caspase-3, -12 and downregulation of Bcl-2. Meanwhile, ZEN activated the endoplasmic reticulum (ER) stress by upregulating ER stress-related molecular sensors (GRP78, ATF6, ATF4, IRE). However, co-treatment with Se effectively blocked ROS generation, improved antioxdative capacity, and reversed apoptosis and ER stress-related genes and protein expression. Taken together, these data suggest that oxidative stress and ER stress play a vital role in ZEN-induced apoptosis, and Se had a significant preventive effect on ZEN-induced apoptosis in chicken spleen lymphocyte via ameliorating the ER stress signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zearalenone (ZEN) [6-(10-hydroxy-6-oxo-trans-1-undecenyl)-bresorcyclic acid lactone], also known as F-2 toxin, is a non-steroid estrogen mycotoxin. It is mainly produced by Fusarium species, such as Fusarium cerealis, Fusarium graminearum, Fusarium culmorum, and Fusarium equiseti, and exists widely in many foods and feedstuffs (Richard 2007). The structures of ZEN and its metabolites are similar to that of 17β-oestradiol, and thus ZEN and its metabolites can competitively bind to estrogen receptors, disturbing steroid metabolism and causing functional changes in reproductive organs in farm animals and human (Olsen et al. 1981; Turcotte et al. 2005). Besides reproductive toxicity, ZEN also exhibits hepatotoxicity, cytotoxicity, genotoxicity, and immunotoxicity (Abid-Essefi et al. 2003; Tiemann et al. 2008; Zinedine et al. 2007). For instance, ZEN increased reactive oxygen species (ROS) production, leading to lipid peroxidation, DNA damages and immunosuppression (Abid-Essefi et al. 2003; Lin et al. 2015; Marin et al. 2011). In chicken, ZEN induced inflammation, oxidative stress, and calcium (Ca2+) imbalance (Gresakova et al. 2012; Wang et al. 2012a, b). Furthermore, ZEA could induce apoptosis via an endoplasmic reticulum (ER) stress-dependent signaling pathway in mouse Leydig cells (Lin et al. 2015).
The endoplasmic reticulum (ER) is a main site for protein synthesis, protein folding, and intracellular calcium (Ca2+) storage in eukaryotic cell, which plays a regulatory role in the cellular stress response (Todd et al. 2008). ER stress can be caused by various factors, such as hypoxia, hunger, calcium imbalance, free radical invasion, or drugs (Schröder and Kaufman 2005), and leads to an accumulation ROS, inflammation, and apoptosis (Guan et al. 2009). In ER stress, unfolded protein response (UPR) is a crucial signal transduction pathways, that is, sensed and activated by three upstream signaling proteins IRE (inositol requiring enzyme), PERK (protein kinase RNA (PKR)-like ER kinase), and ATF 6 (activating transcription factor 6) (Ron 2002). At normal conditions, the three ER stress transducers are in an inactive configuration by binding to the chaperone GRP78 (glucose-regulated protein 78). But chronic or excessive ER stress may break the balance between unfolded proteins and chaperones, and ultimately triggers apoptosis (Choi et al. 2010). As a specific player in the UPR, activated PERK also phosphorylates the α-subunit of the translation initiation factor eIF2 (eukaryotic translation initiation factor-2), increases the expression of ATF4 (activating transcription factor-4), and regulates apoptosis (Jiang et al. 2013). Previous studies have shown the ER stress-mediated cell death pathways in ZEN-treated various cells or tissues (Ben Salem et al. 2015; Lin et al. 2015; Long et al. 2016; Ren et al. 2017). However, little is known about the involvement of ER stress in ZEN-induced apoptosis in chicken.
According to the toxic effects of ZEN, researchers have investigated many chemical and/or biological substances with different properties to eliminate the adverse effects of mycotoxins. Several antioxidants like crocin, quercetin, and vitamin E have a strong protective effect against ZEN-induced toxicity (Abid-Essefi et al. 2003; Ben Salem et al. 2015). As an essential trace element, selenium (Se) plays an important role in the health and performance of animals. Se is involved in the protective effects of cells against excess ROS, and regulation of the immune and reproductive systems due to its antioxidant properties (Long et al. 2016; Peng et al. 2010; Zhou et al. 2009). Se inhibited ultraviolet radiation-induced apoptosis in primary human keratinocytes (Rafferty et al. 2010). In LLC-PK1 cells, Se produced a significant protection against ROS-mediated apoptosis via mitochondrial dysfunction (Zhou et al. 2009). Also, in ZEN-caused reproductive system damage, high levels of Se improved antioxidant ability, and inhibited reproductive cell apoptosis (Long et al. 2016). In chicken, Se could ameliorate cadmium or lead-induced cytotoxicity, oxidative stress, ER stress, and apoptosis in the splenic lymphocytes, kidney, testis, ovary, and liver (Chen et al. 2012; Liu et al. 2015b; Wan et al. 2018; Wang et al. 2018). Moreover, a Se-deficient diet can cause the occurrence of oxidative stress and hepatocyte apoptosis (Yao et al. 2015), but dietary supplementation with Se reduced germ cells apoptosis in the testis during spermatogenesis in chicken (Song et al. 2015).
