Abstract
Recent large-scale randomized clinical trials in Europe and the US demonstrated that maintenance therapy with rituximab significantly improved the progression-free survival (PFS) in indolent B-cell non-Hodgkin lymphoma (B-NHL) patients, especially those with follicular lymphoma (FL). However, rituximab maintenance has not been approved in Japan, because there are no clinical data supporting the benefit of rituximab maintenance in Japanese patients. Therefore, we conducted a single-arm, multicenter bridging study in previously untreated indolent B-NHL patients with high tumor burden. The primary endpoint was 4-year PFS and was expected to be 70 % based on previous studies. Sixty-two patients, including 55 FL patients, were enrolled and received induction therapy with CHOP combined with rituximab (R-CHOP). Fifty-eight patients responding to R-CHOP induction received rituximab at 375 mg/m2 every 8 weeks for 2 years as for the rituximab maintenance arm in the PRIMA study. A 4-year PFS of 69.8 % was obtained (95 % confidence interval 55.9–80.0 %). Rituximab maintenance was well tolerated and common adverse events were infections, neutropenia, and/or leukopenia that were manageable with conventional supportive care. No patients died. These data were compatible with the PRIMA data. R-CHOP induction followed by rituximab is useful in Japanese patients with untreated indolent B-NHL having high tumor burden.
Clinical trial number UMIN000001191
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Rituximab, an anti-CD20 chimeric monoclonal antibody, [1] has been used as monotherapy or in combination with chemotherapy for advanced indolent B-cell non-Hodgkin lymphoma (B-NHL). However, most indolent B-NHLs are incurable as the disease inevitably relapses. Several large-scale randomized clinical studies in Europe and the United States (US) have reported significant improvement in progression-free survival (PFS) with rituximab when used as maintenance therapy in patients with indolent B-NHL or follicular lymphoma (FL) who achieved responses by preceding induction therapy with chemotherapy alone or in combination with rituximab [2–7]. A prospective phase III study conducted by the European Organization for Research and Treatment of Cancer (EORTC) showed improved PFS in patients with recurrent/resistant FL who received rituximab maintenance treatment for 2 years following induction with rituximab in combination with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) [2, 3]. The PRIMA (primary rituximab and maintenance) randomized phase III study also demonstrated an improvement in PFS with rituximab maintenance therapy in patients with previously untreated FL with high tumor burden, although no improvement in overall survival (OS) was seen in comparison with observation alone [8, 9].
While rituximab maintenance has already been approved in Europe and the US, it has been off-label in Japan because its efficacy and safety in Japanese patients have not yet been examined. Therefore, we conducted a non-randomized, single-arm, multicenter phase II study to evaluate whether rituximab maintenance is applicable to Japanese patients with previously untreated indolent B-NHLs bearing high tumor burden.
Materials and methods
Study design and patients
This open-label, single-arm multicenter phase II study between January 2009 and November 2013 was intended to evaluate the effectiveness of rituximab maintenance in Japanese indolent B-NHL with the Groupe d’Etude des Lymphomes Folliculaires (GELF) high tumor burden by comparing its efficacy and safety with those reported in the PRIMA study. All patients provided written informed consent prior to study entry. The study was approved by each institutional review board and was conducted according to the Declaration of Helsinki, Good Clinical Practice guidelines, and other related regulations. The trial was registered at the University Hospital Medical Information Network (No. UMIN000001191).
Patient eligibility
Study patients were aged 20–80 years, with previously untreated CD20-positive indolent B-NHL diagnosed by biopsy performed within 4 weeks of enrollment and classified into small lymphocytic lymphoma, lymphoplasmacytic lymphoma, splenic marginal zone B-cell lymphoma, extra-nodal marginal zone B-cell lymphoma of MALT type, nodal marginal zone B-cell lymphoma, or FL (grade 1, 2, or 3a), according to the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues [10]. Histology was later confirmed by a central pathology review committee consisting of three independent pathology experts. Eligible patients had to have a high tumor burden meeting at least one of the GELF criteria [11, 12]: bulky disease (one lesion >7 cm); presence of B symptoms; raised serum concentrations of lactate dehydrogenase or β2-microglobulin; involvement of three or more regional lymph nodes >3 cm; symptomatic splenic enlargement; and compressive syndrome, ascites or pleural effusion.
