Abstract
MicroRNAs (miRNAs) are a class of small non-coding RNAs approximately 18–22 nucleotides in length, which play an important role in malignant transformation. The roles of miR-192 as an oncogene or tumor suppressor in solid tumors have been previously reported. However, little is known about the role of miR-192 in human acute myeloid leukemia. The results of the present study indicate that miR-192 is significantly downregulated in specimens from acute myeloid leukemia patients. Functional assays demonstrated that overexpression of miR-192 in NB4 and HL-60 cells significantly inhibited cell proliferation compared with that in control cells, and induced G0/G1 cell cycle arrest, cell differentiation, and apoptosis in vitro. Dual-luciferase reporter gene assays showed that miR-192 significantly suppressed the activity of a reporter gene containing the wild type 3′-UTR of CCNT2, but it did not suppress the activity of a reporter gene containing mutated 3′-UTR of CCNT2. QRT-PCR and Western blot assays showed that miR-192 significantly downregulated the expression of CCNT2 in human leukemia cells. Exogenous expression of CCNT2 attenuated the cell cycle arrest induced by miR-192 in NB4 and HL-60 cells. Collectively, miR-192 inhibits cell proliferation and induces G0/G1 cell cycle arrest in AML by regulating the expression of CCNT2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a lethal hematological malignancy characterized by uncontrolled proliferation of abnormal myeloblasts that accumulate in the bone marrow and interfere with programmed differentiation of normal blood cells [1]. Several cytogenetic, molecular, and epigenetic aberrations have been previously reported to contribute to initiation and development of AML [2–4]. Even with the progress of therapeutic alternatives including chemotherapy and hematopoietic stem cell transplant, AML progresses rapidly, especially for those elderly patients who are intolerable to intensive therapy and the average survival is estimated to be less than one year [5]. It is, therefore, urgent to uncover the molecular mechanisms responsible for leukemogenesis and find new therapeutic target of this malignant disease.
MicroRNAs (miRNAs) are a class of small non-coding RNAs with approximately 18–22 nucleotides in length, which were transcribed from the genome but did not give rise to proteins [6, 7]. Evidence is increasing that miRNAs function as regulators of carcinogenesis or drivers of tumor metastasis through their involvement in cell proliferation, differentiation and apoptosis [8–11]. Oncogenic and tumor-suppressive roles of miR-192 in solid tumors have both been previously reported [11–14]. Upregulation of miR-192 was found in multiple cancer types including gastric cancer, esophageal squamous cell carcinoma and pancreatic ductal adenocarcinoma [11–13]. Some other studies have suggested miR-192 as a tumor suppressor in colon cancer [9] and bladder cancer [10]. miR-192 promoted cancer cell proliferation and migration, and suppressed apoptosis via inhibition of Bim [11], Bcl2, Zeb2 and VEGFA [9]. Jin et al. reported recently that miR-192 induced a significant increase in G0/G1 phase and a significant decrease in S phase in bladder cancer cell [10]. However, little is known about the roles of miR-192 in human AML up to now.
Cyclin T2 (CCNT2) belongs to the highly conserved cyclin family, which functions as regulators of cyclin-dependent kinases (CDKs) to control progression of cell cycle [15]. CCNT2 and its kinase partner CDK9 were found to be subunits of the transcription elongation factor p-TEFb [16], which promotes messenger RNA transcriptional elongation through phosphorylation of elongation repressors and RNA polymerase II [17, 18]. CCNT2 also associates with Pkn to play roles in muscle differentiation [19] or interferes with Rb and CDK9 to mediate cellular signals transduction [20]. However, roles of CCNT2 in AML remain unsolved.
In this study, we found that expression of miR-192 was significantly downregulated in AML specimens by qRT-PCR. Moreover, restored expression of miR-192 significantly suppressed cell proliferation and induced G0/G1 cell cycle arrest of AML cells via inhibition of CCNT2 expression. Our data revealed that miR-192 was downregulated in AML and functioned as tumor suppressor via inhibition of CCNT2 expression.
