Abstract
Purpose of Review
The purpose of this review is to determine the role of pulmonary vein (PV) triggers in different types of atrial fibrillation (AF) as well as to determine based on prospective randomized data which other approaches may increase the success rate of radiofrequency ablation of persistent AF.
Recent Findings
Special attention must be paid to detect, diagnose, and optimize management of reversible or treatable causes of persistent AF such as obesity, obstructive sleep apnea, hypertension, hypo- or hyperthyroidism, inflammatory and infectious diseases, and stress. Though the role of PVs is more pronounced in paroxysmal AF than in persistent AF, performing an adequate PV isolation is still a key part in treating persistent AF. There are now numerous techniques to obtain long-lasting pulmonary vein isolation and avoid esophageal damage. Patients with persistent AF will frequently require a more aggressive mapping and ablative approach. Ablation of sites associated with non-PV triggers such as the entire posterior wall, the roof, the anterior part of the left atrial (LA) septum, the left atrial appendage (LAA), the coronary sinus, and the superior vena cava has been shown to improve the freedom from AF at follow-up when combined with PV isolation. We do not encourage the use of empiric lines or complex fractionated atrial electrograms. Several studies have shown the role of empirical LAA electrical isolation in persistent AF.
Summary
When focal ectopic atrial activity is observed after PV isolation, its activation sequence is compared to that of sinus rhythm, allowing quick identification of its origin. For significant non-PV triggers (repetitive isolated beats, focal atrial tachycardias or beats triggering AF/atrial flutter), a more detailed activation mapping is performed in the area of origin. They are subsequently targeted with focal ablation, except for triggers originating from the superior vena cava (SVC), LAA, or coronary sinus, for which complete isolation of these structures is the ablation strategy of choice. We truly believe the LAA and the posterior LA wall deserve special consideration when managing patients with persistent AF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2010, the prevalence of atrial fibrillation (AF) in the USA ranged from 2.7 millions to 6.1 millions, with evidence suggesting an increasing incidence and prevalence worldwide, making AF the most common arrhythmia in the elderly population (more than 65 years) [1, 2]. Several arrythmogenic mechanisms have been postulated; hence, countless treatment strategies have been proposed that include antiarrythmic medications, numerous ablation strategies, and surgical procedures. In this review, we will discuss available literature describing different approaches to treat persistent AF with catheter ablation (CA), ranging from techniques to achieve long-lasting pulmonary vein isolation (PVI), complex fractionated atrial electrograms (CFAEs), focal impulses and rotor modulation (FIRM), posterior wall isolation, left atrial appendage (LAA) isolation, ablation of non-pulmonary vein (PV) triggers, and the role of autonomic modulation.
AF Pathogenesis
It was thought initially that AF was due to a simple disorganized activation focused within the atrium; continued studies have led to two primary theories regarding the mechanisms of AF. The multiple-wavelet hypothesis, described by Moe et al. [3], suggests that AF is due to constant formation of new wavelets occurring through the process of wave splitting, resulting in multiple-wavelet fibrillation. The mother rotor hypothesis, advanced by Jalife et al., suggests that AF is maintained by a single stable rapid reentrant circuit of excitation that is unable to maintain 1:1 conduction throughout the myocardium. These mechanisms appear not to be mutually exclusive in the human heart [3, 4]. Of note, Allessie and de Groot [6] demonstrated with the first quantitative analysis of intra-atrial conduction disturbances during AF that the main feature of the long-standing AF substrate was a significant increase in longitudinal dissociation consisting of lines of block running parallel to the atrium rather than a rotor or foci conduction disturbance itself. De Groot subsequently with the use of high-resolution intraoperative mapping introduced the “double-layer” hypothesis, a direct proof of endo-epicardial asynchrony of the atrium wall during AF in humans [5]. This asynchronous dual layer promotes multiple fibrillation waves that stabilize the fibrillation process through a constant feeding of focal-breakthrough waves [6, 5]. Electromechanical remodeling serves as a critical component in the progression and worsening of AF over time. Multiple mechanisms have been shown to contribute to the latter process, from shortening of atrial refractoriness (electrical remodeling) to chronic atrial stretch that induces histologic changes leading to tissue fibrosis, resulting in the peculiar non-uniform anisotropy and local conduction heterogeneities that facilitate reentry of breakthrough waves in the dilated LA.
Other Triggers of AF
Special attention must be paid to detect, diagnose, and optimize management of treatable or reversible causes of AF that include lifestyle habits (e.g., alcohol abuse, caffeine intake, energy drinks), obesity, obstructive sleep apnea (OSA), hypertension, hypo- or hyperthyroidism, stress, and inflammatory and infectious diseases that may trigger or maintain this arrhythmia. It has been demonstrated that risk factors such as OSA and metabolic syndrome have a cumulative effect in initiation and recurrence of AF after ablation [7].