There are many studies for the toxic effects of ZEN and the protective effects of Se on many organs. In animals, spleen lymphocytes play a crucial role in immune system. Therefore, the disruption of spleen lymphocytes function can adversely affect immunologic function and disease resistance (Hemmi and Ishida 1980). The immune system is a potential target for ZEN, because various cells of the immune system have estrogen receptors (Sakazaki and Ueno 2002). The cytotoxicity of ZEN on spleen lymphocytes has been observed in different species (Chen et al. 2017; Ren et al. 2017). In chicken spleen lymphocytes, ZEN induced intracellular calcium imbalance and changed immune-related gene expression of IL-2, IL-6, and IFN-γ (Wang et al. 2012a, b). However, little is known about its effects on apoptosis and the molecular and cellular mechanisms in chicken spleen lymphocytes. In the current study, we evaluated the potential toxicity mechanism of ZEN in primary chicken spleen lymphocytes and further investigated the protective effects of Se on ZEN-induced cytotoxicity.
Materials and methods
Materials
ZEN, RPMI1640 medium, dimethyl sulfoxide (DMSO), phosphate buffer saline (PBS), acridine orange/ethidium bromide (AO/EB), and sodium selenite were purchased from Sigma (St Louis, Missouri, USA). Fetal bovine serum (FBS) was ordered from Gibco (Grand Island, NY, USA). Cell culture plates were supplied by Mediatech, Inc. (Cornig, Manassas, VA, USA). Annexin V-FITC and propidium iodide (PI) were purchased from ACTGene Inc., (Piscataway, NJ, USA). Quantitative real-time PCR (qPCR) kits were obtained from TaKaRa Bio, Inc. (Dalian, China). Anti-GRP78 antibody, anti-Bcl-2 antibody, anti-caspase-3 antibody, anti-caspase-12 antibody, anti-ATF4 antibody, anti-ATF6 antibody, anti-IRE antibody, and anti-GAPDH antibody were purchased from Santa Cruz Biotechnology (USA). All other reagents were purchased from Sigma, and all the used chemicals were of analytical grade.
Cell culture and treatment
All chickens in the test were obtained from Laboratory Animal Center, College of Veterinary Medicine, Northeast Agricultural University, China. Fifty 60-day-old Hyline cocks were fed food and water ad libitum. This study followed good laboratory practices (GLP), and the use of animal was approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University.
Chicken spleen lymphocytes were prepared and cultured according to the methods of Wang et al. (2012b). Briefly, spleen was taken into a petri dish, washed with sterile cooled PBS, and then ground at low temperature. The mixture was filtered through a 200-mesh sieve to collect spleen cell suspension. The lymphocytes were then collected by centrifuging (400×g, 15 min) in Histopaque 1077 (Sigma) at room temperature and washed twice with cooled PBS. The cells were re-suspended in RPMI 1640 medium (containing 10% FBS and 1% antibiotic-antimycotic solution) and counted using a hemocytometer. When the lymphocytes viability greater than 95% as tested by trypan blue exclusion, the cells were used for in vitro experiments.
The cells were adjusted to a density of 106cell/mL and cultured in 6-well microplates at 2 mL/well under 5% CO2 at 41.5 °C. After 24-h incubation, the cells were distributed into four treatment groups as follows: (1) control (without ZEN and Se), (2) treated with ZEN alone (5.0 μg/mL), (3) treated with Se alone (10−7 mol/mL), and (4) treated with Se (10−7 mol/mL) + ZEN (5.0 μg/mL). Each treatment was performed in quadruplicate and incubated at 41.5 °C in a humidified 5% CO2 environment for 24 h. At the end of experiment, the cells were collected for biochemical and molecular assays, respectively. All experiments were repeated independently three times.
Determination of ROS level
The cells were collected after the treatments and the ROS levels in intracellular were determined using commercial kit (Sigma). In brief, the cells in each group were collected, washed three times with PBS, and then incubated with 2′,7′-dichlorofluorescein diacetate (DCFH-DA) at 37 °C for 30 min. The DCF fluorescence distribution of these cells was detected under an excitation wavelength of 488 nm and an emission wavelength of 530 nm using FACS Calibur flow cytometry (Becton-Dickinson, Franklin Lakes, USA).
