Abstract
High-dose methotrexate therapy (HD-MTX) has been well established for the treatment of childhood acute lymphoblastic leukemia (ALL). The aims of this study were to investigate whether clinical and pharmacogenetic factors influence plasma MTX concentration and renal dysfunction in patients treated with HD-MTX. In a total of 127 courses of HD-MTX in 51 patients with childhood ALL, influence of clinical and pharmacogenetic factors on plasma MTX concentration and HD-MTX-related renal dysfunction was evaluated. Clinical factors included age, gender, duration of HD-MTX continuous-infusion and duration of pre-hydration before HD-MTX. Pharmacogenetic factors included 5 gene polymorphisms within the MTX pathway genes, namely, SLC19A1, MTHFR, ABCC2 and ABCG2. Short duration of pre-hydration before HD-MTX is the most important risk factor for prolonged high MTX concentration (p < 0.001, OR 6.40, 95 % CI 2.39–17.16) and renal dysfunction (p = 0.013, OR 3.15, 95 % CI 1.27–7.80). The T allele at MTHFR C677T was the risk factor for prolonged high MTX concentration (p = 0.009, OR 5.54, 95 % CI 1.54–19.85), but not for renal dysfunction. We found the influence of MTHFR C677T polymorphism on prolonged high MTX concentration. We reconfirmed the importance of adequate pre-hydration before HD-MTX to prevent prolonged high MTX concentration and MTX-related renal dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX) is an antifolate agent which is an important drug for childhood acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma and osteosarcoma [1–3]. High-dose methotrexate therapy (HD-MTX) reduced hematological relapse in childhood ALL [4]. However, HD-MTX causes various toxicities, such as hematological, gastrointestinal, hepatic and renal toxicities [5–8].
Elevated plasma MTX concentration due to unexpected impairment of MTX excretion is known to be predictive for MTX-related renal toxicity [9]. Since more than 90 % of MTX is excreted by urine [10], to prevent HD-MTX-related toxicities, various approaches to supportive care have been established [6]. Examples include hydration to maintain urine output, alkalinization of urine and withholding of several medications, such as probenecid, sulfisoxazole and non-steroidal anti-inflammatory agents (NSAIDs) which are nephrotoxic or may interfere with MTX excretion [6]. Monitoring of plasma MTX concentration and leucovorin rescue is also important for preventing MTX-related toxicities [11].
Furthermore, there are inter-individual differences in MTX-related toxicities even under the same supportive care. Pharmacogenetics would lead to personalized therapy with MTX and another anti-cancer drugs [5, 12–16]. Methylentetrahydrofolate reductase (MTHFR) catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methylentetrahydrofolate [17]. MTX enters the cell through Solute carrier family 19 (folate transporter), member 1 (SLC19A1) also known as reduced folate carrier 1 (RFC1)-mediated active transport and is pumped out of the cell through ATP-binding Cassette (ABC) transporters such as ABCC2 (MRP2) and ABCG2 (BCRP) [17].
The aims of this study were to investigate whether supportive care and polymorphisms in the MTX pathway genes influence the plasma MTX concentration, and renal dysfunction in ALL patients treated with HD-MTX. Short duration of alkalinizing hydration and of HD-MTX continuous-infusion may be risk factors for renal dysfunction due to higher urinary and plasma MTX concentration [6, 18]. But, there was no report on the efficacy of overnight pre-hydration for preventing MTX-related toxicities. We found the importance of overnight pre-hydration for preventing prolonged high MTX concentration and MTX-related renal dysfunction.
Materials and methods
Study population
We retrospectively investigated a total of 51 patients (26 males and 25 females) who were treated at the Yokohama City University Hospital and Yokohama Saiseikai Nanbu Hospital between February 1999 and October 2009. Forty-eight patients had B cell precursor ALL and 3 had T cell ALL. Patients were eligible if they were treated with the Tokyo Children’s Cancer Study Group protocol of L99-15 and L07-16 for standard and intermediate risk groups of ALL patients [19]. These protocols contained three courses of HD-MTX at 3 g/m2/day (at 1–2 weeks intervals). HD-MTX were administered by 12 or 24 h continuous infusion with intrathecal chemotherapy (MTX and hydrocortisone) according to the protocol [19]. A total of 127 courses of HD-MTX in 51 patients were performed (Table 1). Fourteen patients could not complete all three courses of HD-MTX because of renal or hepatic toxicities. Leucovorin rescue (15 mg/m2) was started 36 h after HD-MTX and continued for at least 6 doses every 6 h until routinely measured plasma MTX levels were 0.1 μmol/L or lower. Alkalinizing hydration (2500–3000 ml/m2/day) began before HD-MTX and continued until plasma MTX levels were 0.1 μmol/L or lower.
