Abstract
High-dose methotrexate (HD-MTX) therapy is widely used in patients with acute lymphoblastic leukemia (ALL) and lymphoma. However, some patients experience delayed MTX elimination, which requires treatment suspension or dose reduction to avoid organ damage. This single-center retrospective analysis reviewed the clinical data of 88 children with ALL or non-Hodgkin lymphoma who received a total of 269 courses of HD-MTX therapy between April 2008 and April 2019. HD-MTX was defined as MTX administration at 2.0, 3.0, or 5.0 g/m2 over a 24-h period, and delayed MTX elimination was defined as a serum MTX concentration ≥ 1.0 µmol/L at 48 h after the start of HD-MTX. Clinical factors were compared between courses with and without delayed MTX elimination. MTX elimination was delayed in 21 of the 269 courses (7.8%). Multivariate analysis showed that first HD-MTX course (OR 4.04), lower urine volume per BSA on the first day of HD-MTX administration (< 2,675 mL/m2, OR 5.10), higher total bilirubin (> 0.5 mg/dL, OR 5.11), lower eGFR (< 136 mL/min/1.73 m2, OR 3.90), higher dose of MTX(> 3.0 g/m2, OR 10.8), and lower urine volume per BSA on the next day of starting HD-MTX (< 2,107 mL/m2, OR 3.43) were independent risk factors for delayed MTX elimination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX) is widely used in the treatment of patients with acute lymphoblastic leukemia (ALL) or non-Hodgkin lymphoma (NHL). High-dose MTX (HD-MTX) therapy has therapeutic effects on the whole body, including the central nerve system (CNS) and testes, and is thus used in the phase of intensification therapy for ALL and the phase of CNS prophylaxis for NHL. However, this therapy sometimes induces critical organ damage, including the kidney, liver, and skin possibly due to delayed MTX elimination. As prophylactic measures, patients may receive intravenous hydration, alkalization of urine, and cessation of drugs known to interact with MTX [1]. However, despite these steps, delayed MTX elimination sometimes appears unexpectedly and can require dose reduction or treatment cessation to avoid severe organ damage. The present study was undertaken as a retrospective analysis of risk factors for delayed MTX elimination.
Materials and methods
We analyzed data from 108 pediatric patients (68 males, 40 females) with ALL or NHL who received HD-MTX therapy at Sapporo Hokuyu Hospital between April 2008 and April 2019. HD-MTX was defined as an MTX dosage of 2.0, 3.0, or 5.0 g/m2 administered during a 24-h period. Delayed MTX elimination was defined as a serum MTX concentration ≥ 1.0 µmol/L at 48 h after starting administration as described by Petez et al. [2]. We excluded patients who had been administered MTX at other doses or over other durations. A final total of 88 patients (50 males, 38 females) with 269 courses of HD-MTX were analyzed. The median age of patients at HD-MTX administration was 8.1 years (range 0.6–24.1 years). Seventy-eight patients had ALL (B-precursor ALL, n = 64; T-cell ALL (T-ALL), n = 13; mature B-cell ALL (B-ALL), n = 1). One of the 13 cases of T-ALL was a recurrence T-ALL. Ten patients had NHL (T-cell lymphoblastic lymphoma (T-LBL), n = 5; B-cell LBL (B-LBL), n = 2; diffuse large B-cell lymphoma (DLBCL), n = 2; Burkitt lymphoma, n = 1). HD-MTX was administered once in 2 patients, twice in 35 patients, thrice in 7 patients, and four times in 44 patients.
For each patient, we analyzed background characteristics (sex, age, body mass index (BMI), disease category), number of HD-MTX courses, doses of HD-MTX, combined use of 6-mercaptopurine (6-MP), combined use of triple intrathecal therapy (TIT), whether change in speed during 24-h administration or not (yes: administer 10% of the total dose in the first 30 min, or no: constant speed), fluid volumes on the day of HD-MTX administration and the following day, urine volumes on the day of HD-MTX administration and the following day, and data from blood testing before HD-MTX administration. Renal function before HD-MTX was expressed as estimated glomerular filtration rate (eGFR) as calculated from the equation reported for adults (> 20 years old) by Matsuo et al. [3], and the equation for children and adolescents (2–19 years old) by Uemura et al. [4]. We administered MTX through one lumen of a peripherally inserted central double-lumen catheter and drew blood samples to measure MTX concentrations from the other lumen. Measurements of serum MTX concentrations were performed by enzyme immunoassay. Data were analyzed as of June 2019. This study was approved by the institutional review board committee of Sapporo Hokuyu Hospital.