Determination of antioxidant status
The cells antioxidant status was estimated by measuring the amount of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione (GSH), malondialdehyde (MDA), and catalase (CAT) using diagnostic kits obtained from Nanjing Jiancheng Bioengineering Research Institute (Zhao et al. 2010). Protein concentration of the cells was measured via the method reported by Bradford (1976).
Cell apoptosis assay by dual AO/EB staining and flow cytometry
Dual AO/EB fluorescent staining
After treatments with ZEN and/or Se, the cells were collected and washed with PBS by centrifugation (1500 r/min, 3 min). The cells were then stained with fluorescent staining solution (1 μL) containing 100 μg/mL AO (acridine orange) and 100 μg/mL EB (ethidium bromide) according to the method described by Liu et al. (2015a). The morphology of apoptotic cells was examined and 500 cells in each group were counted within 10 min using a fluorescent microscope (OLYMPUS, Japan).
Flow cytometry
After treatments with ZEN and/or Se, the cells were collected and washed with PBS by centrifugation (1500 r/min, 3 min). The cells were then stained with 10 μL Annexin V-FITC and 5 μL PI according to a previously described procedure (Eray et al. 2015). The fluorescent signal of the cells was measured with FACS Calibur flow cytometry (Becton-Dickinson).
Expression of genes
The total RNA in chicken spleen lymphocyte was isolated using Trizol (Invitrogen, Inc., Carlsbad, CA) according to the manufacturer’s instructions. Isolated RNA was dissolved in RNase-free water (TaKaRa Bio, Inc., Dalian, China). The RNA concentration and purity were estimated by measuring absorbance at 260 and 280 nm on a spectrophotometer (Eppendorf, Inc., Hamburg, Germany). The 260/280 ratio of all samples was between 1.8 and 2.0. Purified RNA was subjected to reverse transcription to cDNA by FastKing RT Kit (With gDNase) (Tiangen Biotech, Beijing, China) following the manufacturer’s instructions (Dai et al. 2015).
The gene expression was analyzed by qPCR using synthesized cDNA. QPCR experiments were performed with ABI PRISM 7500 Detection System (Applied Biosystems, USA). Briefly, for each gene, the qPCR reaction (total volume of 20 μL) contains 2 μL of cDNA, 10 μL of SYBR Premix Ex Taq, 0.4 μL of Rox Reference Dye II, 0.4 μL of forward and reverse primer (10 μM), and 6.8 μL RNase-free water. Cycling conditions was as follows: a denaturation step at 95 °C for 30 s, 40 cycles at 95 °C for 5 s, and 60 °C for 34 s. The specificity of qPCR product and quality of primer were confirmed by melting curve. All qPCRs were performed at least in triplicate. The data was normalized using GADPH as the internal control gene and the relative gene expression was quantified using the 2-ΔΔCt method (Livak and Schmittgen 2001). The specific primers used for genes expression analysis are listed in Table 1.
Western blot analysis
Following treatment, cells for each group were washed with PBS, and total cell lysates were prepared as the method described by Pozio et al. (2002). The protein concertation was determined using a bicinchoninic acid (BCA) assay (Smith et al. 1985). Equal amounts of protein samples were separated by 12% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and transferred onto PVDF (polyvinylidene fluoride) membranes that were blocked with 5% dehydrated skim milk at room temperature for 2 h. Then, the membranes were incubated with the primary antibodies for GRP78 (1:1000), Bcl-2 (1:500), caspase-3 (1:100), caspase-12 (1:500), ATF4 (1:500), ATF6 (1:500), IRE (1:500), and GAPDH (1: 2000) overnight at 4 °C that was followed by incubation with horseradish peroxidase-conjugated secondary antibodies against rabbit IgG (1:1000) at room temperature for 1 h. The blots were visualized with enhanced chemiluminescence using ECL-plus reagent (GE Healthcare, Buckinghamshire, UK). The GAPDH was used as an internal index. The optical density was quantified using Gel-pro Analyzer software (Media Cybernetics, Silver Spring, MD).
Statistical analysis
The results are shown as the mean ± standard error of the mean (SEM). The differences between different groups were analyzed using one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Different lowercase letters indicate that there were statistical significance (P < 0.05) among different groups. Statistical analyses were carried out using SPSS version 20.0 software (SAS Institute Inc., Cary, NC).