Materials and methods
Ethic approval
Approval for our study was obtained from the Ethic Committee at Tongji Hospital. Written informed consent was obtained from all participants prior to the study. The use of the clinical specimens for research purposes was in accordance with the Declaration of Helsinki, federal laws and medical regulations in China.
Patients and cell lines
Bone marrow samples were collected from 10 patients histopathologically and clinically diagnosed as AML according to the French–American–British (FAB) classification criteria [21] in the Tongji Hospital. All the patients were chemotherapy naïve prior to this study. In addition, bone marrows from ten volunteers without malignant hematological diseases were used as normal controls.
The AML cell lines NB4 and HL-60 were purchased from American Type Culture Collection (ATCC, USA). All the cell lines were maintained in RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (HyClone, Logan, Utah, USA), 2 mM l-glutamine and 100 units/ml streptomycin plus 100 units/ml penicillin (Pen/Strep, Sigma-Aldrich, USA) in a humidified incubator at 37 °C in 5% CO2.
RNA extraction and qRT-PCR
Total RNA was extracted from the patient samples and cells with Trizol reagent (Invitrogen) and reversely transcripted to complementary DNA for detection of CCNT2 expression, while total small RNA was obtained using mirVana miRNA isolation kit (Applied Biosystems/Ambion, Austin, TX) according to the manufacturer’s instructions. Expression of miR-192 was detected with a TaqMan miRNA kit (Applied Biosystems) according to the manufacturer’s instructions and U6 was used as the endogenous control. Expression of CCNT2 was determined using an SYBR Mix (Promega) and normalized to that of β-Actin. The primers used are listed in Supplementary Table 1. qRT-PCR was carried out using the ABI PRISM 7500 Sequence Detection System (Applied Biosystems).
Vectors, transfections and luciferase activity assay
A miR-192 expression plasmid (pcDNA-miR-192) and a CCNT2 expression plasmid (pcDNA3.1-CCNT2) containing the coding sequence but lacking the 3′-UTR were obtained from GeneCopoeia™. The 3′-UTR of CCNT2 mRNA and a mutant variant were generated by PCR and cloned to the XbaI site of a pGL3-basic vector (Promega). For the luciferase assays, NB4 and HL-60 cells were co-transfected with the pGL3 reporter vector (250 ng/well), pRL-TK luciferase reporters (25 ng/well) and pcDNA-miR-192 (750 ng/well) or a negative-control (NC) vector using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. A Dual-Luciferase Reporter Assay Kit (Promega) was used to measure the luciferase activities in the indicated cells. The miR-192 mimic and NC oligonucleotides were obtained from Genepharma. Cells were plated in the 6-well plate and transfected with mimic or NC via lipofectamine2000 (Invitrogen) according to the manufacturer’s instructions. 48 h after transfection, cells were collected for qRT-PCR and western blot assays to validate the transfection efficiency or other functional assays.
Cell proliferation and cell cycle assays
Cell proliferation was assessed using CCK-8 assays. Briefly, NB4 and HL-60 cells were plated in the 96-well plate at a density of 500 cells/well before the addition of CCK-8 solution every day. Then, the cells were incubated at 37 °C in 5% CO2 for another 2 h and the absorbance was measured at wavelength of 490 nm on a Synergy™ Multi-Mode Microplate Reader (Biotek, Vermont, USA).
For the cell cycle assays, the indicated cells were synchronized with serum-free medium for 24 h before they were collected and washed with cold PBS twice. Then, the cells were labeled with propidium iodide (PI) containing RNaseA (30 mg/ml) at 37 °C water bath for 30 min. FACS calibers (BD, NJ, USA) were used to detect the DNA content and the data were analyzed with Flowjo software. Each group was performed in triplicates.