Duration of HD-MTX continuous infusion
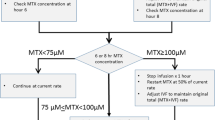
Before October 2005, the first course of HD-MTX was performed by 12 h continuous infusion in order to expect the prolonged high MTX concentration and MTX-related toxicities according to the protocol. If there was no prolonged high plasma MTX concentration at 12 h continuous infusion, the following HD-MTX was performed by 24 h continuous infusion. After November 2005, the first course of HD-MTX was infused by 24 h continuously. Clinical factors included age, gender, duration of MTX continuous infusion (12 h or 24 h) and duration of pre-hydration before HD-MTX.
Pre-hydration
The duration of pre-hydration was divided into following two groups, namely short pre-hydration-shorter than 4 h; i.e. from the morning, and long pre-hydration longer than 12 h; i.e. from the night before (Table 1). Before September 2004, HD-MTX was administered after short pre-hydration according to the protocol that recommended 3-h hydration before HD-MTX. If the prolonged high MTX concentration or renal toxicity were observed after the first course of HD-MTX, long pre-hydrations were selected for the subsequent HD-MTX. After October 2004, HD-MTX was administered along with long pre-hydration. It was confirmed from patient’s medical records that alkalinization of urine was well controlled and no medications, such as sulfisoxazole and NSAIDs, were co-administrated during all courses of HD-MTX. All patients have not had any liver dysfunction, infection or third space fluid sequestration before HD-MTX administration. According to the previous reports, prolonged high MTX concentration was defined as MTX concentration higher than 1.0 μmol/mL 48 h after the infusion [5, 20]. MTX-related renal toxicity was defined as an increase in the serum creatinine level after HD-MTX to more than 1.5 times the pretreatment baseline.
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Yokohama City University School of Medicine and Saiseikai Yokohamasi Nanbu Hospital. Written informed consent for the retrospective pharmacogenetic study was obtained from each patient or his/her guardians.
Genotyping
Genomic DNA was isolated from peripheral blood or bone marrow when the patients were in remission from their current diseases, using the QIAamp DNA Mini kit (Qiagen K.K., Tokyo, Japan). The following 5 single nucleotide polymorphisms (SNPs) within the MTX pathway genes encoding SLC19A1, ABCG2, MTHFR and ABCC2 were selected according to previous reports on the association between gene polymorphisms and MTX-related toxicities [20–23]. Genotyping for SLC19A1 A80G (rs1051266), ABCG2 C421A (rs2231142), MTHFR C677T (rs1801133), MTHFR A1298C (rs1801131) and ABCC2 C-24T (rs717620) were performed using the TaqMan technique (Applied Biosystems, Foster, CA, USA). TaqMan SNP Genotyping Assays was used for MTHFR A1298C, MTHFR C677T and ABCC2 C-24T. Custom TaqMan SNP Genotyping Assays was used for SLC19A1 A80G and ABCG2 C421A [24] (see supplementary table 1). These SNPs were analyzed in real-time PCR by the AB7500 Real Time PCR system (Applied Biosystems, Foster, CA, USA), under the conditions recommended by the manufacturer. Allele discrimination was performed using SDS software version 1.4 (Applied Biosystems).
Statistical analysis
A mixed-effects model for repeated measures was used to assess clinical and genetic factors associated with MTX concentration and MTX-related renal dysfunction [18, 25].
All statistical analyses were carried out using the SAS system version 9 (SAS Institute Inc., Cary, NC, USA).
Results
MTX concentrations
MTX concentrations 48 h after starting HD-MTX were higher than 1.0 μmol/L in 22 (17.3 %) out of 127 courses. With regard to clinical factors, short duration of pre-hydration before HD-MTX was the most important risk factor for prolonged high MTX concentration (p < 0.001, OR 6.40, 95 % CI 2.39–17.16). Thirteen (37.1 %) out of 35 courses with short pre-hydration had prolonged high MTX concentration, whereas 9 (9.2 %) out of 92 courses with long pre-hydration had prolonged high MTX concentration. Higher age was also associated with elevated MTX levels (Table 2).
As for genetic factors, the genotype frequencies for the 5 SNPs of the patients were in Hardy–Weinberg equilibrium (p > 0.05) (see supplementary table 2). MTHFR C677T was associated with MTX concentration (Table 3). The T allele at MTHFR C677T was the risk factor for prolonged high MTX concentration even after adjusting for clinical factors (p = 0.009, OR 5.54, 95 % CI 1.54–19.85). Allele frequency of MTHFR C677T was not significantly different between two types of prehydration before HD-MTX (p = 0.20, Student t test, supplementary table 3).