Results
Level of ROS in chicken spleen lymphocyte
As shown in Fig. 1, ZEN induced a marked increase in the ROS production (39.7%) compared with control group (0.5%), whereas, co-treatment with Se significantly inhibited ROS formation that was reduced by 14.9% (P < 0.05). Se itself had no effect on the intracellular level of ROS (P > 0.05).
The changes of ROS level in chicken splenic lymphocyte treated with Se and ZEN. a Cells were treated with control (untreated), Se, Se + ZEN, ZEN. b Relative ROS level, the data from three independent experiments represent the mean ± SEM, different lowercase letters indicate that there are statistically significance (P < 0.05) among different groups
Antioxidant status in chicken spleen lymphocyte
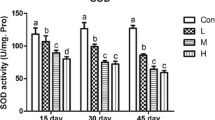
The antioxidant status in chicken splenic lymphocyte was assessed by measuring SOD, GSH-Px, GSH, CAT, and MDA, and the results were depicted in Fig. 2. Compared to control group, treatment with ZEN alone resulted in a significant decrease in the levels of SOD, GSH-Px, GSH, and CAT, and a significant increase in formation of MDA in chicken splenic lymphocyte (P < 0.05). However, treatment with Se in combination with ZEN significantly suppressed the adverse changes of these parameters induced by ZEN alone (P < 0.05). Cells treated with Se alone showed a normal level in these parameters compared to control (P > 0.05).
Nuclear staining for assessment of apoptosis
After treatment with ZEN and/or Se for 24 h, the apoptotic cells were observed by AO/EB staining (Fig. 3a). The apoptotic cells had green or red-orange stained condensation nucleus. In the control group and Se-treated group, no obvious apoptosis was observed, but in treatment with ZEN alone, the number of apoptotic cells significantly increased. Moreover, the cell count showed that the apoptosis rate in treatment with Se + ZEN (37.7 ± 5.42%) was significantly lower than that in treatment with ZEN alone (52.17 ± 4.33%, P < 0.05) (Fig.3b).
Observation of morphological apoptosis in chicken splenic lymphocyte treated with ZEN and Se. a Fluorescence images determined by staining with acridine orange and ethidium bromide (AO/EB). The cells were treated with control (untreated), Se, Se + ZEN, and ZEN. White indicate arrows live cells; blue arrows indicate apoptotic cells; red arrows indicate necrotic cells
Flow cytometry for assessment of apoptosis
To further confirm the ZEN-induced apoptosis and anti-apoptosis of Se in chicken splenic lymphocyte, cells were treated with Se and ZEN and detected with flow cytometry-based annexin V-FITC/PI double staining. As shown in Fig. 4, more than 94% of the cells were living cells in control group and Se-treated group. Compared with the control group, the apoptosis cells were significantly increased to 42.1% and mainly showed late apoptosis (II) in treatment with ZEN alone (P < 0.05). But in the group treated with Se in combination with ZEN, the percentage of apoptotic cells was significantly decreased by 10.5% (P < 0.05).
Changes of apoptosis in chicken splenic lymphocyte treated with ZEN and Se. Apoptosis was evaluated by flow cytometry using annexin V/PI staining. a The cells were treated with control (untreated), Se, Se + ZEN and ZEN. Quadrants: I, necrotic cells; II, late apoptosis cells; III, living cells; and IV, early apoptosis cells. b Columns, mean of three experiments, and the data represent the mean ± SEM; different lowercase letters indicate that there are statistically significance (P < 0.05) among different groups
The expression of apoptosis-related genes and proteins
As shown in Fig. 5, compared with the control group, the treatment with ZEN alone significantly upregulated the expression of caspase-3 and caspase-12 genes and proteins, and downregulated the Bcl-2 gene and protein expression. In contrast, when the splenic lymphocyte were co-treated with Se and ZEN, the mRNA and protein levels of caspase-3 and caspase-12 were decreased, and the mRNA and protein levels of Bcl-2 was increased compared with that in the ZEN-treated groups (P < 0.05).
The expression of ER stress-related genes and proteins
To examine the participation of the ER stress response in chicken splenic lymphocyte apoptosis, the mRNA and protein levels of GRP78, ATF4, ATF6, and IRE were analyzed by qPCR and Western Blot (Fig. 6). The mRNA and protein levels of GRP78, ATF4, ATF6, and IRE significantly increased in treatments with ZEN alone compared to control groups (P < 0.05), whereas the upregulation of the four genes and proteins was significantly suppressed in treatment with Se + ZEN (P < 0.05). Moreover, there was no difference between control and Se-treated group.