Patients were also required to have adequate organ function (bone marrow, liver, kidney, heart, and lung), a performance status (PS) of ≤2 on the Eastern Cooperative Oncology Group (ECOG) scale, and a life expectancy of at least 6 months at the time of study enrollment.
Patients with grade 3b FL or mantle cell lymphoma, histologically transformed from indolent B-NHL, central nervous system involvement, or any other active malignancy were excluded from the study. Patients were also excluded if they had human immunodeficiency virus infection, were sero-positive for hepatitis B virus or hepatitis C virus infection, had any underlying infectious disease or medical conditions known to affect life expectancy, had a previous history of monoclonal antibody therapy, or had allergy or sensitivity to mouse-related proteins. Nursing or pregnant patients were also excluded.
Treatment schedule
Patients were assessed for their baseline characteristics prior to initial study treatment, including a physical examination, laboratory testing, and computed tomography (CT) assessment of tumor burden. In the induction phase of the study, patients were treated with rituximab and CHOP chemotherapy as follows: six cycles of standard CHOP therapy (cyclophosphamide 750 mg/m2 i.v. on day 1; doxorubicin 50 mg/m2 i.v. on day 1; vincristine 1.4 mg/m2 [capped at 2 mg] i.v. on day 1; and prednisone 100 mg orally on days 1–5) and rituximab (375 mg/m2) on day 1 of each CHOP cycle, with two additional infusions every 3 weeks after the last R-CHOP cycle. Patients who achieved a partial response (PR) or better after the induction phase stepped into the maintenance phase of the study, where patients received 12 infusions of rituximab monotherapy at 375 mg/m2 i.v. once every 8 weeks, starting 8 weeks after the last induction treatment, for 2 years. After completion of rituximab maintenance, patients were followed for 18 months or until tumor progression, whichever was longer. Thus, the patients were studied up to 4 years (48 months, 192 weeks) from study entry until last observation. All patients received premedication with acetaminophen 400 mg and d-chlorpheniramine maleate 2 mg orally 30 min before each rituximab administration to minimize infusion-related reactions.
Patient evaluation and study endpoints
CT was used to assess tumor responses at study entry, after completion of induction therapy, and every 6 months during rituximab maintenance and during post-maintenance follow-up period. Tumor response was evaluated by a central CT review committee consisting of 3 radiologists according to the International Workshop NHL Response Criteria [13].
The primary endpoint of the study was the 4-year PFS rate from study entry. Secondary endpoints included the 4-year OS rate, overall response rate [ORR; complete response (CR) plus partial response (PR)] and CR rate at the end of maintenance therapy.
Adverse events (AEs) including abnormal laboratory values were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 from the Japan Clinical Oncology Group and the Japan Society of Clinical Oncology (JCOG/JSCO). All AEs observed were examined by extramural committee for severity and relationship to rituximab.
Statistical analysis
Based on previous studies, we expected to observe a 4-year PFS of 70 % with a two-sided 95 % confidence interval (CI) no lower than 50 % [2, 4, 8, 14–16]. A total of 50 patients were required to achieve at least 80 % power to show the expected 4-year PFS with two-sided 5 % significance in accordance with Fleming’s one-stage procedure (e.g., α = 0.05, 1 − β = 0.8) [17]. We assumed that up to 8 patients (15 %) would be lost to follow-up and/or eligibility violation; thus, 58 patients were planned for enrollment.
All statistical analyses for efficacy were performed using the full analysis set (FAS, all enrolled patients). PFS and OS rates were estimated by the Kaplan–Meier method [18].
Baseline pretreatment factors affecting PFS were analyzed in FAS population by univariate and multivariate analysis using Cox proportional hazard regression model. The proportional hazard assumption was graphically checked using the log–log survival plot. The safety analysis population set was defined as patients who received at least one dose of rituximab.
SAS software version 9.2 (SAS Institute Inc., NC, USA) was used for all analyses. P values <0.05 were considered significant.