Cell differentiation and cell apoptosis assays
Differentiated percentage of indicated cells was determined with flow cytometry analysis using CD11b and CD15 antibodies (BD Pharmingen, San Jose, CA, USA). Briefly, cells were resuspended in 1 ml buffer containing previously indicated antibodies and incubated for 60 min at 4° in the dark. After two washes with PBS, the cells were resuspended in 0.5 ml of 3% FBS-PBS and analyzed by flow cytometry. Cell apoptosis was determined with the Annexin V/PI kit (Beyotime Technology) according to the manufacturer’s instructions.
Western blot analysis
Proteins were extracted from cells with the RIPA lysis buffer with protease (1:100) and phosphatase inhibitors (1:100) and the concentration was measured by BCA Protein Assay Kit (Pierce). Western blot analysis was conducted according to previously reported method [22]. Antibodies used in our study include p21, p27, p16 (Cell Singling, Boston, MA, USA) and CCNT2 (Abcam, Cambridge, Massachusetts, USA).
Animal study
All BALB/c nude mice (4 weeks old, female) were maintained under pathogen-free conditions. Our animal experiments were approved by the Animal Care Committee of Tongji Medical College. NB4 cells (5 × 106) with stable overexpression of miR-192 or control cells were injected subcutaneously into the left flank of the nude mice (n = 4/group). The tumors volumes were monitored every other day after injection. All mice were killed 26 days afterwards. The tumors were dissected out and weight of each xenograft was recorded.
Statistical analysis
All results were shown as the mean ± standard error of the mean (SEM). All statistical analyses were carried out with GraphPad Prism Software (GraphPad Software, USA). The relationship between relative miR-192 expression and CCNT2 was evaluated using the Pearson’s correlation coefficient test. P < 0.05 was considered significant.
Results
MiR-192 was downregulated in AML patient samples
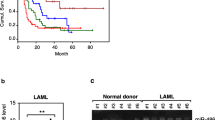
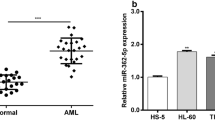
As shown in Fig. 1, bone marrow samples from AML patients had significantly lower expression level of miR-192 as compared to that from health control patients (Fig. 1, * P < 0.05). We, therefore, hypothesized that downregulation of miR-192 may be involved in leukemogenesis of AML.
MiR-192 inhibited proliferation in AML cells
To test our hypothesis, miR-192 expressing vectors were introduced into NB4 and HL-60 cells. As shown in Fig. 2a, b, qRT-PCR showed significantly increased miR-192 expression after transfection compared with that in the control group (* P < 0.05) in NB4 and HL-60 cell lines. Moreover, forced expression of miR-192 significantly inhibited cell proliferation in both NB4 and HL-60 cells compared with the control cells as assessed by CCK-8 kits (Fig. 2c, d). Next, in the subcutaneous nude mice model, overexpression of miR-192 significantly suppressed tumor growth in vivo (Fig. 2e, f). Altogether, our data revealed that the anti-proliferation activity of miR-192 may account for tumor-suppressive roles in AML cells.