HD-MTX treatment
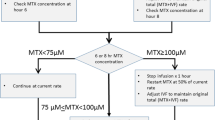
In our hospital, HD-MTX was administered by continuous drip infusion started at 10:00 am and continuing until 10:00 am the next day, together with intravenous hydration at a rate > 3,000 mL/m2/day with 4.3% glucose and NaHCO3 32 mEq/L, initiated 12 h before starting HD-MTX administration and maintained for 48 h after starting the HD-MTX administration. We prescribed acetazolamide every 12 h during HD-MTX. Trimethoprim–sulfamethoxazole was discontinued 1 week before starting HD-MTX. Protocols prohibited other drugs that affect elimination of MTX, such as NSIADs and antibiotics. Urine pH was checked every 8 h with urine test paper. If the urine pH was < 7.0, NaHCO3 solution (32 mEq/L, 20 mL) was administered. We performed leucovorin (LV) rescue (15 mg/m2) three times, at 42, 48, and 54 h after starting HD-MTX administration. We surveyed serum MTX concentration at 24 and 42 h after starting HD-MTX. If serum MTX concentration is exceeding 150 µmol/L at 24 h, we provide additional LV at 36 h. If serum MTX concentration is exceeding 1.0 µmol/L at 42 h, we provide additional leucovorin (LV) rescue (15 mg/m2) at 48 h. Details of HD-MTX regimen (combined use of drugs, LV rescue at 36 h, and change in speed during 24 h administration) within 48 h after starting HD-MTX are shown in Table 1.
Statistical analyses
We compared a number of analyzed factors between courses with delayed MTX elimination (≥ 1.0 µmol/L at 48 h after the start of MTX administration) and without delayed MTX elimination (< 1.0 µmol/L) to explore factors related to delayed MTX elimination, using Fisher’s exact test and the Mann–Whitney U test. Receiver operating characteristic analyses and multivariate stepwise regression were performed on factors showing significance on univariate analyses (p < 0.1) to explore independent risk factors. Changes in serum MTX concentration at 48 h after starting administration in each patient during HD-MTX courses were analyzed using the Freidman test. Differences in MTX concentration among courses were analyzed using the Wilcoxson signed rank test (p-value adjustment method: Holm). Values of p < 0.05 were considered to be statistically significant. Statistical analyses were performed using EZR ver 1.37 on R commander programed by Y. Kanda [5].
Results
Figure 1 depicts the transition of serum MTX concentrations at 48 h after starting administration of HD-MTX in each patient from the first course through to the fourth course. In the total of 269 HD-MTX courses, serum MTX concentration at 48 h after starting administration ranged from 0.07 to 39.8 µmol/L (median 0.35 µmol/L). Delayed MTX elimination (≥ 1.0 µmol/L at 48 h after the start of MTX administration) was observed in 21 of the 269 courses (7.8%) and 16 of the 88 patients (18%). Among the HD-MTX courses, delayed MTX elimination was observed in 9 of 88 patients (10%) with the only first course, in 4 of 88 patients (4.5%) with subsequent (second through fourth) course, and in 3 of 88 patients (3.4%) with first and subsequent courses. We compared the frequency of delayed MTX elimination between first course and subsequent courses, and there are significant difference (12 of the 88 (13.6%) in first course vs 9 of the 181 (4.9%) in subsequent courses, p = 0.026). Analysis showed that serum MTX concentrations in each patient differed significantly among HD-MTX courses (p < 0.01). Significant differences were identified between first and fourth courses (p = 0.018), but not between other courses (1st vs 2nd p = 0.44; 1st vs 3rd p = 0.14; 2nd vs 3rd p = 0.56; 2nd vs 4th, p = 0.56; 3rd vs 4th, p = 0.82). Only 1 patient, in whom serum MTX was 39.8 µmol/L at 48 h after starting administration, suffered from renal dysfunction that needed dialysis and plasma adsorption. The HD-MTX dose in that patient was reduced from 5.0 g/m2 to 2.0 g/m2 and no complications were noted subsequently. Another case that happened delayed MTX elimination at first and second courses suffered from severe oral mucositis and bone marrow suppression, and the HD-MTX doses at third and fourth courses in that patient were reduced from 5.0 g/m2 to 3.0 g/m2 and no complications were noted subsequently. The other cases suffered from delayed MTX elimination were not reduced doses of subsequent HD-MTX.