Results
Patient disposition and baseline characteristics
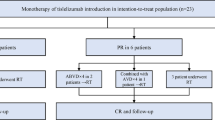
Figure 1 shows the patient disposition. Overall, 62 patients were enrolled and underwent induction therapy. Of these, 59 patients completed the 6 cycles of R-CHOP plus 2 additional cycles of rituximab monotherapy in the induction phase. Three patients were discharged during the course of induction therapy for the following reasons: one patient withdrew consent [this patient was later diagnosed as having diffuse large B-cell lymphoma (DLBCL) by central pathology review and was treated as a protocol violation]; treatment-related toxicity (prolonged leukopenia and neutropenia), n = 1; and serious deep venous thrombosis not related to rituximab, n = 1. All 59 patients who completed the induction therapy achieved PR or better tumor responses and stepped into the maintenance phase, except one patient who was positive for antibody against hepatitis B virus surface antigen (anti-HBs) and discharged from the study. Fifty-two of the 58 patients completed the maintenance phase and 6 patients discontinued the study because of lymphoma progression, n = 4; grade 3 AEs (back pain) not related to rituximab, n = 1; medical judgment because of ineligible pathology (diagnosed as having DLBCL by central pathology review), n = 1. Among 52 patients who completed rituximab maintenance, nine patients developed tumor progression and 43 patients completed 18 months of post-maintenance follow-up.
Table 1 shows baseline characteristics of the FAS. Sixty-one of 62 enrolled patients (98 %) fulfilled at least of one of the GELF high tumor burden criteria [11, 12]. Thirty-six patients (58 %) had bone marrow involvement. Fifty-two patients (84 %) had stage III or IV disease. The median age was 58.5 years (range 36–77 years). Baseline Follicular Lymphoma International Prognostic Index (FLIPI) scores categorized patients as low risk (zero-to-one risk factor) n = 21; intermediate risk (two risk factors) n = 20; and high risk (three-to-five risk factors) n = 21 [19]. Three patients were diagnosed as having composite lymphoma mixed with FL grade 3a and DLBCL by central pathology review, but were considered to be eligible by extramural review because they had an indolent clinical course correlated with FL, and thus, stayed in the study. Overall, 92 % (57/62) of patients enrolled had confirmed indolent B-NHL and 89 % (55/62) of patients had FL.
Efficacy endpoints
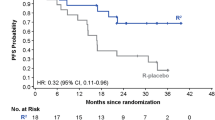
Four-year PFS and 4-year OS from the date of enrollment were 69.8 % (95 % CI 55.9–80.0) and 100 %, respectively (Fig. 2a).
Table 2 shows the response rates after induction therapy and maintenance therapy. The ORR was 95.2 % (59/62; 95 % CI 86.5–99.0) after induction therapy and 82.8 % (48/58; 95 % CI 70.6–91.4) after maintenance therapy. Fourteen of 21 patients who achieved PR after induction therapy converted to CR during maintenance therapy. Sixteen patients experienced disease progression during the maintenance phase and follow-up period.
Factors affecting PFS
Baseline pretreatment factors affecting PFS were analyzed by univariate and multivariate analysis. In addition to conventional patient characteristics such as gender, ECOG PS, bone marrow involvement, extra-nodal involvement, and FLIPI score, we also selected the peripheral blood absolute lymphocyte count/absolute monocyte count ratio (ALC/AMC ratio) at study enrollment for analysis [20, 21]. As shown in Table 3 and Fig. 2b, PFS was significantly affected by FLIPI in both univariate and multivariate analyses, and patients in the low/intermediate-risk group had a significantly higher PFS rate over the 4-year period (80.7 %, 95 % CI 63.7–90.3) compared with the high-risk group (50.0 %, 95 % CI 27.1–69.2; p = 0.014; by log-rank test). By univariate analysis, PFS was significantly associated with ECOG PS, ALC/AMC ratio, and FLIPI score. As a result of the model selection by multivariate Cox proportional hazards regression analyses, FLIPI high risk was an independent unfavorable factor, whereas ECOG PS was not selected as a factor because of multicollinearity with FLIPI score.