PVI
Haïssaguerre and colleagues demonstrated that the PVs play a crucial role in the initiation of AF, and special attention should be paid to obtain long-lasting entrance-and-exit block to completely isolate these structures from the LA [8]. Results from a recently published study by Santangeli et al. suggest that the PVs are the main AF trigger sites in patients with persistent and long-standing persistent AF (LSPAF) [9], supporting the key role of an adequate PVI in persistent AF. Prevention of any tachyarrhythmia after PVI is of great concern for electrophysiologists. Ouyang et al. showed that approximately 80% of recurrent atrial tachycardias had PV reconnection after the procedure [10]. It has been established by several studies that wide antral PVI is more efficacious than ostial PVI in achieving freedom from any atrial tachyarrhythmia recurrence at long-term follow-up (Fig. 1) [11]. In addition, antral PVI is associated with minimal risk of PV stenosis and lower risk of phrenic nerve damage.
Electroanatomic mapping of the left atrium (right lateral view) showing wide antral circumferential ablation to try to isolate the right pulmonary veins (red and blue dots). Using this type of approach, there is a lower risk of damaging the right phrenic nerves (yellow dots), which might run more anteriorly in some patients. Likewise, using this approach, the risk of pulmonary vein stenosis is almost negligible
Adequate catheter-tissue contact force (CF) is critical to deliver transmural lesions and to prevent conduction recovery. Several indirect parameters have been used as surrogates: tactile feedback, local electrogram amplitude and morphology changes, imaging of the catheter tip stability with fluoroscopy or intracardiac echocardiography, and catheter tip impedance monitoring. Presently, with CF-sensing catheters, the quality of ablation lesions has dramatically improved. The prospective multicenter SMART-AF trial, which evaluated the safety and effectiveness of an irrigated CF-sensing catheter in patients with paroxysmal AF, revealed in multivariate analyses that when the CF employed was between investigator-selected working ranges more than 80% of the time during CA, outcomes were 4.25 times more likely to be successful (p = 0.0054) [12]. The contribution of real-time CF sensing to the CA outcomes was demonstrated by a significantly higher success rate (81 vs. 66%) in patients where the investigators stayed within the selected CF range, suggesting that consistent and stable catheter-tissue contact is necessary for effective transmural lesions [12]. This study used the Thermocool SmartTouch® catheter with an average contact force per procedure of 18 ± 9 g [13, 14]. Similar results were obtained using the TactiCath CF ablation catheter in the multicenter randomized trial for AF (TOCCASTAR) [15].
Assessing Effectiveness: Unexcitability, Voltage Map, and Impedance Drop
Evidence supports that PVI is one of the standard treatments for paroxysmal AF. Nevertheless, arrhythmia recurrence is high when conduction gaps are present. Recently, acute endpoints in addition to bidirectional block on each PV have been proposed. A prospective two-center randomized trial showed that bipolar pacing (10 mA and 2 ms) to ensure unexcitability along the PVI line improved long-term single-procedure success when compared with demonstration of bidirectional block alone (82.7 vs. 52%, p = 0.001). Moreover, the use of bipolar voltage mapping at the edge of the acute or chronic PVI ablation site can identify an atrial unexcitable dense scar, which might be a rigorous tool for validating PVI (Fig. 2) [16]. Cutoff values to detect the scar in the acute setting are 0.45 mV for the LAA/left PV ridge and 0.2 mV for other locations around the PVs, whereas for redo procedures, chronic scar cutoff values are 0.2 mV for the LAA/left PV ridge and 0.15 mV for other locations [17]. It is well established that tissue heating during CA application results in an impedance decline at the catheter tip. As reported by Reichlin et al. in which a greater CF was associated with an initial impedance drop, monitoring of the latter is an indicator of catheter contact and may help to improve formation of durable ablation lesions [18]. Subsequently, the same group demonstrated that PVI guided by an initial impedance decrease was achievable and resulted in PVI concurrent with or before completion of the ablation ring in 94% of patients. Single procedure efficacy after 1 year of follow-up was 84% [19].
Unmasking Dormant Conduction: Adenosine Administration
The phenomenon associated with the use of adenosine for unmasking dormant conduction is believed to be mediated by mechanisms such as shortening of the action potential, changes in refractoriness, and hyperpolarization of dormant PV myocytes. Cellularly, these effects are caused by activation of the inward rectifying K+ current [20], as well as trigger PV ectopy [21]. Identification and targeting of dormant PV conduction is a safe and highly controversial strategy since opposite results with regard to long-term outcomes have been reported in literature. In the international multicenter, randomized ADVICE trial, which tested adenosine-guided PVI for the treatment of paroxysmal AF, adenosine administration unmasked dormant PV conduction in 53% of patients. Sixty-nine percent of patients with additional adenosine-guided ablation were free from symptomatic atrial tachyarrhythmia compared with 42% of patients with no further ablation, corresponding to an absolute risk reduction of 27% (p < 0·0001) and a relative risk reduction of 56% (p < 0·0001) [22]. However, a second randomized controlled trial, the UNDER-ATP, found no significant reduction in the 1-year incidence of recurrent atrial tachyarrhythmias by adenosine-guided PVI compared with conventional PVI [23].
