Abstract
This study was conducted to evaluate the efficacy and safety of rituximab and Bortezomib in relapsed or refractory indolent B cell non-Hodgkin’s lymphoma (NHL). Treatments consisted of rituximab 375 mg/m2, i.v. on days 1, 8, 15, and 22 of cycle 1 and on day one of cycles 2–5, bortezomib 1.6 mg/m2, given by intravenous injection (3-s to 5-s bolus) on days 1, 8, 15, and 22 of a maximum of five cycles. The primary end points were the overall survival (OS) and progression-free survival (PFS). Secondary endpoints included response rate (ORR; CR) and toxicities. From January 2008 to December 2010, 60 successive patients at Tianjin cancer hospital lymphoma department were enrolled in this study. All patients were recurrent or refractory indolent B cell NHL, including follicular lymphoma grades 1–2 (n = 35), small lymphocytic lymphoma/chronic lymphocytic leukemia (LL/CLL; n = 16) and marginal zone lymphoma (n = 9). The median follow-up time was 30 months (range 12–48). The overall response rate was 70.0 %, with a CR/CRu rate of 31.7 %. The 2-year OS and PFS of all patients were 75.0 and 41.0 %, respectively. Grade 3–4 neutropenia and thrombocytopenia occurred in 10 and 3.3 % of patients, respectively. Higher IPI and refractory disease were independently associated with worse survival and PFS. RB chemotherapy in patients with refractory or relapsed indolent B cell NHL was effective with low toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indolent B cell lymphomas tend to be slow growing but are incurable. Recent epidemiological data suggest that the frequency is rising in our country. The 5-year survival is 60–70 %, but as many as one-third of these lymphomas will transform to a higher grade form of lymphoma, usually diffuse large B cell. Because the natural course of these diseases is characterized by a relatively long median survival, patients receive multiple cytotoxic therapies during the course of their disease [1]. Repeated chemotherapy frequently results in multidrug resistance, and patients die due to rapid tumor progression [2]. Lymphoma cells over expressing BCL2 are resistant to apoptosis and establish intimate relationships with their microenvironment. To circumvent multidrug resistance, new drugs are necessary, which are not influenced by the multidrug mechanisms directed toward alkylating agents and anthracyclines.
Bortezomib (VELCADE) is a small-molecule proteasome inhibitor, developed as an agent to treat human malignancies. The antineoplastic effect of bortezomib likely involves several distinct mechanisms, including inhibition of cell growth and survival pathways, induction of apoptosis, inhibition of expression of genes that control cellular adhesion, migration, and angiogenesis. Notably, bortezomib induces apoptosis in cells that over express BCL2 [3]. Bortezomib is approved for treatment of multiple myeloma and, additionally in the USA, for the treatment of patients with mantle cell lymphoma after at least one previous therapy [4]. Bortezomib is thought to be efficacious in multiple myeloma via its inhibition of NF-κB activation, its attenuation of interleukin-6-mediated cell growth, a direct apoptotic effect, and possibly antiangiogenic and other effects [5]. In multiple phase II studies performed in follicular lymphoma, bortezomib as a single agent seemed to show variable activity. The overall response rate (ORR) ranged from 16 to 41 %, with few complete remissions (CRs). Biweekly schedules eventually seemed to be associated with higher response rates; however, this did not translate into significant prolonged progression-free survival (PFS) [6].
Rituximab is a chimeric antibody directed against the CD20 antigen present on human B cells. The antibody is able to kill B cells due to antibody-dependent cytotoxicity (ADCC), induction of apoptosis, and complement activation. Rituximab also inhibits NF-κB in cell lines of B cell non-Hodgkin’s lymphoma, associated with chemo-sensitisation effects [7]. In the pivotal trial, rituximab produced an overall response rate in relapsed and refractory indolent lymphomas of 50 % when used as a single agent [8]. Bortezomib increases CD20 expression in rituximab-resistant cell lines in vitro [9]. Bortezomib and rituximab had additive activity in preclinical models of lymphoma [10]. Bortezomib in combination with rituximab alone or plus other chemotherapy drugs was active and generally well tolerated in early phase studies in follicular lymphoma and other subtypes of non-Hodgkin’s lymphoma [11]. In a phase III clinical trial, Bertrand Coiffier [12] reported that rituximab combined with bortezomibin in treatment of patients with relapsed or refractory follicular lymphoma, the median progression-free survival was 12.8 months, the ORR was 63 %, with a CR/CRu rate of 25 %.