Discussion
ZEN, as a fusariotoxin, is harmful to animal health including immunotoxicity, oxidative stress and apoptosis (Zinedine et al. 2007). However, there are few reports of the effects of ZEN on apoptosis in chicken spleen lymphocyte. This study extends the knowledge of ZEN toxicity on chicken.
ZEN is a potent inducer of ROS in animals (Ben Salem et al. 2017; Wang et al. 2014). Excessive ROS can consume antioxidant components (SOD, CAT, GPx, and GSH), and reduce antioxidant capacity (Newsholme et al. 2016). In the present study, we demonstrate that ZEN triggered oxidative stress in spleen lymphocytes as evidenced by the over-production of intracellular ROS. Meanwhile, the levels of antioxidant components (SOD, CAT, GPx, and GSH) were remarkably depressed in treatment with ZEN alone. ZEN-induced oxidative stress also attacked polyunsaturated fatty acids and induced lipid peroxidation that resulted in damage to cell structures (Jia et al. 2015). In our work, the increased MDA level indicated a lipid peroxidation formation in ZEN-treated group. These results are consistent with the findings of increased lipid peroxidation level with the concomitant decrease in antioxidative capacity showed a pronounced oxidative stress, and the decrease of GPx level may be due to the conjugation with ZEN or its metabolites (Borutova et al. 2008). Moreover, ZEN could impair glutathione system balance that resulted in decrease of GSH level in a time and concentration-dependent manner (Hassen et al. 2007).
The activation of apoptosis is an important molecular consequence of ZEN toxicity (Shen et al. 2015). In the present study, the results of apoptosis morphologically form AO/EB fluorescent staining and flow cytometry analysis showed that ZEA-induced apoptosis in primary chicken spleen lymphocytes. Similar results were also reported in other ZEN-treated porcine splenic lymphocytes, cardiac cells, and Leydig cells (Ben Salem et al. 2017; Ren et al. 2017; Wang et al. 2014). In apoptotic responses of cells (mitochondrial-mediated or death receptor-dependent), caspases play crucial roles. Caspase-3 is the major executioner caspase in apoptosis, and caspase-12 mainly mediates ER-specific apoptosis (Earnshaw et al. 1999). Many investigations have demonstrated that ZEN could trigger apoptosis via caspase-dependent pathway in some cells. Caspase-3, -9 were activated in ZEN-toxicated rat Leydig cells, indicating that ZEN participated in the activation of the caspase cascade to induce apoptosis (Wang et al. 2014). Caspase-3, and -8 protein levels in ZEN-treated porcine splenic lymphocytes led to the activation of caspases and initiation of cell apoptosis (Ren et al. 2017). In the present study, we also discovered the upregulation of caspase-3, and -12 upon ZEN-treatment, suggesting that ZEN could induce caspase-dependent apoptosis in chicken spleen lymphocytes, and the apoptosis pathway might be related to ER stress.
Bcl-2, as an effector molecule in the apoptotic response, is a potent anti-apoptotic protein. Bcl-2 was suppressed in many types of cell apoptosis induced by different stimuli, such as oxidative stress, drugs, or virus (Liao et al. 1997; Takahashi et al. 2004). Similarly, Bcl-2 was involved in ZEN-induced apoptosis, and the level was decreased in rat Leydig cells (Lin et al. 2015). In vivo, chronic ZEN administration downregulated the Bcl-2 gene expression in mice reproductive system (Long et al. 2016). In line with previous reports, our study showed that ZEN downregulated Bcl-2 gene and protein expression that triggered apoptosis in chicken spleen lymphocytes. Our results also suggest that the balance of Bcl-2 level might be a key step for ZEN induction of apoptosis and eventually cell death.
Growing evidence suggests that oxidative stress leads to excessive oxidative modifications of proteins that induce ER stress and UPR activation (Anantharam et al. 2008; Verfaillie et al. 2012). Generally, the UPR is an adaptive cellular response and can restore homeostasis in the ER by expressing numerous chaperone proteins (e.g., GRP78) and transcription factors (e.g., ATF 4 and ATF6). But, prolonged or overloading ER stress restrains the UPR scavenging capacity, and eventually leads to cell apoptosis (Bánhegyi et al. 2010). Here, we observed the activation of ER stress as evidenced by upregulation of molecular sensors including GRP78, IRE, ATF6, and ATF4 after treatment with ZEN, and correspondingly, the caspase-12 was also upregulated and apoptosis occurred. These results suggested that the ER stress signaling pathway played a vital role in the regulation of ZEN-induced apoptosis in chicken spleen lymphocytes. Similar results were reported in other studies. ER stress was the mechanism involved in ZEN-induced apoptotic death in human leukemic cells, where ZEN upregulated GRP78 and calreticulin, and then triggered apoptosis (Banjerdpongchai et al. 2010). HCT116 cells treated with ZEN resulted in induction of ER stress as demonstrated by increase of GRP78, ATF6, and ATF4 protein and mRNA levels (Ben Salem et al. 2015). Similar findings were also confirmed in mouse Leydig cells, showing that treatment with ZEN caused apparent dose-dependent upregulation in the protein levels of GRP78, CHOP, and caspase-12 (Lin et al. 2015). These observations suggest that activation of ER stress may be a common feature of mycotoxin toxicity.