Transition of serum MTX concentration for each patient during the course of HD-MTX administration. Differences in serum MTX concentrations among HD-MTX courses were assessed using the Freidman test (p < 0.01). The Wilcoxon signed rank test revealed differences between first and fourth courses (p = 0.018), but not between other courses (1st vs 2nd p = 0.44; 1st vs 3rd p = 0.14; 2nd vs 3rd p = 0.56; 2nd vs 4th, p = 0.56; 3rd vs 4th, p = 0.82)
We then evaluated potential risk factors for delayed MTX elimination. Univariate analysis extracted the following variables as risk factors for delayed MTX elimination (Table 2): NHL (6 of the 21 (28.5%) with delayed MTX elimination vs 24 of the 248 (9.6%) without delayed MTX elimination, p = 0.022); change in speed (5 of the 21 (23.8%) with delayed MTX elimination vs 21 of the 248 (8.4%) without delayed MTX elimination, p = 0.039); older age (median age 12.0 years with delayed MTX elimination vs 7.0 years without delayed MTX elimination, p < 0.01); higher dose of MTX (median dose, 5.0 g/m2 with delayed MTX elimination vs 3.0 g/m2 without delayed MTX elimination, p < 0.01); first HD-MTX course (median number of courses, first time with delayed MTX elimination vs second time without delayed MTX elimination, p < 0.01); higher total bilirubin (median 0.5 mg/dL with delayed MTX elimination vs 0.4 mg/dL without delayed MTX elimination, p < 0.01); lower eGFR (median, 130 mL/min/1.73 m2 with delayed MTX elimination vs 144 mL/min/1.73 m2 without delayed MTX elimination, p = 0.09), lower fluid volume per body surface area (BSA) on the day of starting HD-MTX administration (median volume 3,175 mL/m2 with delayed MTX elimination vs 3,387 mL/m2 without delayed MTX elimination, p = 0.021); lower urine volume per BSA on the day of starting HD-MTX administration (median 2,613 mL/m2 with delayed MTX elimination vs 3,151 mL/m2 without delayed MTX elimination, p < 0.01) and on the next day of starting HD-MTX (median, 2,369 mL/m2 with delayed MTX elimination vs 2,648 mL/m2 without delayed MTX elimination, p < 0.01).
Multivariate analyses were performed on these variables to identify independent risk factors. First HD-MTX course (OR 4.04, p = 0.0014), lower urine volume per BSA on the day of starting HD-MTX administration (< 2,675 mL/m2, OR 5.10, p = 0.0054), higher total bilirubin (> 0.5 mg/dL, OR 5.11, p = 0.0081), lower eGFR (< 136 mL/min/1.73 m2, OR 3.90, p = 0.02), higher dose of MTX(> 3.0 g/m2, OR 10.8, p = 0.024), and lower urine volume per BSA on the next day of starting HD-MTX (< 2,107 mL/m2, OR 3.43, p = 0.043) were identified as independent risk factors (Table 3). We showed the number of courses and the proportion of courses with delayed MTX elimination about each independent risk factors; first HD-MTX courses were 88 (12/88, 13.6%) vs subsequent courses were 181 (9/181, 4.9%); the courses that urine volume per BSA on the day of starting HD-MTX administration was < 2,675 mL/m2 were 68 (13/68, 19.1%) vs the courses that urine volume per BSA on the day of starting HD-MTX administration was > 2,675 mL/m2 were 201 (8/201, 3.9%); the courses that total bilirubin was < 0.5 mg/dL were 167 (7/167, 4.1%) vs the courses that total bilirubin was > 0.5 mg/dL were 102 (14/102, 13.7%); the courses that eGFR was > 136 mL/min/1.73 m2 were 165 (8/165, 4.8%) vs the courses that eGFR was < 136 mL/min/1.73 m2 were 104 (13/104, 12.5%); the courses that dose of MTX was 2.0 g/m2 were 116 (1/116, 0.8%) vs the courses that dose of MTX was 3.0 g/m2 were 73 (7/73, 9.5%) vs the courses that dose of MTX was 5.0 g/m2 were 80 (13/80, 16.2%); the courses that urine volume per BSA on the next day of starting HD-MTX was > 2,107 mL/m2 were 225 (12/225, 5.3%) vs the courses that urine volume per BSA on the next day of starting HD-MTX was < 2,107 mL/m2 were 44(9/44, 20.4%).