In this study, we retrospectively analyzed a series of 60 patients presenting with relapsed/refractory indolent lymphoma treated by rituximab, bortezomib (RB) regimen in our department between 2008 and 2010. We evaluated the efficacy and toxicity of RB regimen.
Patients and methods
Patients
Between January 2008 and December 2010, sixty refractory and relapsed CD20-positive patients with indolent lymphoma were enrolled in the study. All the histological diagnoses, including CD20 positivity, were confirmed with immunohistochemistry. Patients who met the following criteria were considered eligible for inclusion in the study: (1) nodal biopsy confirming diagnosis of follicular lymphoma grade 1–2, small lymphocytic lymphoma/chronic lymphocytic leukemia(SLL/CLL), and marginal zone lymphoma as defined by the World Health Organization (WHO) classification [13]; (2) Eastern Cooperative Oncology Group (ECOG) performance status <3; (3) ≥18 years of age; (4) Ann Arbor stage of III–IV disease; (5) at least one site of disease measurable in two dimensions using clinical examination, CT scan, or MRI scans; (6) normal cardiac, renal, pulmonary, and hepatic function unless abnormal because of disease involvement; (7) granulocyte count ≥4.0 × 109/L and platelet count ≥100 × 109/L, except in cases of bone marrow (BM) involvement; and (8) Patients were rituximab naive or rituximab sensitive (response to and time to progression ≥6 months for previous rituximab-containing treatment). Exclusion criteria were as follows: HIV-positive or hepatitis B or C virus-positive status.
Treatment
The rituximab/bortezomib schedule was as follows: rituximab 375 mg/m2 i.v. on days 1, 8, 15, and 22 of cycle 1, and on day one of cycles 2–5, bortezomib 1.6 mg/m2, given by intravenous injection (3-s to 5-s bolus) on days 1, 8, 15, and 22 of a maximum of five cycles. If the patient had neutrophil count >1.0 × 109/L; platelet count >50 × 109/L, treatment was restarted at the initial dose after the neutrophil and platelet counts recovered with granulocyte colony stimulating factor (G-CSF) provided. If the patient had neutrophil count <1.0 × 109/L; platelet count <50 × 109/L, the following cycle was delayed up to 3 weeks until the blood cell recovered to normal level, and bortezomib was administered at 1.3 mg/m2 with G-CSF given in prevention and platelet and erythrocyte concentrates administered if necessary. Bortezomib dose modifications were required for prespecified non-hematological toxic effects.
Patient monitoring and assessment
The following evaluations and procedures were performed during the pretreatment screening period: medical history and baseline laboratory and imaging studies. Evaluations performed during and after treatment included physical examination, CBC counts, serum chemistry, and imaging studies (CT scan or MRI) for disease assessment. Treatment response was assessed at 4 weeks after two cycles of primary chemotherapy. Reassessment carried out for tumor response was performed 4 weeks after the last treatment and then every 3 months during the first year, every 6 months in successive years.
Determination of CR, CRu, partial response (PR), stable disease (SD), and progressive disease (PD) was defined using a modification of the International Workshop NHL Response Criteria published by Cheson et al. [14]. A complete remission (CR) was defined as the complete disappearance of the disease for at least 8 weeks. CRu was defined as unconfirmed CR. A partial remission was defined as a 50 % reduction of all measurable lesions maintained for at least 4 weeks without increase in size of any area of known malignant disease. Progressive disease was defined as any new lesion or an increase of ≥50 % of previously involved sites from the nadir, and patients not included in any of these categories were considered to have stable disease.
Complete response rate (CRR) = number of CR patients/Total number of evaluable patients × 100 %.
Overall response rate = (CR + PR)/Total number of evaluable patients × 100 %.
The primary end point was overall survival (OS) and PFS. Secondary end points were overall response rate, complete response rate, safety, and tolerability. Overall survival was defined as the period from the treatment start date to the date of patient died for any cause or observation end. Progression-free survival was defined as the period from the treatment start date to the progression date.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 software. The responses between groups were compared by χ2 tests; Kaplan–Meier method was used to calculate survival rate and draw the survival curves. Log rank test was used to compare the survival rate between two groups. Cox’s proportional hazards analysis was used to calculate hazard ratios and the 95 % confidence interval. Multivariate analysis was performed using Cox regression model. p < 0.05 was considered statistically significant.