Se, an effective antioxidant agent, is known to improve antioxidant capacity and provide protection from ROS-induced cell damage (Tapiero et al. 2003). Indeed, our results showed that co-treatment with Se and ZEN effectively attenuated the ROS generation, lipid peroxidation formation, and antioxidant components depletion, suggesting Se could restrain the ZEN-induced oxidative stress and reduce ZEN toxicity by scavenging free radical in chicken spleen lymphocytes. Interestingly, the phenomenon was supported in previous studies, where Se could enhance the decrease of SOD, CAT, GSH, and GSH-Px levels, and suppress MDA formation as induced by lead and cadmium in chicken (Jiao et al. 2017; Liu et al. 2015b). Moreover, in ZEN-induced toxicity, Se had protective effects on reproductive system damage in mice that might be associated with recovering activity of antioxidant enzymes and decreasing free radical-mediated lipid peroxidation (Long et al. 2016).
Furthermore, Se appears to be mediated through its anti-apoptosis effect to protect against tissue or cell damage. Previous studies have demonstrated that Se significantly ameliorated apoptosis and the changes of apoptosis-related genes or proteins via decreasing the expression of the ER stress-related genes or proteins GRP78, GRP94, ATF4, ATF6, and IRE in cadmium or lead-treated chicken kidney and ovary (Liu et al. 2015b; Wan et al. 2018; Wang et al. 2018). Also, our results showed that Se clearly inhibited ER stress and apoptosis induced by ZEN exposure. An important observation in this study is that Se restrained the expression of ER stress-related genes and proteins (GRP78, ATF6, ATF4, and IRE), upregulated the Bcl-2 gene and protein, and attenuated the casapase-12 and -3 activities and apoptosis ratio. These findings, taken together, indicated that ER stress signal pathway played important role in protection of Se against ZEN-induced apoptosis.
Conclusion
This study provided new insight into the molecular mechanisms of ZEN toxicity in chicken spleen lymphocytes, and a way to reduce the toxicity of ZEN. We demonstrated that ZEN increased ROS generation, induced ER stress and triggered apoptosis in chicken spleen lymphocytes. Further, we also observed that Se protect cells from ZEN toxicity by inhibiting ER stress. We speculate that antioxidant treatment might be helpful to prevent ZEN-related apoptosis through ER stress signaling pathway in chicken spleen lymphocytes.
Abbreviations
- ZEN:
-
Zearalenone
- ROS:
-
Reactive oxygen species
- ER:
-
Endoplasmic reticulum
- IRE:
-
Inositol requiring enzyme
- PERK:
-
Protein kinase RNA (PKR)-like ER kinase
- ATF 6:
-
Activating transcription factor 6
- GRP78:
-
Glucose-regulated protein 78
- ATF4:
-
Activating transcription factor 4
- Se:
-
Selenium
- SOD:
-
Superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- CAT:
-
Catalase
- AO/EB:
-
Acridine orange/Ethidium bromide
References
Abid-Essefi S, Baudrimont I, Hassen W, Ouanes Z, Mobio TA, Anane R, Creppy EE, Bacha H (2003) DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: prevention by vitamin E. Toxicology 192:237–248
Anantharam V, Kanthasamy A, Choi CJ, Martin DP, Latchoumycandane C, Richt JA, Kanthasamy AG (2008) Opposing role of prion protein in oxidative stress- and ER stress-induced apoptotic signaling. Free Radic Biol Med 45:1530–1541
Bánhegyi G et al (2010) Endoplasmic reticulum stress. Ann N Y Acad Sci 1113:58–71
Banjerdpongchai R, Kongtawelert P, Khantamat O, Srisomsap C, Chokchaichamnankit D, Subhasitanont P, Svasti J (2010) Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J Hematol Oncol 3:50
Ben Salem I, Prola A, Boussabbeh M, Guilbert A, Bacha H, Abid-Essefi S, Lemaire C (2015) Crocin and quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 20:927–938. https://doi.org/10.1007/s12192-015-0613-0
Ben Salem I, Boussabbeh M, Da Silva JP, Guilbert A, Bacha H, Abid-Essefi S, Lemaire C (2017) SIRT1 protects cardiac cells against apoptosis induced by zearalenone or its metabolites α- and β-zearalenol through an autophagy-dependent pathway. Toxicol Appl Pharmacol 314:82–90. https://doi.org/10.1016/j.taap.2016.11.012