Limiting Factors for Ablation
It is important to recognize the proximity of the LA to the esophagus and to understand the significance of the large contact area between these two structures, which always presents a challenge for the electrophysiologist. Potential complications such as atrial-esophageal esophageal fistulas are a concern and sometimes a limiting factor when attempting PVI [24,25,26]. The mean vertical contact length is on average 4.4 cm, and the mean distance between the anterior wall of the esophagus and the endocardium is only approximately 2.6 mm. Morphological changes of the periesophageal connective tissue and the posterior wall of the LA are diagnosed in almost one third of patients by endosonography [27]. Hence, a reduction in maximum CA power and duration on the entire posterior LA has been pursued in an attempt to avoid these complications [28]. Buch et al. were pioneers in protecting the esophagus by inflating a balloon catheter (18-mm by 4-cm balloon dilation catheter, Meditech, Boston Scientific) in the pericardial space. This technique was initially described as epicardial ablation of ventricular tachycardia [29]. More recently, the same technique has been applied to protect the esophagus and right phrenic nerve during AF ablation in a porcine model and in humans [30, 31]. The distance between the esophagus and posterior LA after balloon inflation increased by 12.3 ± 4.0 mm, considerably attenuating the luminal esophageal temperature increase during endocardial radiofrequency ablation (RFA) (6.1 vs. 1.2 °C; p < 0001) [30]. Similarly, after displacement of the right phrenic nerve with the intrapericardial balloon, nerve capture was abolished in 91% of sites previously stimulated by pacing [30].
PV and Its Role in Persistent AF
Despite the data supporting the well-established role of PVI in patients with persistent AF, single-procedure success rate remains low at 1 year in this patient population and even lower in those with long-standing persistent AF (LSPAF more than 1 year), 39 and 20%, respectively [32]. This success rate has not been shown to improve over time, according to a study performed by Tilz et al., which discussed the 5-year outcomes of a sequential ablation strategy in patients with LSPAF. Single and multiple ablation procedure successes were 20% and 45%, respectively. Interestingly, 50% of the patients received PVI alone [33, 34]. The role of PV in persistent AF appears to be passive, since either they have been found to be silent or their cycle length (CL) has been greater than the LAA CL. Some investigators have shown good AF success rates just by performing substrate ablation without touching these veins [35]. Haïssaguerre et al. recently evaluated the CL gradient between PVs and the LA in an attempt to identify the subset of patients where PVs still play an important role in maintenance and perpetuation of this arrhythmia. The PV-to-LA CL gradient was quantified by the ratio of fastest PV to LAA AF CL over 1 min. Patients with persistent AF in whom a gradient of PV to LA less than 69% was found, were more prone to terminate after PVI or limited substrate ablation. After a follow-up of 29 months, freedom from any arrhythmia recurrence was achieved in most patients with the fastest PV/LAA ratio less than 69% as opposed to the remaining population (80 vs. 43%; P < 0.001) [36]. There is frequently a trend to ablate until AF termination is achieved at the expense of facing a higher risk of complications. Albeit Haïssaguerre et al. showed that AF termination during the procedure is a strong predictor of long-term success and there was no difference between groups in terms of arrhythmia recurrence probably because patients in whom AF was terminated acutely required more extensive ablation and had a higher incidence of atrial tachycardia at follow-up [37]. Seeking AF termination might indeed increase procedural and fluoroscopy time as well as complications. These findings were corroborated by two studies a year later [37, 38].
Several studies reporting results on CA of persistent AF have been published to date. Most of these studies are observational with heterogeneous variables, methodologies, and end points. As a consequence, the optimal ablation approach for persistent AF is still to be determined [39].
Subtypes of Linear Ablation
The prevention of macro-reentrant circuit formation has long been performed in persistent AF; compartmentalizing the atria through ablation lines was not long ago a strategy to create electrical block. These lines included roof line, mitral isthmus, and anterior line. A meta-analysis comparing the main six case series using PV antral isolation in conjunction with linear substrate modification reported a wide range of success from 11 to 74% [39]. The highly variable results might reflect the significant difference in methodology and endpoints of each study as well as operator experience and the fashion with which these lines are actually performed. Whether defragmentation of these additional targets after PVI with linear lesions is superior to the actual ablation of non-PV triggers is also open for debate. Some studies have demonstrated that substrate modification with purely anatomical lesions without any specific attempt to ablate trigger sites of AF has been associated with a higher recurrence rate [40, 41].
CFAEs
It has been proven that AF is a reflection of various specific patterns of conduction. In the 1990s, the CFAE concept emerged, suggesting that reentry sites of electrical waves help perpetuate this arrhythmia. Typically, they arise from the sites of electromechanical boundaries with low conduction, and have distinctive properties including a CL length less than 120 ms, low voltage, and heterogeneous distribution [42, 43]. Subsequently, Nademanee et al. in 2004 defined CFAEs as fractionated electrograms composed of two or more deflections, and/or perturbation of the baseline with continuous deflection of a prolonged activation complex over a 10-s recording period, with a CL (less than 120 ms) over a 10-s recording period. Therefore, some investigators suggested an incremental benefit to target CFAEs when added to PVI alone [44].