Treatment toxicity was evaluated according to the WHO criteria.
Results
Patients and response to therapy
From January 2008 to December 2010, sixty refractory and relapsed CD20-positive patients with indolent lymphoma at Tianjin cancer hospital lymphoma department were enrolled in the study. All patients were diagnosed by pathology according to the WHO (2008) criteria. All patients had evaluable lesions. Median age at the time of treatment was 55 years (range 18–65 years). All patients were refractory and relapsed indolent lymphoma who have been treated with CHOP-like and fludarabine-based regimen with or without rituximab previously. The characteristics of 60 patients are listed in Table 1.
Short term efficacy
All patients received chemotherapy of RB regimen as salvage therapy for up to five cycles. All patients were evaluable for response. At the end of treatment, 19 patients (31.7 %) achieved CR/CRu, 23 PR (38.3 %), OR rate was 70.0 % (95 % CI 43.6–97.2 %), 13.3 % patients remaining in stable disease while 16.7 % having disease progression, as shown in Table 2.
The OR rate of the relapsed patients was significantly higher than the refractory patients (43.3 vs. 26.7 %, p = 0.018). The CR rate of the relapsed patients was significantly higher than the refractory patients (21.7 vs. 10.0 %, p = 0.026; Table 2).
Survival analysis and prognostic factors
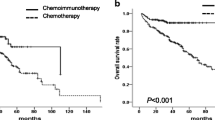
All the 60 patients were followed up until December 2011. The median follow-up time was 30 months (range 12–48); ten patients (16.7 %) died, eight patients died of disease progression, and two patients died of other diseases. The 2-year OS and PFS of all patients were 75.0 and 41.0 %, respectively (Fig. 1). OS and PFS of relapsed patients were significantly higher than that of refractory patients: 2-year OS was 84.0 versus 58.0 % (p = 0.006), and 2-year PFS was 61.0 versus 18.0 % (p = 0.002; Figs. 2, 3). According to the IPI at the start of RB regimen, OS and PFS of patients with low or low-intermediate risk was more favorable as compared with that of the high or high-intermediate risk group: 2-year OS was 78.0 versus 42.0 % (p = 0.002), and 2-year PFS was 72.0 versus 38.0 % (p = 0.009; Figs. 4, 5).
A univariate analysis showed that ECOG (p = 0.014), IPI score (p = 0.009), bone marrow involvement (p = 0.034), disease status (p = 0.002), and time to disease recurrence or progression (p = 0.023) were related to patients’ 2-year PFS (Table 1). A multivariate analysis showed that IPI score (p = 0.004) and disease status (0.000) were independent factors related to patients’ 2-year PFS (Table 3).
Toxicity
The toxicities of RB regimen included hematologic and non-hematologic toxicity. The major hematologic toxicity was myelosuppression. Grade 3 or 4 neutropenia, anemia, and thrombocytopenia were observed in six (10 %), four (6.7 %), and two patients (3.3 %), respectively. Neutrophils count were recovered to normal level after G-CSF administration. The duration of myelosuppression is short, and blood cell support is only rarely required. Only one patient developed febrile neutropenia during chemotherapy, who improved after subsequent neutrophil recovery and appropriate use of antibiotics. The major non-hematologic toxicities were nausea, fatigue, diarrhea, and peripheral sensory neuropathy. The symptom was relieved when appropriate administration was given. Peripheral neuropathy events were reported in ten patients (16.7 %), including two patients (3.3 %) with events of grade 3 or higher. Most events were peripheral sensory neuropathy. Two patients modified dose of bortezomib to 1.3 mg/m2 because of peripheral neuropathy. The adverse effects related to rituximab were hypersensitivity (fever, chills, rush, and hypotension) and were moderate in most patients. During the first rituximab infusion, nine patients (15.0 %) had hypersensitivity. Subsequent rituximab infusions were well tolerated without recurrence of adverse reactions. Of the infections, 12 (20 %) patients had infections during chemotherapy, and 2 (3.3 %) patients had grade 3–4 infection. Patients recovered by appropriate use of G-CSF and antibiotics. No toxicity-related death was observed. (Table 4).