MTX-related renal toxicity
Twenty-eight (22.0 %) in a total of 127 courses of HD-MTX had renal toxicity. With regard to clinical factors, short duration of pre-hydration and higher age were associated with MTX-related renal toxicity (Table 4). Fourteen (40.0 %) in a total of 35 courses with short pre-hydration had renal dysfunction, whereas only 14 (15.2 %) in a total of 92 courses with long pre-hydration had renal dysfunction (p = 0.013, OR 3.15, 95 % CI 1.27–7.80).
As for association between renal dysfunction and prolonged high MTX concentration, 20 (90.9 %) in a total of 22 courses with prolonged high MTX concentration had increased serum creatinine levels, whereas 10 (9.5 %) in a total of 105 courses with normal MTX concentration had increased creatinine levels.
There was no association between genetic factors and MTX-related renal toxicity (Table 5).
Discussion
High-dose methotrexate therapy (HD-MTX) has improved treatment outcome for childhood ALL [4]. However, concerns with HD-MTX exist including many toxicities which are sometimes lethal [5, 6], but some toxicities may be avoidable by proper supportive care. Therefore, supportive care is important in patients treated with HD-MTX, even in the era of pharmacogenetics.
First, we investigated the influence of supportive care on prolonged high MTX concentration in HD-MTX-treated patients. We found that short duration of pre-hydration before HD-MTX was the most important risk factor for prolonged high MTX concentration. Although it was recommended that hydration should begin 12 h before HD-MTX and continue for 24–48 h [6], there was no report on the efficacy of long pre-hydration (from the night before) in preventing prolonged high MTX concentration and MTX-related renal dysfunction. Here, we have shown the efficacy of long pre-hydration from the night before. Although pre-hydration from the night before may lead to loss of sleep caused by frequent urination, adequate pre-hydration before HD-MTX should be performed to prevent renal toxicity. The influence of long pre-hydration on preventing prolonged high MTX concentration and MTX-related renal dysfunction may be associated with urine output before HD-MTX, although we did not address an association between urine output before HD-MTX and prolonged high MTX concentration/MTX-related renal dysfunction. With increasing age, a decrease in the MTX clearance and higher MTX levels with ALL patients have been reported [26, 27]. We also found that higher age was associated with elevated MTX levels and MTX-related renal dysfunction.
Second, we found that the T allele at MTHFR C677T was associated with prolonged high MTX concentration. This result was consistent with a previous report that the T allele at MTHFR C677T was associated with high MTX concentration 48 h after HD-MTX in Japanese childhood ALL patients [20]. MTHFR impacts the pharmacokinetics and pharmacodynamics of HD-MTX, yet MTHFR is not thought to be responsible for the metabolism or transport of MTX [22, 28]. Further studies are required to determine exactly how MTHFR function affects pharmacokinetics and pharmacodynamics of MTX. However, MTHFR C677T polymorphism was not a significant factor in patients who received long duration of pre-hydration (p = 0.44). Therefore, supportive care (especially long pre-hydration) may be more important than genetic background in HD-MTX therapy.
Third, we investigated the influence of clinical and genetic factors on MTX-related renal dysfunction. Prolonged high MTX concentration 48 h after HD-MTX was significantly associated with renal dysfunction in the present study. However, it is difficult to say whether prolonged high MTX concentration is the cause or outcome of renal dysfunction. Although MTHFR C677T was associated with MTX concentration 48 h after HD-MTX, there was no association between genetic factors and renal dysfunction. This is striking given that other factors influence the susceptibility of patients with high MTX concentration to renal toxicity. In terms of HD-MTX courses with normal MTX concentration, 9.5 % of courses had increased serum creatinine level. Again, the MTX concentration does not appear to be the sole determinant of susceptibility to renal toxicity. Therefore, SNPs or mutations at the MTX pathway genes that were not studied here may influence MTX-related renal toxicity.
One of the limitations of this study (besides the small study population) is the selection bias. At the first course of HD-MTX, the 12-h continuous infusion of HD-MTX was performed only before October 2005. Because the prolonged high MTX concentration and MTX-related renal toxicity in 24-h continuous infusion could not be expected using the precedence of 12-h continuous infusion, 24-h continuous infusion was performed from the first course of HD-MTX, after November 2005. Short pre-hydration was also performed only before September 2004. Therefore, there may be a selection bias. We compared the characteristics of patients between two hydration arms in supplementary table 3. Twelve-hour continuous infusion was associated with short pre-hydration (p < 0.01) because short pre-hydration and 12 h continuous infusion were concurrently selected mainly before 2004. By the continuous infusion time-stratified analysis, short pre-hydration was the risk factor for the prolonged high MTX concentration in both subgroups (12 h; OR = 2.9, p = 0.13, 24 h; OR = 6.1, p < 0.01) (supplementary table 4). Therefore, long pre-hydration is probably one of the important supportive care for preventing the prolonged high MTX concentration and renal toxicity, although the prospective research to validate the pre-hydration’s effect for preventing the prolonged high MTX concentration and MTX-related renal toxicity is needed.