Discussion
Although HD-MTX is accepted as an effective treatment for leukemia and lymphoma, long-term exposure to high concentrations of MTX sometimes induces lethal liver and renal dysfunction. As a result, monitoring of serum MTX concentration is very important, and clarification of risk factors for delayed MTX elimination before treatment would be clinically useful. Moreover, HD-MTX is usually used in a repetitive fashion over the course of chemotherapy, and patients who develop delayed MTX elimination would thus require modifications to subsequent doses of HD-MTX. In this context, we retrospectively analyzed risk factors for delayed MTX elimination.
Well-known risk factors for delayed MTX elimination include dehydration, acid urine, renal dysfunction, drugs that impair MTX clearance, and extravascular fluid collections including ascites, pleural effusions, and intracranial fluid [1, 6,7,8,9,10,11,12,13]. The present study revealed first HD-MTX course, lower urine volume per BSA on the day and the next day of starting HD-MTX, high dose of HD-MTX, higher total bilirubin, and lower eGFR as risk factors for delayed MTX elimination.
Lower urine volume per BSA on the day and the next day of starting HD-MTX are unsurprising risk factors for delayed MTX elimination, because almost 90% of MTX is excreted from the kidneys [14]. Previous studies have shown that creatinine clearance (CrCL) is related to delayed MTX elimination [15, 16], but another study found no such relationship [17]. Our investigation showed that eGFR, which correlates approximately with CrCL [3, 4], was related to delayed MTX elimination. Unfortunately, the amount of urine creatinine data is insufficient and we could not discuss about CrCL and 24 h CrCL. Our protocols recommend infusing intravenous fluids at > 3,000 mL/m2/day (to a maximum of 4,500 mL/m2/day) and maintaining a balance under + 400 mL/m2/12 hours. Relatively few studies have provided suggestions on the urine volume necessary to minimize the risk of delayed MTX elimination. Lower urine volume per BSA on the next day of starting HD-MTX might be not useful to predict and prevent delayed MTX elimination at 48 h. However, if urine volume per BSA on the day of starting HD-MTX is insufficient, we could change fluid volume and dose of acetazolamide and so on to prevent delayed MTX elimination.
We identified higher dose of HD-MTX as a risk factor for delayed MTX elimination, consistent with the previous reports [12, 18]. The cutoff level of ≥ 3.0 g/m2 was also consistent with results from one of those studies [18]. In the present study, administration of the first course of HD-MTX was extracted as a significant risk factor for delayed MTX elimination. This finding was unexpected, and reasons for this are unclear. We did not change intentionally dose of HD-MTX except for two cases as mentioned above, and we did not change dose and number of LV from protocol in all cases. Among 9 patients who suffered from delayed MTX elimination at only first course, fluid volume per BSA did not change between first course and subsequent courses (median volume 3,455 mL/m2 with at first course vs 3,631 mL/m2 at subsequent courses, p = 0.20). We did not change intentionally fluid volume or dose of acetazolamide, so there are no pretreatment factors that correlate with urine volume the day after administration. One possible explanation might be low plasma folate concentrations before starting HD-MTX due to insufficient folate dosage as pretreatment. In fact, plasma folate concentrations before administration of HD-MTX have been reported to be lower than those after administering HD-MTX [19, 20]. In one case report, severe encephalopathy was described in association with the first but not the second course of HD-MTX, with plasma folate concentrations low before the first course, but tenfold higher before the second course [21]. Low folate concentrations before treatment may induce delayed MTX elimination leading to organ damage, especially under conditions of renal dysfunction, although some reports have described folate status before HD-MTX treatment as unrelated to acute toxicity [19, 20]. More research into the relationship between pretreatment folate concentrations and delayed MTX elimination is thus needed.
One previous study showed no significant correlations between MTX concentrations at 48 h after starting MTX administration in the first and second HD-MTX courses in each patient [22], while our study showed first HD-MTX course as a risk factor for delayed MTX elimination. Therefore, delayed MTX elimination occurring with the first course does not mean this event will occur with subsequent courses.