Discussion
Patients with relapsed or refractory indolent NHL have limited options and poor prognosis. Treatment of patients with relapsed or refractory indolent lymphomas is becoming increasingly difficult when such immunochemotherapy like R-CHOP, R-CVP, and R-FCM has been applied already in previous therapies. Multiple efficient second-line treatments are available, but new therapeutic options are needed, especially in those patients who develop resistance to standard therapy or experience histologic transformation. Because overall survival has been markedly prolonged over the last decade [15], cumulative or long-term treatment toxicities have become important to consider to preserve patient quality of life and avoid therapy-related death.
Proteasome inhibitors such as bortezomib have a wide spectrum of demonstrated activity against cancer cells, including nuclear factor κB inhibition and modulation as well as pro- and antiapoptotic pathway and cell-cycle modification [16]. Preclinical data have been generated in the investigation of the mechanisms of action of bortezomib in lymphoma cell lines, mainly in mantle cell and diffuse large B cell lymphoma cell lines [17].
Di Bella et al. reported [18] the efficacy and safety of single-agent bortezomib in patients with relapsed or refractory indolent lymphoma, fifty-nine patients were treated with bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 for up to eight 21-day cycles; ORR was 13.3 %, CR/CRu was 7.6, 64.2 % patients received stable disease, and 22.6 % patients with progressive disease. 1-year survival was 73 % and 2-year survival was 58 %; median survival was 27.7 months (range 1.4–30.9 months); median progression-free survival was 5.1 months (range 0.2–27.7 months), Treatment-related grade 3 or 4 adverse events included: thrombocytopenia (20 %), fatigue (10 %), neutropenia (8.5 %), and neuropathy and diarrhea(6.8 % each). This study demonstrates that bortezomib has modest activity against relapsed or refractory indolent lymphoma; it has the potential for combination with other agents in low-grade lymphomas.
The phase II VERTICAL study [19] reported that seventy-three patients received five 35-day cycles of bortezomib, bendamustine, and rituximab: Bortezomib was given at a dose of 1.6 mg/m2 on days 1, 8, 15, and 22, bendamustine 50, 70, or 90 mg/m2 on days 1 and 2, and rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle one and day one of subsequent cycles, up to five cycles. The ORR was 88 % (including 53 % complete response). Median duration of response was 11.7 months (95 % CI 9.2–13.3). Median progression-free survival was 14.9 months (95 % CI 11.1–23.7). Toxicities were manageable; myelosuppression was the main toxicity (25 and 14 % of patients experienced grade 3–4 neutropenia and grade 3–4 thrombocytopenia, respectively). Transient grade 3–4 neuropathy occurred in 11 % of patients.
Jonathan et al. reported [20] the efficacy and toxicity of bendamustine, rituximab, and bortezomib in patients with relapsed indolent and mantle cell non-Hodgkin’s lymphoma, bendamustine was given 90 mg/m2 on days 1 and 4; rituximab 375 mg/m2 on day 1, and bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11. Twenty-eight-day cycles were planned. Of 29 patients evaluable for efficacy, 24 (83 %) achieved an objective response (with CR rate 52 %). With median follow-up of 24 months, 2-year progression-free survival is 47 % (95 % CI 25–69 %). Common non-hematologic adverse events were generally grade 1 or grade 2 and included nausea (50 %), neuropathy (47 %), fatigue (47 %), constipation (40 %), and fever (40 %).
Our results showed that RB is effective in patients with relapsed/refractory indolent B cell lymphoma. In the entire population, ORR was 70.0 %, with a CR/CRu rate of 31.7 %. 2-year OS was 75.0 %, and 2-year PFS was 41.0 %. The results from this study showed that addition of weekly bortezomib to rituximab therapy had the similar results as bortezomib, bendamustine, and rituximab regimen, and the toxic effects were tolerable.
In this study, high or high-intermediate risk of IPI and refractory disease were independently associated with worse survival and PFS. Secondary IPI score at the start of R-B therefore might be an effective way to predict patient’s outcome as primary IPI at the first diagnosis. Moreover, the outcome of patients with refractory disease was significantly worse as compared to that of relapsed patients, as most published studies have found.