Due to several studies on CFAEs reporting contradictory results, a meta-analysis conformed of eight controlled studies compared the effect of CFAEs ablation with PVI vs. PVI alone in patients with paroxysmal and non-paroxysmal AF. This study found slight benefit with the addition on CFAEs ablation to PVI with a relative risk (RR) of 1.15 (CI 1.2–1.31, p = 0.03) [45].
Although the STAR AF trial was the first randomized control study showing that CFAEs ablation guided by automated mapping software could have an additive benefit over PVI alone, but does not suffice as an ablation strategy itself [46•], more recently, the results from the STAR AF II trial have been published [47]. In this large multicenter randomized trial, participants with persistent AF received PVI vs. PVI plus CFAEs ablation vs. PVI plus empiric linear ablation. PVI was performed by PV antral isolation, while PVI plus CFAEs was performed by PVI followed by mapping and ablation of CFAEs during AF, identified by 3D mapping software, and PVI plus lines were performed by PVI followed by a LA roof line and a line along the mitral valve isthmus. Surprisingly, after following these patients for 18 months, freedom from AF occurred in 59% of the PVI group, 48% of the PVI plus CFAEs group, and 44% of the PVI plus lines group with no statistical difference among groups (p = 0.15). Freedom from atrial fibrillation after two procedures occurred in 72% of the PVI group, 60% of the PVI plus CFAEs group, and 58% of the PVI plus lines group (p = 0.18 among groups) [47].
Non-pulmonary Triggers for AF
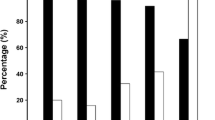
Enough evidence to support the key role of PV as triggers of paroxysmal AF has been published, but this is not the case for persistent AF or LSPAF, where other foci have been described. We recently published data from the AATAC multicenter randomized trial, in which patients with persistent AF and left ventricular ejection fraction less than 40% were randomly assigned to undergo CA for AF or receive amiodarone [48••]. After a 24-month follow-up, 70% of patients in the RFA group were recurrence free after an average of 1.4 procedures, in comparison with 34% in the amiodarone group (p < 0.001). More importantly, higher success was reported in patients undergoing PVI and posterior wall isolation in comparison with PVI alone (79 and 36%, respectively, p < 0.001) (Fig. 3). There was no significant difference between PVI alone and amiodarone [48••]. In general, ablation of AF triggers appears to be associated with a higher success rate [49]. PV trigger-directed ablation should be preferred to a blind substrate modification based on several studies [50,51,52]. Ablation of non-PV triggers has been shown to improve the freedom from AF when combined with PVIs [53,54,55,56,57,58]. The most commonly foci are mapped to the CS (73.8%), interatrial septum (IAS) (50.0%), LAA (38.1%), (SVC) (28.6%), and mitral valve annulus (4.8%) [9]. With a specific catheter setup, when a single ectopic beat is observed, its activation sequence is compared to that of the sinus rhythm, allowing to quickly identify its area of origin: (a) right atrium (RA), (b) IAS area, (c) CS, and (d) LAA. For significant non-PV triggers (repetitive isolated beats, focal atrial tachycardia, or beats triggering AF/atrial flutter), a more detailed activation mapping is performed. They are subsequently targeted with focal ablation, except for triggers originating from the SVC, LAA, or CS, for which complete isolation of these structures is the ablation strategy of choice (Fig. 3).
Left Atrial Appendage Isolation
Anatomically, the LAA has a trabecular tubular shape with variable morphologies and derives from the embryonic left atrium as an outgrowth of the PVs [59]. Special consideration needs to be addressed to this structure, particularly in managing persistent AF and LSPAF [60]. At least 30% of patients with persistent AF have triggers in this area. Our group demonstrated that if complete isolation of the LAA is achieved, only 15% of patients will have recurrences compared to 74% without ablation [61]. Lakkireddy et al. prospectively studied 40 patients who underwent AF ablation with LARIAT vs. AF ablation alone; results after 1-year follow-up were promising, given that less recurrence was observed in the LARIAT group vs. the control (34 vs. 55%, p = 0.025). Similarly, repeated ablation was seen in fewer patients of the LARIAT group (15 vs. 35%, p = 0.04) [62]. Evidence that supports the incremental benefit of the LARIAT procedure as an adjunctive therapy is currently under investigation in the aMAZE trial (LAA Ligation Adjunctive to PVI for Persistent or LSPAF NCT02513797). LAA electric isolation is reported to improve persistent AF according to an investigation by Panikker et al. They concluded that AF ablation, mechanical occlusion, and LAA electric isolation can be performed simultaneously with freedom from AF higher in the intervention group vs. ablation alone (95 vs. 63%, p = 0.036) [63]. We sought to assess the effectiveness of empirical use of LAA electrical isolation in LSPAF patients in the BELIEF study (NCT01362738). Patients were randomly assigned to receive LAA isolation plus ablation vs. the standard approach. At the 12-month follow-up, 25 (28%) in the control group and 48 (56%) in the group where LAA was isolated were recurrence free after a single procedure (p = 0.001). During repeat procedures, empirical electrical LAA isolation was performed in all patients. After an average of 1.3 procedures, cumulative success at the 24-month follow-up was reported in 49 (56%) in the control group and in 65 (76%) in the LAA isolation group (p = 0.003). In conclusion, after single and redo procedures, empirical electrical isolation of the LAA improved long-term freedom from atrial arrhythmias without increasing complications (Fig. 4) [64••].