Overexpression of miR-192 inhibited proliferation of AML cells. qRT-PCR assays of NB4 (a) and HL-60 (b) cells transfected with vectors expressing miR-192. Data were normalized to those of U6. Effect of miR-192 overexpression on cell proliferation was measured by CCK-8 kit after miRNAs were introduced into NB4 (c) and HL-60 (d) cells. e Tumor growth curve of indicated cells was created. F Weight of the dissected tumors was recorded. *P < 0.05 indicates a significant difference from the NC cells
MiR-192 induced G0/G1 arrest, cell differentiation and apoptosis in AML cells
Next, to explore whether the anti-proliferation effects of miR-192 were associated with aberrant cell cycle progression, we examined cell cycle distribution in two cell lines (NB4 and HL-60) in response to restored expression of miR-192 via flow cytometry. To avoid the baseline discordance, the serum was removed from the medium of NB4 and HL-60 cells transfected with miR-192 or control vectors before the cell cycle was assessed by flow cytometry. As shown in Fig. 3a, we found significantly increased ratio of G0/G1 phase and significantly decreased ratio of S phase in miR-192-overexpressed cells compared with that in the control cells. Moreover, expression of several checkpoint genes was detected after overexpression of miR-192. Western blot analysis showed that miR-192 overexpression resulted in remarkable increase of p21, p27 and p16 in NB4 and HL-60 cells (Fig. 3b). To further explore the effects of miR-192 on cell differentiation and apoptosis, flow cytometer assays were utilized. Overexpression of miR-192 significantly induced cell differentiation as evidenced by elevated expression of CD15 and CD11b (Fig. 3c). Apoptotic analysis indicated that the percentage of Annexin V negative cells was significantly increased after enforced expression of miR-192 (Fig. 3d). Collectively, our data indicated that miR-192 induced G0/G1 arrest, cell differentiation and apoptosis in AML cells.
Overexpression of miR-192 induced G0/G1 arrest in AML cells. a The cell cycle distribution of NB4 and HL-60 cells, which was transfected with miR-192 for 48 h and then cultured in non-serum mediums for 24 h, and cell cycle phase distribution was analyzed by flow cytometry after PI staining. The percentage of cells in each phase is indicated. b Western blot analysis of p21, p27 and p16 after transfection with miR-192 expressing vector in NB4 and HL-60 cells. c Flow cytometer analysis of CD15 and CD11b expression in NB4 and HL-60 cells after transfection of miR-192. d Flow cytometer analysis of apoptotic percentage in NB4 and HL-60 cells after transfection of miR-192. *P < 0.05 indicates a significant difference from the NC cells
CCNT2 was a direct molecular target of miR-192
It is generally acknowledged that miRNAs function by directly binding to 3′ untranslated regions (3′-UTR) of target genes [7]. We, therefore, predicted the possible target molecules of miR-192 by applying several publicly available algorithms (TargetScan, PicStar and miRDB, Fig. 4a) and identified 13 genes as the target of miR-192 (Supplementary Table 2), among which CCNT2 was reported to be associated with cell cycle control and selected for further analysis (Fig. 4b). As shown in Fig. 4b, CCNT2 possesses potential binding sites of miR-192 in the 3′-UTR. To verify direct interaction and repression effects between miR-192 and CCNT2, we constructed a Renilla luciferase reporter vector expressing the 3′-UTR of CCNT2 containing miR-192 target site or a mutated target site (Fig. 4b), which were termed as CCNT2-WT-UTR and CCNT2-Mu-UTR, respectively. Next, luciferase reporter assays were performed in NB4 and HL-60 cells. Transfection with CCNT2-WT-UTR resulted in a significant reduction in the luciferase activity of the reporter construct in the presence of miR-192, which was not observed after transfection with CCNT2-Mu-UTR in NB4 and HL-60 cells (Fig. 4c, d, * P < 0.05). Furthermore, the mRNA level of CCNT2 in NB4 and HL-60 cells was significantly decreased in miR-192-overexpressed cells (Fig. 4e, * P < 0.05). Accordingly, the level of CCNT2 protein was significantly inhibited in miR-192 mimics-transfected NB4 and HL-60 cells (Fig. 4f). These data provided evidence that CCNT2 is a direct target of miR-192 in AML cells.