Besides, RB regimen was quite tolerable. Grade 3 or 4 neutropenia, anemia, and thrombocytopenia were observed in six (10 %), four (6.7 %), and two patients (3.3 %), respectively, which was much lower than the incidence of the other bortezomib-based second-line regimen, such as phase II VERTICAL study [19]. The duration of myelosuppression is short. Only one patient developed febrile neutropenia during chemotherapy. Peripheral neuropathy events were reported in ten patients(16.7 %), including two patients (3.3 %) with events of grade 3 or higher, which was also lower than other reports [19, 20]. All neuropathies were transient. None of these adverse events were associated with serious conditions or subsequent treatment-related death. According to the non-hematologic toxicity, such as nausea, diarrhea, and constipation, it was mild and controllable.
In conclusion, our study showed the RB regimen was highly effective in patients with relapsed or refractory indolent lymphomas. The toxicity of such therapy was relatively low. However, the treatment for patients with high IPI score or primary refractory disease is still insufficient. For these patients, novel therapeutic approaches are therefore warranted.
References
Coiffier B, Thieblemont C, Felman P, Salles G, Berger F. Indolent non-follicular lymphomas: characteristics, treatment and outcome. Semin Hematol. 1999;336:198–208.
Johnson PW, Rohatiner AZ, Whelan JS, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20-year study from a single center. J Clin Oncol. 1995;13:140–7.
Kunami N, Katsuya H, Nogami R, et al. Promise of combining a Bcl-2 family inhibitor with bortezomib or SAHA for adult T-cell leukemia/lymphoma. Anticancer Res. 2014;34(10):5287–94.
Di Bella N, Taetle R, Kolibaba K, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–80.
Holkova B, Kmieciak M, Perkins EB, et al. Phase I trial of bortezomib (PS-341; NSC 681239) and “nonhybrid” (Bolus) infusion schedule of alvocidib (Flavopiridol; NSC 649890) in patients with recurrent or refractory indolent B-cell neoplasms. Clin Cancer Res. 2014;20(22):5652–62.
Ribrag V, Tilly H, Casasnovas O, et al. Efficacy and toxicity of two schedules of bortezomib in patients with recurrent or refractory follicular lymphoma: a randomised phase II trial from the Groupe d'Etude des Lymphomes de l’Adulte (GELA). Eur J Cancer. 2013;49(4):904–10.
Knapp CM, Whitehead KA, et al. In pursuit of a moving target: nanotherapeutics for the treatment of non-Hodgkin B-cell lymphoma. Expert Opin Drug Deliv. 2014;95(4):1–15.
Ichikawa K, Noguchi M, Koike M, et al. Rituximab plus a CHOP-like regimen, central nervous system prophylaxis, and contralateral testicular irradiation for localized primary testicular diffuse large B-cell lymphoma lead to prolonged progression-free survival. Int J Hematol. 2014;100(4):370–8.
Czuczman MS, Olejniczak S, Gowda A, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–70.
Furtado M, Johnson R, Kruger A. Addition of bortezomib to standard dose chop chemotherapy improves response and survival in relapsed mantle cell lymphoma. Br J Haematol 2014 Aug 22.
Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–12.
Bertrand Coiffi er, Evgenii A Osmanov, Xiaonan Hong, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol 2011;12: 773–84.
Bose P, Batalo MS, Holkova B, et al. Bortezomib for the treatment of non- Hodgkin’s lymphoma. Expert Opin Pharmacother. 2014;15(16):2443–59.
Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having non-Hodgkin lymphoma from the 1990s to the early 21st century. Arch Intern Med. 2008;168:469–76.
Goda AE, Erikson RL, Sakai T, et al. Preclinical evaluation of bortezomib/dipyridamole novel combination as a potential therapeutic modality for hematologic malignancies. Mol Oncol 2014 Sep 6. pii: S1574-7891(14)00205-1.
Craig M, Hanna WT, Cabanillas F, et al. Phase II study of bortezomib in combination with rituximab, cyclophosphamide and prednisone with or without doxorubicin followed by rituximab maintenance in patients with relapsed or refractory follicular lymphoma. Br J Haematol. 2014;166(6):920–8.
Nicholas DB, Raymond T, Kathryn K, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115:475–80.
Fowler N, Kahl BS, Lee P, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: the phase II VERTICAL study. J Clin Oncol. 2011;29(25):3389–95.
Jonathan WF, Julie MV, Jennifer LK, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood. 2011;117:2807–12.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yun, H., Zhang, H.l. & Wang, Hq. Rituximab and bortezomib (RB): a new effective regimen for refractory or relapsed indolent lymphomas. Med Oncol 32, 353 (2015). https://doi.org/10.1007/s12032-014-0353-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0353-5