Borutova R, Faix S, Placha I, Gresakova L, Cobanova K, Leng L (2008) Effects of deoxynivalenol and zearalenone on oxidative stress and blood phagocytic activity in broilers. Arch Anim Nutr 62:303–312
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen X, Zhu YH, Cheng XY, Zhang ZW, Xu SW (2012) The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17:14565–14572
Chen P, Liu T, Jiang S, Yang Z, Huang L, Liu F (2017) Effects of purified zearalenone on selected immunological and histopathologic measurements of spleen in post-weanling gilts. Anim Nutr 3:212–218. https://doi.org/10.1016/j.aninu.2017.04.008
Choi HH, Shin DM, Kang G, Kim KH, Park JB, Hur GM, Lee HM, Lim YJ, Park JK, Jo EK, Song CH (2010) Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett 584:2445–2454
Dai L, Li Z, Yu J, Ma M, Zhang R, Chen H, Pham T (2015) The CYP51F1 gene of Leptographium qinlingensis: sequence characteristic, phylogeny and transcript levels. Int J Mol Sci 16:12014–12034
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
Eray M, Mättö M, Kaartinen M, Andersson L, Pelkonen J (2015) Flow cytometric analysis of apoptotic subpopulations with a combination of annexin V-FITC, propidium iodide, and SYTO 17. Cytometry 43:134–142
Gresakova L, Borutova R, Faix S, Placha I, Cobanova K, Kosikova B, Leng L (2012) Effect of lignin on oxidative stress in chickens fed a diet contaminated with zearalenone. Acta Vet Hung 60:103–114. https://doi.org/10.1556/AVet.2012.009
Guan L, Han B, Li Z, Hua F, Huang F, Wei W, Yang Y, Xu C (2009) Sodium selenite induces apoptosis by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis 14:218–225
Hassen W, Ayed-Boussema I, Oscoz AA, Lopez AC, Bacha H (2007) The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 232:294–302
Hemmi H, Ishida N (1980) The immune response of splenic lymphocytes after cimicifugoside treatment in vitro and pretreatment in vivo. J Pharmacobiodyn 3:643–648
Jia R, Han C, Lei JL, Liu BL, Huang B, Huo HH, Yin ST (2015) Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat Toxicol 169:1–9
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y, Xu C (2013) ATF4 activation by the p38MAPK-eIF4E axis mediates apoptosis and autophagy induced by selenite in Jurkat cells. FEBS Lett 587:2420–2429
Jiao X, Yang K, An Y, Teng X, Teng X (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res 24:7555–7564
Liao CL, Lin YL, Wang JJ, Huang YL, Yeh CT, Ma SH, Chen LK (1997) Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol 71:5963–5971
Lin P, Chen F, Sun J, Zhou J, Wang X, Wang N, Li X, Zhang Z, Wang A, Jin YP (2015) Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress-dependent signalling pathway. Reprod Toxicol 52:71–77
Liu K, Liu PC, Liu R, Wu X (2015a) Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res 21:15–20
Liu L, Yang B, Cheng Y, Lin H (2015b) Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol Trace Elem Res 167:308–319
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Long M, Yang S, Wang Y, Li P, Zhang Y, Dong S, Chen X, Guo J, He J, Gao Z, Wang J (2016) The protective effect of selenium on chronic Zearalenone-induced reproductive system damage in male mice. Molecules 21:1687
Marin DE, Taranu I, Burlacu R, Manda G, Motiu M, Neagoe I, Dragomir C, Stancu M, Calin L (2011) Effects of zearalenone and its derivatives on porcine immune response. Toxicol In Vitro 25:1981–1988
Newsholme P, Cruzat VF, Keane KN, Carlessi R, Bittencourt PIHD (2016) Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 473:4527–4550
Olsen M, Pettersson H, Kiessling KH (1981) Reduction of zearalenone to zearalenol in female rat liver by 3 alpha-hydroxysteroid dehydrogenase. Basic Clin Pharmacol Toxicol 48:157–161
Peng X, Bai CM, Chen T, Hengmin C (2010) Effects of low dietary selenium on immune function, total count of erythrocyte and content of hemoglobin in chickens. Chin Vet Sci 40:945–948
Pozio E, Sofronic-Milosavljevic L, Morales MAG, Boireau P, Nöckler K (2002) Evaluation of ELISA and Western Blot Analysis using three antigens to detect anti- Trichinella IgG in horses. Vet Parasitol 108:163–178