CCNT2 was identified as a putative target gene of miR-192. a Venn diagrams showing the number of potential targets of miR-192, as predicted by three databases: TargetScan, PicStar and miRDB. b Sequences of the indicated 3′-UTR of CCNT2 and its potential binding site of miR-192 are shown. Luciferase activity due to the interaction between miR-192 and the luciferase constructs containing wild type or mutant 3′-UTR of CCNT2 in NB4 (c) and HL-60 (d) cells. Each Renilla luciferase reading was normalized to that obtained for the control firefly luciferase. e Relative mRNA level of CCNT2 in NB4 and HL-60 cells after overexpression of miR-192. f Western blot analysis of CCNT2 in NB4 and HL-60 cells after overexpression of miR-192. *P < 0.05 indicates a significant difference from the NC cells
CCNT2 overexpression reversed cell cycle arrest in AML induced by miR-192 upregulation
Finally, we asked whether CCNT2 might mediate miR-192-induced cell cycle arrest in AML. A vector containing the coding sequence of CCNT2 without the 3′UTR (pcDNA-CCNT2) was generated and introduced into NB4 and HL-60 cells. qRT-PCR analysis showed that pcDNA-CCNT2 significantly upregulated the expressions of CCNT2 in both NB4 and HL-60 cells (Fig. 5a, * P < 0.05). Concordantly, western blot analysis showed that pcDNA-CCNT2 restored expression of CCNT2 repressed by miR-192 mimics in both NB4 and HL-60 cells (Fig. 5b). Moreover, overexpression of CCNT2 was found in our patient cohort, which was further confirmed by the results from the Halferlach and Stegmaier data sets available in Oncomine database (Fig. 5c), demonstrating that CCNT2 may be involved in the pathogenesis and progression of AML. Furthermore, mRNA expression level of CCNT2 and miR-192 in the bone marrow samples of AML patients from our hospital correlated inversely, suggesting regulation of CCNT2 by miR-192 in AML cells (Fig. 5d). To further confirm the direct involvement of CCNT2 in the anti-tumor activity of miR-192 in AML, pcDNA-CCNT2 was introduced into the miR-192-overexpressed NB4 and HL-60 cells. Flow cytometric assays showed that CCNT2 overexpression significantly attenuated the G0/G1 arrest induced by miR-192 upregulation (Fig. 5e, * P < 0.05). Therefore, our data revealed that CCNT2 overexpression could reverse the anti-neoplastic effects of miR-192 on AML cell cycle transition, further confirming that CCNT2 was the downstream target gene of miR-192 in AML.
CCNT2 mediated the anti-tumor activity of miR-192 in AML. a Relative mRNA level of CCNT2 in NB4 and HL-60 cells after overexpression of miR-192 with or without co-transfection with pcDNA-CCNT2. b Western blot analysis of CCNT2 in NB4 and HL-60 cells after overexpression of miR-192 with or without co-transfection with pcDNA-CCNT2. c CCNT2 expression in patient samples from our hospital and in other cancer microarray data sets (Halferlach and Stegmaier) available from Oncomine (https://www.oncomine.com//). “AML” means the bone marrow specimen from the AML patients and “Normal” means the bone marrow samples from donors without malignant hematological diseases (Data in Tongji Hospital and in Halferlach’s study), whereas “AML” means bone marrow or peripheral blood samples from AML patients and “Normal” means the monocyte from peripheral blood specimen of health donors in Stegmaier’s study. d Expression of miR-192 and CCNT2 correlated inversely in the patient samples (r = −0.691, P < 0.05). e Cell cycle distribution in NB4 and HL-60 cells after overexpression of miR-192 with or without co-transfection with pcDNA-CCNT2. * P < 0.05 indicates a significant difference from the NC cells
Discussion
Evidences regarding the roles of miRNAs in human disease including cancer are increasingly emerged [7], whereby both oncogenic and/or tumor-suppressive characteristics have been described [13, 23]. Regulation network between miRNAs and messenger RNA has become a hotspot in cancer study [9]. Several miRNAs including miR-144 [24], miR-143 [25], miR-181b [26] and miR-204 [23] are downregulated in leukemia, acting as negative regulators of tumor growth [9, 24] or therapy sensitivity [25, 26], while others have reported upregulation of miR-126-5p [27], miR-181a [22], miR-221/222 [28] in human hematologic tumors.