Left lateral (left panel) and anterior (right panel) views of a three-dimensional electroanatomic map of the left atrium. The patient, who has long-standing persistent atrial fibrillation, underwent extensive ablation with pulmonary vein (PV) antrum and posterior wall isolation in a prior procedure. He had recurrences, and non-PV triggers were found in the CS and LAA. Lasso and ablation catheter in the LAA. Intracardiac electrograms depicting significant atrial activation delay into the LAA. The first beat shows the near and far fields. The second beat illustrates the moment when the LAA is isolated (loss of near-field electrogram)
FIRM
The role of cardiac electric rotors as the main drivers in AF shows conflicting evidence in animal and human models.
The CONFIRM trial by Narayan et al. was the first to hypothesize that AF is regulated by electrical rotor and focal impulses. FIRM were detected in 97% of a 107 cohort of patients, who underwent ablation. AF termination was achieved in 86% of FIRM-guided cases vs. 20% of conventional ablation (p < 0.001). During a median follow-up of 273 days after a single procedure, FIRM-guided cases had higher freedom from AF (82.4 vs. 44.9%, p < 0.001) [65, 66]. An extended analysis of the same group reported an 80.3% AF freedom if those sources were ablated, compared with 18.2% if sources were missed (p < 0.001) [67]. This benefit persisted over time, as reported by an extended analysis of the same cohort of patients. Those receiving rotor modulation maintained higher freedom from AF than those in the conventional group (77.8 vs. 38.5%, p = 0.001) [68].
Similar results were reproduced among 78 patients in a multicenter study in 2014. The elimination of patient-specific AF rotors was associated with an increased freedom from AF at approximately 1-year follow-up [69]. Nevertheless, results published by Buch et al. differed from prior studies, in which FIRM-identified rotor sites did not exhibit specific atrial characteristics and RFA at these sites, in conjunction with PVI, resulted in AF termination in only 17% of the patients [70, 71].
Autonomic Modulation
The LA ganglionated plexus (GP) has been implicated in the pathogenesis of AF. However, ablation of this anatomic structure has provided mixed results. In patients in sinus rhythm, synchronized high-frequency stimulation can be used to identify GP sites producing PV ectopy. Ablation at these sites abolishes the PV ectopic response to stimulation [72]. Katritsis et al. conducted a randomized clinical trial evaluating autonomic denervation added to PVI in patients with paroxysmal AF. The investigators demonstrated the addition of ablation on the main GP to PVI confers a significantly higher success rate compared with either PVI or GP alone at 2-year follow-up using implantable loop recorders [73]. Nevertheless, this approach has not been widely implemented clinically as a result of studies revealing ablation of these GP might have paradoxical pro-arrhythmic effects increasing the AF burden and AF inducibility [74, 75].
Epicardial CA for Persistent AF
It has been proposed that electrical dissociation between the epicardium and endocardium can produce electrical wave points affecting the atrium. Recent mapping studies in patients with persistent AF are consistent with this finding. The presence of endomysial fibrosis within the epicardial layer (result of AF itself) can disrupt electrical connections between muscle bundles increasing this dissociation even more [76]. The atrial epicardium has unique characteristics, a shorter refractory period, and more frequent and repetitive response induction by programmed stimulation [77]. High-density epicardial mapping of the posterior LA wall (PLAW), LAA, and right superior PV-LA junction in 18 patients with persistent AF undergoing open-heart surgery showed this arrhythmia had heterogeneous patterns of atrial activation involving primarily multiple, unstable, and disorganized activity [78]. Parallel, an approach that combined surgical epicardial/endocardial ablation, is associated with an increased complication rate and has a lack of outcome improvement when compared to extensive endocardial ablation only in patients with LSPAF [79]. More data are still needed in this field given the controversy of available literature.