Rafferty TS, Beckett GJ, Walker C, Bisset YC, Mckenzie RC (2010) Selenium protects primary human keratinocytes from apoptosis induced by exposure to ultraviolet radiation. Clin Exp Dermatol 28:294–300
Ren Z, Deng H, Deng Y, Liang Z, Deng J, Zuo Z, Hu Y, Shen L, Yu S, Cao S (2017) Combined effects of deoxynivalenol and zearalenone on oxidative injury and apoptosis in porcine splenic lymphocytes in vitro. Exp Toxicol Pathol 69:612–617. https://doi.org/10.1016/j.etp.2017.05.008
Richard JL (2007) Some major mycotoxins and their mycotoxicoses—an overview. Int J Food Microbiol 119:3–10
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Investig 110:1383–1388
Sakazaki H, Ueno HK (2002) Estrogen receptor alpha in mouse splenic lymphocytes: possible involvement in immunity. Toxicol Lett 133:221–229
Schröder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569:29–63
Shen Y, Yang J, Zhao J, Xiao C, Xu C, Xiang Y (2015) The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: a survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp Cell Res 334:207–218
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Song R, Yao X, Shi L, Ren Y, Zhao H (2015) Effects of dietary selenium on apoptosis of germ cells in the testis during spermatogenesis in roosters. Theriogenology 84:583–588
Takahashi A, Masuda A, Sun M, Centonze VE, Herman B (2004) Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm). Brain Res Bull 62:497–504
Tapiero H, Townsend DM, Tew KD (2003) The antioxidant role of selenium and seleno-compounds. Biomed Pharmacother 57:134–144
Tiemann U, Brüssow KP, Dannenberger D, Jonas L, Pöhland R, Jäger K, Dänicke S, Hagemann E (2008) The effect of feeding a diet naturally contaminated with deoxynivalenol (DON) and zearalenone (ZON) on the spleen and liver of sow and fetus from day 35 to 70 of gestation. Toxicol Lett 179:113–117
Todd DJ, Lee AH, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8:663–674
Turcotte JC, Hunt PJ, Blaustein JD (2005) Estrogenic effects of zearalenone on the expression of progestin receptors and sexual behavior in female rats. Horm Behav 47:178–184
Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19:1880–1891
Wan N, Xu Z, Liu T, Min Y, Li S (2018) Ameliorative effects of selenium on cadmium-induced injury in the chicken ovary: mechanisms of oxidative stress and endoplasmic reticulum stress in cadmium-induced apoptosis. Biol Trace Elem Res 184:463–473
Wang YC, Deng JL, Xu SW, Peng X, Zuo ZC, Cui HM, Wang Y, Ren ZH (2012a) Effects of zearalenone on calcium homeostasis of splenic lymphocytes of chickens in vitro. Poult Sci 91:1956–1963
Wang YC et al (2012b) Effects of zearalenone on IL-2, IL-6, and IFN-γ mRNA levels in the splenic lymphocytes of chickens. Sci World J 2012(2012-5-2):567327
Wang Y, Zheng W, Bian X, Yuan Y, Gu J, Liu X, Liu Z, Bian J (2014) Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells. Toxicol Lett 226:182–191
Wang X, An Y, Jiao W, Zhang Z, Han H, Gu X, Teng X (2018) Selenium protects against lead-induced apoptosis via endoplasmic reticulum stress in chicken kidneys. Biol Trace Elem Res 182:354–363
Yao L, Du Q, Yao H, Chen X, Zhang Z, Xu S (2015) Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals 28:255–265
Zhao Z et al (2010) Mesenteric lymph reperfusion may exacerbate brain injury in a rat model of superior mesenteric artery occlusion shock. Neural Regen Res 5:683–689
Zhou YJ, Zhang SP, Liu CW, Cai YQ (2009) The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK1 cells. Toxicol in Vitro 23:288–294
Zinedine A, Soriano JM, Moltó JC, Mañes J (2007) Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol 45:1–18
Funding
This work received financial support from the National Key Research and Development Program of China (No. 2017YFD0502200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Xiao, Y., Xu, S., Zhao, S. et al. Protective effects of selenium against zearalenone-induced apoptosis in chicken spleen lymphocyte via an endoplasmic reticulum stress signaling pathway. Cell Stress and Chaperones 24, 77–89 (2019). https://doi.org/10.1007/s12192-018-0943-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-018-0943-9