Higher total bilirubin was also extracted as a risk factor for delayed MTX elimination in our study. So far, no reports appear to have discussed the relationship between delayed MTX elimination and higher total bilirubin. Most serum MTX is eliminated from kidneys, with a small proportion excreted unchanged into the bile and entering the enterohepatic circulation. Biliary excretion of MTX has not been considered clinically important [23], but may be involved in delayed MTX elimination when a patient who received HD-MTX therapy shows severe disorder of bilirubin excretion. We revealed that median total bilirubin are 0.5 mg/dL with delayed MTX elimination and 0.4 mg/dL without delayed MTX elimination, but the difference of total bilirubin between 0.4 and 0.5 is not clinically significant. Therefore, we additionally analyzed with Fisher’s exact test about whether total bilirubin exceeding standard value by age [24] is the risk of delayed MTX elimination or not. There are three cases that total bilirubin exceeded standard value by age, and one of the three cases suffered from delayed MTX elimination. We analyzed that total bilirubin exceeding standard value by age is not significant risk factor for delayed MTX elimination (1 of the 21 (5.0%) with delayed MTX elimination vs 2 of the 248 (0.8%) without delayed MTX elimination, p = 0.217). However, the number of cases that total bilirubin exceeded standard value by age might be insufficient statistically, and more research is needed. Similar to MTX, 6-MP is a drug affecting nucleic acid metabolism. This agent is sometimes used during HD-MTX therapy. The combination of 6-MP and MTX causes increases in 6-MP concentration [25], but few studies have evaluated whether 6-MP represent a risk factor for delayed MTX elimination during HD-MTX. In the present study, combined use of 6-MP was not extracted as a risk factor relating to delayed MTX elimination even in univariate analysis. Drugs that impair MTX clearance are trimethoprim–sulfamethoxazole, NSAIDs, penicillin, amphotericin, aminoglycosides, proton-pump inhibitors (PPI), and so on [7, 8, 13]. Our protocols warn against combine use of these drugs with MTX during HD-MTX, and no patients were used. Urine pH is the important factor of MTX elimination, and target pH is more than 7 [1, 26]. Urine pH was checked every 8 h with urine test paper in our hospital, but there are some missed data. Therefore, these data are not reliable to analyze, and we could not include urine pH among analyzing factors. However, all urine pH except for one time was more than 7. In that case, subsequent urine pH was above 8 and elimination of HD-MTX was not delayed. Therefore, we considered that urine pH may not affect the result of this research. Some reports showed that single nucleotide polymorphism (SNP), such as SLCO1B1, affected delayed MTX elimination, but we did not checked SNP in this research [27].
Conclusion
This study disclosed first HD-MTX course, lower urine volume per BSA on the day and the next day of starting HD-MTX, high dose of HD-MTX, higher total bilirubin, and lower eGFR as independent risk factors for delayed MTX elimination. The real significance of some of these factors in the delayed elimination of MTX was not properly explained in the present study. More precise analyses seem necessary in the future.
References
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471–82.
Petez C, Wang YM, Sutow WW, Herson J. Significance of the 48-hour plasma level in high-dose methotrexate regimens. Cancer Clin Trials. 1978;1(2):107–11.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–33.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Yanagimachi M, Goto H, Kaneko T, Naruto T, Sasaki K, Takeuchi M, Tanoshima R, Kato H, Yokosuka T, Kajiwara R, Fujii H, Tanaka F, Goto S, Takahashi H, Mori M, Kai S, Yokota S. Influence of pre-hydration and pharmacogenetics on plasma methotrexate concentration and renal dysfunction following high-dose methotrexate therapy. Int J Hematol. 2013;98:702–7.
Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, Sawada Y, Kohda Y. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67:44–9.
Yamamoto K, Sawada Y, Matsushita Y, Moriwaki K, Bessho F, Iga T. Delayed elimination of methotrexate associated with piperacillin administration. Ann Pharmacother. 1997;31:1261–2.
de Miguel D, García-Suárez J, Martín Y, Gil-Fernández JJ, Burgaleta C. Severe acute renal failure following high-dose methotrexate therapy in adults with hematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23:3762–6.
Pauley JL, Panetta JC, Schmidt J, Kornegay N, Relling MV, Pui CH. Late-onset delayed excretion of methotrexate. Cancer Chemother Pharmacol. 2004;54:146–52.
Wright KD, Panetta JC, Onar-Thomas A, Reddick WE, Patay Z, Qaddoumi I, Broniscer A, Robinson G, Boop FA, Klimo P Jr, Ward D, Gajjar A, Stewart CF. Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol. 2015;75:27–35.