Systematic Approach for Substrate Ablation
High success rates in LSPAF ablation require a meticulous PVI, with particular attention to LA triggers (e.g., CS and LAA) and discretionary right atrial ablation for right atrial triggers (e.g., SVC). With this approach, a single procedure success rate has been achieved, ranging from 38 to 62% at the 18-months follow-up. Added benefit is achieved when repeat procedures are performed (70 to 88%) [37, 80,81,82,83]. Empirical isolation of the posterior LA wall between the right and left PVs is performed in all patients undergoing ablation for persistent or LSPAF (Fig. 3). From an embryologic, anatomic, and electrophysiological standpoint, it should be considered an extension of the PVs and its isolation has been proven to improve outcomes in paroxysmal and non-paroxysmal AF patients [84]. After completing PVI, LA posterior wall isolation can be achieved either by linear ablation (box lesion) or electrogram-based ablation (Fig. 3). With linear ablation, a roof and floor line are created to connect the superior and inferior PVs, respectively. It is not easy to create a single continuous transmural lesion, and even a single gap in the ablation line results in reconnection of the whole posterior wall. To minimize this, it is important to create multiple lines, with lesions overlapping each other, especially in thicker areas where RF can be safely applied. In electrogram-based ablation, all potentials identified moving the multielectrode catheter along the posterior wall are sequentially targeted. Reconnection of the whole posterior wall is unlikely and is usually limited to areas close to the esophagus, where RF energy delivery is limited. The endpoint is to achieve electrical isolation, as documented by the absence of any electrical activity in the posterior wall and unexcitability with high output pacing. Following PVI and posterior wall isolation, entrance and exit blocks are verified in all four PVs as well as the posterior wall, with isoproterenol (pure β-agonist, with inotropic, dromotropic, and chronotropic effects (β1) as well as peripheral vasodilatory effects (β2) at 20–30 μg/min for 10–15 min to reveal reconnection of the PV and, most importantly, induce latent non-PV triggers. Therefore, given its pharmacodynamics, a drop in blood pressure is anticipated and a vasopressor, such as phenylephrine (pure α1), is given and titrated to maintain the systolic blood pressure at ~120 mmHg. Finally, mapping non-PV triggers is guided by multiple catheters positioned along both the right and left atria: a multielectrode mapping catheter in the left superior PV recording the far-field LAA activity, an ablation catheter in the right superior PV that records the far-field intra-atrial septum, and a 20-pole catheter with electrodes spanning from the SVC to the CS. With this catheter setup, the activation sequence of even a single ectopic beat is compared to that of the sinus rhythm, allowing their identification to their area of origin [85].
Conclusion: Future Perspectives
The results from the EMBRACE and CRYSTAL AF investigation have recently been an eye opener for the medical community since they clearly proved that AF is a much bigger problem than we already thought [86, 87]. Greater efforts are to be made in order to better understand the mechanism causing AF and to potentially have a much more potent and curative armamentarium for these patients. Pre-procedural cardiac MRI (CMRI) might be helpful in evaluating the degree of atrial scar tissues given the fact that it has shown to have strong prognostic implications in terms of the AF success rate. Results from the elegantly conducted DECAAF study suggest that patients with more than 30% of atrial tissue fibrosis detected by delayed enhancement CMRI in the LA will have approximately 70% AF recurrences by 16 months after ablation [88]. Although it has been shown that CA in persistent AF achieves significantly greater freedom from recurrent AF than medical therapy in several studies as demonstrated by a recent meta-analysis, we frequently encounter these patients only years after they have developed the arrhythmia when there are fewer chances of obtaining a satisfactory result due in part to advance electrical and mechanical remodeling. [89]. At this moment in time, there are data supporting posterior wall isolation plus non-PV trigger CA in addition to PVI could increase the success rate in patients with persistent and LSPAF.
AF, atrial fibrillation; CA, catheter ablation; CFAEs, complex fractionated atrial electrograms; CF, contact force; CL, cycle length; CS, coronary sinus; FIRM, focal impulse rotor modulation; LA, left atrium; LAA, left atrial appendage; OSA, obstructive sleep apnea; PVI, pulmonary vein isolation; PV, pulmonary veins; RA, right atrium; SVC, superior vena cava.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5.
Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25.
Moe GK. Evidence for reentry as a mechanism of cardiac arrhythmias. Rev Physiol Biochem Pharmacol. 1975;72:55–81.
Lee S, Sahadevan J, Khrestian CM, Durand DM, Waldo AL. High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: restudying the Moe hypothesis. J Cardiovasc Electrophysiol. 2013;24:328–35.
de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops P, van de Woestijne P, Bekkers J, Kik C, Bogers A, Allessie M. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circulation Arrhythmia and electrophysiology. 2016;9
Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circulation Arrhythmia and electrophysiology. 2010;3:606–15.
Mohanty S, Mohanty P, Di Biase L, Bai R, Trivedi C, Santangeli P, Santoro F, Hongo R, Hao S, Beheiry S, Burkhardt D, Gallinghouse JG, Horton R, Sanchez JE, Bailey S, Hranitzky PM, Zagrodzky J, Natale A. Longterm outcome of catheter ablation in atrial fibrillation patients with coexistent metabolic syndrome and obstructive sleep apnea: impact of repeat procedures versus lifestyle changes. J Cardiovasc Electrophysiol. 2014;25:930–8.
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, Supple GE, Garcia FC, Dixit S, Callans DJ, Marchlinski FE. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016;13:374–82.
Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double lasso technique. Circulation. 2005;111:127–35.
Proietti R, Santangeli P, Di Biase L, Joza J, Bernier ML, Wang Y, Sagone A, Viecca M, Essebag V, Natale A. Comparative effectiveness of wide antral versus ostial pulmonary vein isolation: a systematic review and meta-analysis. Circulation Arrhythmia and electrophysiology. 2014;7:39–45.
Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, Kantipudi C, Mansour MC, Melby DP, Packer DL, Nakagawa H, Zhang B, Stagg RB, Boo LM, Marchlinski FE. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. 2014;64:647–56.
Nakagawa H, Kautzner J, Natale A, Peichl P, Cihak R, Wichterle D, Ikeda A, Santangeli P, Di Biase L, Jackman WM. Locations of high contact force during left atrial mapping in atrial fibrillation patients: electrogram amplitude and impedance are poor predictors of electrode-tissue contact force for ablation of atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2013;6:746–53.
Perna F, Heist EK, Danik SB, Barrett CD, Ruskin JN, Mansour M. Assessment of catheter tip contact force resulting in cardiac perforation in swine atria using force sensing technology. Circulation Arrhythmia and electrophysiology. 2011;4:218–24.
Reddy VY, Dukkipati SR, Neuzil P, Natale A, Albenque JP, Kautzner J, Shah D, Michaud G, Wharton M, Harari D, Mahapatra S, Lambert H, Mansour M. Randomized, controlled trial of the safety and effectiveness of a contact force-sensing irrigated catheter for ablation of paroxysmal atrial fibrillation: results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) study. Circulation. 2015;132:907–15.
Steven D, Sultan A, Reddy V, Luker J, Altenburg M, Hoffmann B, Rostock T, Servatius H, Stevenson WG, Willems S, Michaud GF. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J Am Coll Cardiol. 2013;62:44–50.
Kapa S, Desjardins B, Callans DJ, Marchlinski FE, Dixit S. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:1044–52.
Reichlin T, Knecht S, Lane C, Kühne M, Nof E, Chopra N, Tadros TM, Reddy VY, Schaer B, John RM, Osswald S, Stevenson WG, Sticherling C, Michaud GF. Initial impedance decrease as an indicator of good catheter contact: insights from radiofrequency ablation with force sensing catheters. Heart Rhythm. 2014;11:194–201.
Reichlin T, Lane C, Nagashima K, Nof E, Chopra N, Ng J, Barbhaiya C, Tadros T, John RM, Stevenson WG, Michaud GF. Feasibility, efficacy, and safety of radiofrequency ablation of atrial fibrillation guided by monitoring of the initial impedance decrease as a surrogate of catheter contact. J Cardiovasc Electrophysiol. 2015;26:390–6.
Cheung JW, Ip JE, Chung JH, Markowitz SM, Liu CF, Thomas G, Lee JM, Lessner SJ, Lerman BB. Differential effects of adenosine on pulmonary vein ectopy after pulmonary vein isolation: implications for arrhythmogenesis. Circulation Arrhythmia and electrophysiology. 2012;5:659–66.
Cheung JW, Chung JH, Ip JE, Markowitz SM, Liu CF, Thomas G, Lerman BB. Time course of adenosine-induced pulmonary vein reconnection after isolation: implications for mechanism of dormant conduction. Pacing and clinical electrophysiology : PACE. 2012;35:556–63.
Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, Arentz T, Deisenhofer I, Veenhuyzen G, Scavee C, Jais P, Puererfellner H, Levesque S, Andrade JG, Rivard L, Guerra PG, Dubuc M, Thibault B, Talajic M, Roy D, Nattel S, Advice trial investigators. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. 2015;386:672–9.
Kobori A, Shizuta S, Inoue K, Kaitani K, Morimoto T, Nakazawa Y, Ozawa T, Kurotobi T, Morishima I, Miura F, Watanabe T, Masuda M, Naito M, Fujimoto H, Nishida T, Furukawa Y, Shirayama T, Tanaka M, Okajima K, Yao T, Egami Y, Satomi K, Noda T, Miyamoto K, Haruna T, Kawaji T, Yoshizawa T, Toyota T, Yahata M, Nakai K, Sugiyama H, Higashi Y, Ito M, Horie M, Kusano KF, Shimizu W, Kamakura S, Kimura T, Investigators U-AT. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: the UNmasking Dormant Electrical Reconduction by Adenosine TriPhosphate (UNDER-ATP) trial. Eur Heart J. 2015;36:3276–87.
Schmidt M, Nölker G, Marschang H, Gutleben KJ, Schibgilla V, Rittger H, Sinha AM, Ritscher G, Mayer D, Brachmann J, Marrouche NF. Incidence of oesophageal wall injury post-pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace. 2008;10:205–9.
Di Biase L, Saenz LC, Burkhardt DJ, Vacca M, Elayi CS, Barrett CD, Horton R, Bai R, Siu A, Fahmy TS, Patel D, Armaganijan L, Wu CT, Kai S, Ching CK, Phillips K, Schweikert RA, Cummings JE, Arruda M, Saliba WI, Dodig M, Natale A. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circulation Arrhythmia and electrophysiology. 2009;2:108–12.
Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso F, Torracca L, Benussi S, Alfieri O, Hong R, Lau W, Hirata K, Shikuma N, Hall B, Morady F. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–6.