Immunoglobulins
Serum concentrations of immunoglobulins (IgG, IgA, and IgM) that decreased from baseline during the induction and maintenance phase tended to recover during the follow-up period. Mean serum concentrations (mg/dL) of IgG/IgA/IgM at baseline, post-maintenance therapy, and after 2 years follow-up were 1,151/176/95, 798/104/44, and 828/133/53, respectively. No patients received supplemental immunoglobulins.
Other findings
One of 16 patients who experienced a recurrence/relapse had a histological transformation to high grade B-NHL from FL grade 3a. Peripheral blood CD19+ and CD20+ cells were immediately depleted after the first cycle of induction treatment, and depletion persisted through the maintenance phase. The depleted CD19+ and CD20+ cells remained nearly undetectable until approximately 12 months after the end of the maintenance phase, and then gradually recovered. Development of human anti-CD20 antibodies (HACA) was examined in 58 patients but was not detected throughout the study.
Safety analysis
Safety was assessed for all 62 patients who received at least one infusion of rituximab. A total of 7,018 AEs including laboratory value abnormalities were observed throughout the study (4230 events in induction phase, 2119 in maintenance phase, and 669 in follow-up period). Of these, 477 were grade 3 or 4 in severity, but no grade 5 (death) events were reported.
Table 4 shows the AEs of grade 3 that occurred in two or more patients and all AEs of grade 4. Most grade 3 or 4 AEs were hematological toxicities represented by leukopenia/neutropenia and were more frequently observed during the induction phase than during the maintenance phase and follow-up period. Nine of 12 patients who developed grade 3 or 4 febrile neutropenia during induction treatment received G-CSF. Four patients with grade 3 or 4 leukopenia/neutropenia during maintenance or follow-up received G-CSF (three patients during maintenance and one patient during maintenance and follow-up period).
Grade 3 or 4 non-hematologic toxicities were also frequently observed during the induction phase. Infections were the most common AEs and were observed in 52 patients throughout the study. Most infections were of grade 1 or 2 in severity, and grade 3 infections were neutropenic infection, cystitis, herpes simplex, pneumonia, pulmonary mycosis, pyelonephritis, and disseminated herpes zoster. Secondary malignancy, lung adenocarcinoma stage IA was found in one patient who received CT examination for assessment of lymphoma response during the follow-up period, for which endoscopic excision was applied and removed successfully.
Twenty-nine serious adverse events (SAEs) were reported in 19 patients; of these, 18 SAEs were suspected to be treatment-related. All treatment-related AEs and SAEs were recovered and remitted. One patient discontinued the study because of treatment-related toxicity (prolonged neutropenia during induction treatment). No deaths were reported in this study.
Discussion
We conducted a multicenter phase II study of rituximab maintenance in Japanese patients with newly diagnosed indolent B-NHL having GELF high tumor burden, with reference to the PRIMA study. Our study design was similar to the PRIMA study in terms of patient eligibility and treatment schedule, and we expected to obtain a 4-year PFS of 70 % starting from study enrollment in accord with previous rituximab maintenance studies in the Europe and US and R-CHOP combination study in Japan [2, 4, 8, 14–16].
Sixty-two patients were enrolled and 43 patients were free from progression at 4-years yielding 69.8 % (95 % CI 55.9–80.0 %) of 4-year PFS as originally expected. In the PRIMA study, a PFS of 74.9 % was obtained with a median follow-up of 36 months from initiation of rituximab maintenance in FL patients [8]. Considering that 88.7 % (55/62) of our patients had FL, the 4-year PFS we observed in our study is comparable to that in the PRIMA study [8]. The 4-year PFS of 70 % in the current study is longer than that reported in a previous R-CHOP study without rituximab maintenance in Japan [22]. An ORR of 95.2 % with CR rate of 61.3 % was obtained after R-CHOP induction therapy. These responses are consistent with the results of a US study of low-grade or follicular B-NHL patients receiving R-CHOP [23] and our previous study in which an ORR of 94 % with CR rate of 66 % was obtained [14]. Fourteen of 21 patients (67 %) who achieved PR after induction treatment converted to CR during maintenance in our study, similar to the PRIMA study in which 72/139 patients (52 %) converted from PR to CR [8]. Thus, our findings, taken together with previous studies, suggest that maintenance use of rituximab after first-line remission induction is useful to prolong PFS in Japanese patients.