Up to now, studies of biological roles of miR-192 were focused on human solid tumors [9–13, 29]. MiR-192 is significantly reduced in liver metastasis of colon cancer and has considerable therapeutic potentials for colon cancer patients [9]. Moreover, downregulation of miR-192 was observed in bladder cancer and induced cell cycle arrest in G0/G1 via reduced expression of CCND1 [10]. In our study, we observed downregulation of miR-192 in AML samples compared with normal counterparts. Further function assays revealed that overexpression of miR-192 in the AML cell lines significantly suppressed proliferation and induced G0/G1 arrest. However, in some other types of human cancer, miR-192 shows tumor-promoting activities. For instance, Li et al. found that miR-192 was upregulated in ESCC tissues and cell lines and promoted tumor progression and inhibited cell apoptosis [11]. Oncogenic roles of miR-192 were further evidenced by recent reports which found that the overexpression of miR-192 promotes tumor growth and progression in human pancreatic ductal adenocarcinoma [12] and facilitates tumorigenesis in gastric cancer [13]. Taken together, miR-192 plays distinct roles in different types of cancers and acts as a tumor suppressor in human AML.
Dysregulation of cell cycle may result in imbalance between cell proliferation and cell differentiation [15], thus leading to tumorigenesis and modulation of cell cycle progression is an important mechanism for the action of tumor-associated genes, including miRNAs [7]. Liu et al. reported that the pro-neoplastic roles of miR-181a in AML cells are associated with acceleration of G1/S transition via negative regulation of ATM [22]. MiR-192 promoted cell cycle progression from the G0/G1 to the S phase by regulating key factors in these processes [29]. However, miR-192 also induced a G0/G1 and G2/M cell cycle arrest of bladder cancer cells [10] and proximal tubular epithelial cells [30], respectively. Our data confirmed the cell cycle-modulation activity of miR-192 in AML, yet with a G0/G1 cell cycle arrest in AML cells after restoration of miR-192. Differences may be attributed to the target genes in different cells. Dicer1 [29] and Bim [11] have been reported to be regulated by miR-192, while in our study, CCNT2, a cyclin playing important roles in the gene transcription, was found to be regulated by miR-192. Altogether, regulation of cell cycle transition by miR-192 is cell type specific depending on the downstream proteins.
In conclusion, as to our knowledge, we found for the first time that miR-192 was downregulated in AML compared with that in healthy controls. Functionality of miR-192 acting as a tumor suppressor makes this new molecule a useful biomarker in cancer diagnosis and management. Further investigations on miR-192 regulation are warranted.
References
Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. New Engl j med. 1999;341:1051–62.
Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–48.
Kern W, Haferlach C, Haferlach T, Schnittger S. Monitoring of minimal residual disease in acute myeloid leukemia. Cancer. 2008;112:4–16.
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33.
Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907.
Singh S, Chitkara D, Kumar V, Behrman SW, Mahato RI. miRNA profiling in pancreatic cancer and restoration of chemosensitivity. Cancer Lett. 2013;334:211–20.
Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–9.
Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY, Qiu MZ, et al. Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis. 2013;34:803–11.
Geng L, Chaudhuri A, Talmon G, Wisecarver JL, Are C, Brattain M, et al. MicroRNA-192 suppresses liver metastasis of colon cancer. Oncogene. 2014;33:5332–40.
Jin Y, Lu J, Wen J, Shen Y, Wen X. Regulation of growth of human bladder cancer by miR-192. Tumour Biol. 2015;36:3791–7.
Li S, Li F, Niu R, Zhang H, Cui A, An W, et al. Mir-192 suppresses apoptosis and promotes proliferation in esophageal aquamous cell caicinoma by targeting Bim. Int J Clinic Exp Pathol. 2015;8:8048–56.
Zhao C, Zhang J, Zhang S, Yu D, Chen Y, Liu Q, et al. Diagnostic and biological significance of microRNA-192 in pancreatic ductal adenocarcinoma. Oncol Rep. 2013;30:276–84.