In summary, we found the influence of MTHFR C677T on prolonged high MTX concentration. We found the importance of supportive care, especially adequate pre-hydration from the night before to prevent prolonged high MTX concentration and MTX-related renal toxicity.
References
Allegra CJ. Antifolates. In: Chabner BA, Collins JM, editors. Cancer chemotherapy: principles and practice. Philadelphia: Lippincott Company; 1990. p. 110–53.
Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19:341–6.
Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309:1094–104.
Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–68.
Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146:489–503.
Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703.
Goto H, Inukai T, Inoue H, et al. Acute lymphoblastic leukemia and Down syndrome: the collaborative study of the Tokyo Children’s Cancer Study Group and the Kyushu Yamaguchi Children’s Cancer Study Group. Int J Hematol. 2011;93:192–8.
Iwatani K, Fujii N, Deguchi S, Tanimoto M. Subacute methotrexate-related leukoencephalopathy with stroke-like presentation. Int J Hematol. 2012;96:683–4.
Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297:630–4.
Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41:36–51.
Bleyer WA. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4:87–101.
Sakaeda T. MDR1 genotype-related pharmacokinetics: fact or fiction? Drug Metab Pharmacokinet. 2005;20:391–414.
Yanagimachi M, Naruto T, Hara T, et al. Influence of polymorphisms within the methotrexate pathway genes on the toxicity and efficacy of methotrexate in patients with juvenile idiopathic arthritis. Br J Clin Pharmacol. 2011;71:237–43.
Sugimoto K, Murata M, Onizuka M, et al. Decreased risk of acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation in patients with the 5,10-methylenetetrahydrofolate reductase 677TT genotype. Int J Hematol. 2008;87:451–8.
Kong JH, Mun YC, Kim S, et al. Polymorphisms of ERCC1 genotype associated with response to imatinib therapy in chronic phase chronic myeloid leukemia. Int J Hematol. 2012;96:327–33.
Johnson SK, Heuck CJ, Albino AP, et al. The use of molecular-based risk stratification and pharmacogenomics for outcome prediction and personalized therapeutic management of multiple myeloma. Int J Hematol. 2011;94:321–33.
Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007;26:153–81.
Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–72.
Manabe A, Ohara A, Hasegawa D, et al. Significance of the complete clearance of peripheral blasts after 7 days of prednisolone treatment in children with acute lymphoblastic leukemia: the Tokyo Children’s Cancer Study Group Study L99-15. Haematologica. 2008;93:1155–60.
Imanishi H, Okamura N, Yagi M, et al. Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52:166–71.
Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–6.
Kishi S, Cheng C, French D, et al. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood. 2007;109:4151–7.
Rau T, Erney B, Gores R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther. 2006;80:468–76.
Dervieux T, Furst D, Lein DO, et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50:2766–74.
Fitzmaurice GM, Laird NM, Ware JH. Linear mixed effect models. In: Fitzmaurice GM, Laird NM, Ware JH, editors. Applied longitudinal analysis. New York: Wiley; 2004. p. 187–236.
Csordas K, Hegyi M, Eipel OT, Muller J, Erdelyi DJ, Kovacs GT. Comparison of pharmacokinetics and toxicity after high-dose methotrexate treatments in children with acute lymphoblastic leukemia. Anticancer Drugs. 2013;24:189–97.
Groninger E, Proost JH, de Graaf SS. Pharmacokinetic studies in children with cancer. Crit Rev Oncol Hematol. 2004;52:173–97.
Chiusolo P, Reddiconto G, Casorelli I, et al. Preponderance of methylenetetrahydrofolate reductase C677T homozygosity among leukemia patients intolerant to methotrexate. Ann Oncol. 2002;13:1915–8.
Acknowledgments
We are grateful to Teddy Kamata for his careful linguistic assistance with this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yanagimachi, M., Goto, H., Kaneko, T. et al. Influence of pre-hydration and pharmacogenetics on plasma methotrexate concentration and renal dysfunction following high-dose methotrexate therapy. Int J Hematol 98, 702–707 (2013). https://doi.org/10.1007/s12185-013-1464-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-013-1464-z