Despite the superior PFS with the addition of rituximab maintenance therapy compared with the standard induction treatment for advanced indolent B-NHL, it remains unclear whether there is a benefit in terms of OS. The OS in the PRIMA study was approximately 90 % in both the observation and rituximab maintenance arms, and was not statistically different between the arms [8]. Because indolent B-NHL has a relatively slow-growing nature with a decade-long life expectancy, a longer follow-up period is necessary to observe the effects of rituximab maintenance on OS [9].
Safety of rituximab maintenance observed in our study was also consistent with that reported in the PRIMA study. In our current study, hematological toxicities and infections were commonly observed and 72 % of patients developed infections or infestations of any grade during the maintenance period. In the PRIMA study, infections were reported to be the most common AEs and grade 2–4 infections were significantly more common in the rituximab maintenance arm (39 %) than in the observation arm (24 %). Frequencies of grade 3/4 infections were similar between our study and PRIMA (5 and 4 %, respectively). Because we routinely employed sulfamethoxazole/trimethoprim as prophylaxis for Pneumocystis jirovecii infection in the induction phase (82 % in all patients) and afterwards by investigator direction, no P. jirovecii infections were observed in our study. All observed treatment-related AEs were manageable with standard supportive therapy and did not prevent the continuation of study treatment.
There was no difference in terms of secondary malignancies in the rituximab maintenance and observation arms in the PRIMA study [8]. Similarly, we observed only one case of lung adenocarcinoma during the follow-up period, and this patient underwent successful surgical resection for lung cancer.
Pretreatment factors affecting PFS in the present study included FLIPI, ECOG PS, and ALC/AMC ratio by univariate analysis. Only a high FLIPI score was independently associated with a shorter PFS by multivariate analysis. This result is consistent with analyses reported by the PRIMA study and other studies [8, 24]. The primary limitations of this study were its relatively small size and non-randomized design. However, the prospective nature of the trial, its long maintenance phase and follow-up period, as well as the consistency of the efficacy and safety results with those of several large-scale, randomized trials, including PRIMA and several other European and US studies, support the validity of our findings.
However, it remains unclear whether indolent B-NHL patients with low tumor burden and patients with non-FL could also benefit from rituximab maintenance, and this should be addressed by further investigations.
In conclusion, R-CHOP induction therapy followed by 2 years of rituximab maintenance every 8 weeks is a useful therapeutic option in Japanese patients with untreated indolent B-NHL with high tumor burden as defined by GELF criteria, with a 4-year PFS of nearly 70 %. The treatment regimen was feasible for routine practice, was well tolerated, and is also applicable to Japanese patients.
References
Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45.
van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–301.
van Oers MH, Van Glabbeke M, Giurgea L, Klasa R, Marcus RE, Wolf M, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–8.
Hochster H, Weller E, Gascoyne RD, Habermann TM, Gordon LI, Ryan T, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 study. J Clin Oncol. 2009;27:1607–14.
Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin’s lymphoma—a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23:1088–95.
Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood. 2004;103:4416–23.
Vidal L, Gafter-Gvili A, Salles G, Dreyling MH, Ghielmini M, Hsu Schmitz SF, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: an updated systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2011;103:1799–806.
Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51.
Seymour JF, Feugier P, Offner F, Lopez-Guillermo A, Belada D, Xerri L, et al. Updated 6 year follow-up of the PRIMA Study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline immunochemotherapy. Blood. 2013;122:509 (abstract).
Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001.
Decaudin D, Lepage E, Brousse N, Brice P, Harousseau JL, Belhadj K, et al. Low-grade stage III–IV follicular lymphoma: multivariate analysis of prognostic factors in 484 patients—a study of the Groupe d’ Etude des Lymphomes de l’Adulte. J Clin Oncol. 1999;17:2499–505.
Solal-Céligny P, Lepage E, Brousse N, Tendler CL, Brice P, Haïoun C, et al. Doxorubicin-containing regimen with or without interferon alfa-2b for advanced follicular lymphomas: final analysis of survival and toxicity in the Groupe d’Etude des Lymphomes Folliculaires 86 Trial. J Clin Oncol. 1998;16:2332–8.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244.