Chen Q, Ge X, Zhang Y, Xia H, Yuan D, Tang Q, et al. Plasma miR-122 and miR-192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol Rep. 2014;31:1863–70.
Feng S, Cong S, Zhang X, Bao X, Wang W, Li H, et al. MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res. 2011;39:6669–78.
Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15:196–212.
Peng J, Zhu Y, Milton JT, Price DH. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–62.
Baumli S, Lolli G, Lowe ED, Troiani S, Rusconi L, Bullock AN, et al. The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation. EMBO J. 2008;27:1907–18.
Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414:322–5.
Cottone G, Baldi A, Palescandolo E, Manente L, Penta R, Paggi MG, et al. Pkn is a novel partner of cyclin T2a in muscle differentiation. J Cell Physiol. 2006;207:232–7.
Simone C, Bagella L, Bellan C, Giordano A. Physical interaction between pRb and cdk9/cyclinT2 complex. Oncogene. 2002;21:4158–65.
Dick FR, Armitage JO, Burns CP. Diagnostic concurrence in the subclassification of adult acute leukemia using French-American-British criteria. Cancer. 1982;49:916–20.
Liu X, Liao W, Peng H, Luo X, Luo Z, Jiang H, et al. miR-181a promotes G1/S transition and cell proliferation in pediatric acute myeloid leukemia by targeting ATM. J Cancer Res Clin Oncol. 2016;142:77–87.
Butrym A, Rybka J, Baczynska D, Tukiendorf A, Kuliczkowski K, Mazur G. Low expression of microRNA-204 (miR-204) is associated with poor clinical outcome of acute myeloid leukemia (AML) patients. J Exp Clinic Cancer Research. 2015;34:68.
Jin J, Wang Y, Xu Y, Zhou X, Liu Y, Li X, et al. MicroRNA-144 regulates cancer cell proliferation and cell-cycle transition in acute lymphoblastic leukemia through the interaction of FMN2. J Gene Med 2016. doi:10.1002/jgm.2898
Shen JZ, Zhang YY, Fu HY, Wu DS, Zhou HR. Overexpression of microRNA-143 inhibits growth and induces apoptosis in human leukemia cells. Oncol Rep. 2014;31:2035–42.
Lu F, Zhang J, Ji M, Li P, Du Y, Wang H, et al. miR-181b increases drug sensitivity in acute myeloid leukemia via targeting HMGB1 and Mcl-1. Int J Oncol. 2014;45:383–92.
Shibayama Y, Kondo T, Ohya H, Fujisawa S, Teshima T, Iseki K. Upregulation of microRNA-126-5p is associated with drug resistance to cytarabine and poor prognosis in AML patients. Oncol Rep. 2015;33:2176–82.
Rommer A, Steinleitner K, Hackl H, Schneckenleithner C, Engelmann M, Scheideler M, et al. Overexpression of primary microRNA 221/222 in acute myeloid leukemia. BMC Cancer. 2013;13:364.
Feinberg-Gorenshtein G, Guedj A, Shichrur K, Jeison M, Luria D, Kodman Y, et al. MiR-192 directly binds and regulates Dicer1 expression in neuroblastoma. PLoS One. 2013;8:e78713.
Jenkins RH, Davies LC, Taylor PR, Akiyama H, Cumbes B, Beltrami C, et al. miR-192 induces G2/M growth arrest in aristolochic acid nephropathy. Am J Pathol. 2014;184:996–1009.
Acknowledgements
The authors thank the local doctors and the patients who participated in our study. The project was supported by the Hubei Provincial Natural Science Foundation of China (No. 2011CDD193).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have declared no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ke, S., Li, Rc., Lu, J. et al. MicroRNA-192 regulates cell proliferation and cell cycle transition in acute myeloid leukemia via interaction with CCNT2. Int J Hematol 106, 258–265 (2017). https://doi.org/10.1007/s12185-017-2232-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2232-2