Samantha R, Larry B, Nelly A, Debra A, Sean M, Dan D. Hypoalbminemia is sgnificantly associated with increased clearance time of high dose methotrexate in patients being treated for lymphoma or leukemia. Ann Hematol. 2016;95:2009–15.
Hall JJ, Bolina M, Chatterley T, Jamali F. Interaction between low-dose methotrexate and nonsteroidal anti-inflammatory drugs, penicillins, and proton pump inhibitors. Ann Pharmacother. 2017;51:163–78.
Sasaki K, Tanaka J, Fujimoto T. Theoretically required urinary flow during high-dose methotrexate infusion. Cancer Chemother Pharmacol. 1984;13:9–13.
Xu WQ, Zhang LY, Chen XY, Pan BH, Mao JQ, Song H, Li JY, Tang YM. Serum creatinine and creatinine clearance for predicting plasma methotrexate concentrations after high-dose methotrexate chemotherapy for the treatment for childhood lymphoblastic malignancies. Cancer Chemother Pharmacol. 2014;73:79–86.
Skärby T, Jönsson P, Hjorth L, Behrentz M, Björk O, Forestier E, Jarfelt M, Lönnerholm G, Höglund P. High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol. 2003;51:311–20.
Joannon P, Oviedo I, Campbell M, Tordecilla J. High-dose methotrexate therapy of childhood acute lymphoblastic leukemia: lack of relation between serum methotrexate concentration and creatinine clearance. Pediatr Blood Cancer. 2004;43:17–22.
Xu W, Tang Y, Song H, Shi S, Yang S. Retrospective study on elimination delay of methotrexate in high-dose therapy of childhood acute lymphoblastic leukemia in China. J Pediatr Hematol Oncol. 2007;29:688–93.
Laila H, Anders M, Lars M, Lars S, Kirsten S. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73:106–14.
Sterba J, Dusek L, Demlova R, Valik D. Pretreatment plasma folate modulates the pharmacodynamics effect of high-dose methotrexate in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma: ‘folate over rescue’ concept revisited. Clin Chem. 2006;52:692–700.
Valik D, Sterba J, Bajciova V, Demlova R. Severe encephalopathy induced by the first but not the second course of high-dose methotrexate mirrored by plasma homocysteine elevations and preceded by extreme differences in pretreatment plasma folate. Oncology. 2005;69:269–72.
Tsurusawa M, Gosho M, Mori T, Mitsui T, Sunami S, Kobayashi R, Fukano R, Tanaka F, Fujita N, Inada H, Koh K, Takimoto T, Saito A, Fujimoto J, Nakazawa A, Horibe K; lymphoma committee of the Japanese Pediatric Leukemia/lymphoma Study Group. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood non-Hodgkin lymphoma. Pediatr Blood Cancer. 2015; 62:279–284.
Locasciulli A, Mura R, Fraschini D, Gornati G, Scovena E, Gervasoni A, Uderzo C, Masera G. High-dose methotrexate administration and acute liver damage in children treated for acute lymphoblastic leukemia. A prospective study. Haematologica. 1992;77(1):49–53.
Innocenti F, Danesi R, Di Paolo A, Loru B, Favre C, Nardi M, Bocci G, Nardini D, Macchia P, Del Tacca M. Clinical and experimental pharmacokinetic interaction between 6-mercaptopurine and methotrexate. Cancer Chemother Pharmacol. 1996;37:409–14.
Toshiaki T, Atsushi Y, Kiyoshi I. Reference intervals of clinical tests in children determined by a latent reference value extraction method. J Jpn Pediatr Soc. 2008;112:1117–32.
Drost SA, Wentzell JR, Giguère P, McLurg DL, Sabloff M, Kanji S, Nguyen TT. Outcomes associated with reducing the urine alkalinization threshold in patients receiving high-dose methotrexate. Pharmacotherapy. 2017;37:684–91.
Ramsey LB, Panetta JC, Smith C, et al. Genomewide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakano, T., Kobayashi, R., Matsushima, S. et al. Risk factors for delayed elimination of high-dose methotrexate in childhood acute lymphoblastic leukemia and lymphoma. Int J Hematol 113, 744–750 (2021). https://doi.org/10.1007/s12185-020-03071-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03071-w