Ogura M, Morishima Y, Kagami Y, Watanabe T, Itoh K, Igarashi T, et al. Randomized phase II study of concurrent and sequential rituximab and CHOP chemotherapy in untreated indolent B-cell lymphoma. Cancer Sci. 2006;97:305–12.
Tobinai K, Ogura M, Itoh K, Kinoshita T, Hotta T, Watanabe T, et al. Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7-year follow-up results. Cancer Sci. 2010;101:2579–85.
Forstpointner R, Unterhalt M, Dreyling M, Böck HP, Repp R, Wandt H, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular. Blood. 2006;108:4003–8.
Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–51.
El Kaplan, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Solal-Coligny P, Roy P, Colombat P. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65.
Behl D, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, et al. Absolute lymphocyte count predicts therapeutic efficacy of rituximab therapy in follicular lymphomas. Br J Haematol. 2007;137:409–15.
Kumagai S, Tashima M, Fujikawa J, Iwasaki M, Iwamoto Y, Sueki Y, et al. Ratio of peripheral blood absolute lymphocyte count to absolute monocyte count at diagnosis is associated with progression-free survival in follicular lymphoma. Int J Hematol. 2014;99:737–42.
Watanabe T, Tobinai K, Shibata T, Tsukasaki K, Morishima Y, Maseki N, et al. Phase II/III study of R-CHOP-21 versus R-CHOP-14 for untreated indolent B-cell non-Hodgkin’s lymphoma: JCOG 0203 trial. J Clin Oncol. 2011;29:3990–8.
Czuczman MS, Grillo-López AJ, White CA, Saleh M, Gordon L, LoBuglio AF, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–76.
Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-López AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711–6.
Acknowledgments
This study was supported by Zenyaku Kogyo (Tokyo, Japan). The authors thank the patients and their families and all the investigators, including the physicians, nurses, clinical research coordinators (CRC), and laboratory technicians in the participating institutions of this multicenter trial. The authors are grateful to Drs K. Toyama (Tokyo Medical College, Tokyo, Japan), N. Horikoshi (Juntendo University School of Medicine, Tokyo, Japan), and M. Mori (Japanese Red Cross Medical Center, Tokyo, Japan) for their critical review of the clinical data as members of the Independent Data and Safety Monitoring Committee. The authors are also grateful to Drs S. Nakamura (Nagoya University Graduate School of Medicine, Nagoya, Japan), and Y. Matsuno (Hokkaido University Hospital, Sapporo, Japan) for their histopathological review as members of the Central Pathology Review Committee, and M. Matsusako (St. Luke’s International Hospital, Tokyo, Japan) for their central radiological review as members of the CT Review Committee. The authors also acknowledge K. Endo, H. Harada, T. Ito, I. Okugaito, M. Watanabe, M. Abe, T Oba, S. Kamiyama, and T. Kayo (Zenyaku Kogyo) for their help with data collection and statistical analysis.
The authors would like to thank Ms Reiko Yamaura and Dr J. Ludovic Croxford of Edanz Group Ltd. for providing English writing support, which was funded by Zenyaku Kogyo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tadahiko Igarashi and Kiyoshi Ando received research funding from Zenyaku Kogyo. Masafumi Taniwaki and Kazuhito Yamamoto received research funding from Chugai Pharmaceutical. Hirokazu Nagai received honoraria from Chugai Pharmaceutical. Yasuo Ohashi is a board member and stockholder of Statcom and received honoraria from Chugai Pharmaceutical. Kensei Tobinai received research funding from Zenyaku Kogyo and Chugai Pharmaceutical, and honoraria from Zenyaku Kogyo. The remaining authors have no conflict of interests to disclose.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s12185-016-2155-3.
About this article
Cite this article
Igarashi, T., Ogura, M., Itoh, K. et al. Japanese phase II study of rituximab maintenance for untreated indolent B-cell non-Hodgkin lymphoma with high tumor burden. Int J Hematol 104, 700–708 (2016). https://doi.org/10.1007/s12185-016-2097-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2097-9