Zellerhoff S, Ullerich H, Lenze F, Meister T, Wasmer K, Monnig G, Kobe J, Milberg P, Bittner A, Domschke W, Breithardt G, Eckardt L. Damage to the esophagus after atrial fibrillation ablation: just the tip of the iceberg? High prevalence of mediastinal changes diagnosed by endosonography. Circulation Arrhythmia and electrophysiology. 2010;3:155–9.
Shah D, Dumonceau JM, Burri H, Sunthorn H, Schroft A, Gentil-Baron P, Yokoyama Y, Takahashi A. Acute pyloric spasm and gastric hypomotility: an extracardiac adverse effect of percutaneous radiofrequency ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:327–30.
Buch E, Vaseghi M, Cesario DA, Shivkumar K. A novel method for preventing phrenic nerve injury during catheter ablation. Heart Rhythm. 2007 Jan;4(1):95–8. Epub 2006 Sep 22.
Nakahara S, Ramirez RJ, Buch E, Michowitz Y, Vaseghi M, de Diego C, Boyle NG, Mahajan A, Shivkumar K. Intrapericardial balloon placement for prevention of collateral injury during catheter ablation of the left atrium in a porcine model. Heart Rhythm. 2010 ;7:81–7.
Buch E, Nakahara S, Shivkumar K. Intra-pericardial balloon retraction of the left atrium: a novel method to prevent esophageal injury during catheter ablation. Heart Rhythm. 2008;5:1473–5.
Cheema A, Dong J, Dalal D, Marine JE, Henrikson CA, Spragg D, Cheng A, Nazarian S, Bilchick KC, Almasry I, Sinha S, Scherr D, Halperin H, Berger R, Calkins H. Circumferential ablation with pulmonary vein isolation in permanent atrial fibrillation. Am J Cardiol. 2007;99:1425–8.
Burkhardt JD, Di Biase L, Natale A. Long-standing persistent atrial fibrillation: the metastatic cancer of electrophysiology. J Am Coll Cardiol. 2012;60:1930–2.
Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, Ouyang F. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg sequential ablation strategy. J Am Coll Cardiol. 2012;60:1921–9.
Seitz J, Horvilleur J, Curel L, Lacotte J, Maluski A, Ferracci A, Bremondy M, Rosier A, Monchi M, Penaranda G, Faure J, Beurtheret S, Pisapia A. Active or passive pulmonary vein in atrial fibrillation: is pulmonary vein isolation always essential? Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11:579–86.
Pascale P, Shah AJ, Roten L, Scherr D, Komatsu Y, Ramoul K, Daly M, Denis A, Derval N, Sacher F, Hocini M, Jais P, Haissaguerre M. Pulmonary veins to left atrium cycle length gradient predicts procedural and clinical outcomes of persistent atrial fibrillation ablation. Circulation Arrhythmia and electrophysiology. 2014;7:473–82.
O'Neill MD, Wright M, Knecht S, Jais P, Hocini M, Takahashi Y, Jonsson A, Sacher F, Matsuo S, Lim KT, Arantes L, Derval N, Lellouche N, Nault I, Bordachar P, Clementy J, Haissaguerre M. Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J. 2009;30:1105–12.
Elayi CS, Di Biase L, Barrett C, Ching CK, al Aly M, Lucciola M, Bai R, Horton R, Fahmy TS, Verma A, Khaykin Y, Shah J, Morales G, Hongo R, Hao S, Beheiry S, Arruda M, Schweikert RA, Cummings J, Burkhardt JD, Wang P, Al-Ahmad A, Cauchemez B, Gaita F, Natale A. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm. 2010 ;7:1216–23.
Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:835–46.
Jais P, Shah DC, Takahashi A, Hocini M, Haissaguerre M, Clementy J. Long-term follow-up after right atrial radiofrequency catheter treatment of paroxysmal atrial fibrillation. Pacing and clinical electrophysiology : PACE. 1998;21:2533–8.
Garg A, Finneran W, Mollerus M, Birgersdotter-Green U, Fujimura O, Tone L, Feld GK. Right atrial compartmentalization using radiofrequency catheter ablation for management of patients with refractory atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:763–71.
Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–80.
Konings KT, Smeets JL, Penn OC, Wellens HJ, Allessie MA. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–41.
Gerstenfeld EP, Sahakian AV, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–82.
Hayward RM, Upadhyay GA, Mela T, Ellinor PT, Barrett CD, Heist EK, Verma A, Choudhry NK, Singh JP. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm. 2011;8:994–1000.
• Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Guerra PG, Nair G, Torrecilla EG, Khaykin Y. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010;31:1344–56. This randomized clinical trial demostrates that empiric lines such as mitral isthmus and roof lines as well as CFAEs ablation is not superior to PVI alone in patients with LSPAF.
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, Investigators SAI. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22.
•• Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–44. Multicenter randomized trial which proved that patients with persistent AF and left ventricular ejection fraction <40% had a better prognosis if treated with catheter ablation when compared with amiodarone. More importantly, higher success was reported in patients undergoing PVI and posterior wall isolation in comparison with PVI alone. There was no significant difference between PVI alone and amiodarone
Di Biase L, Santangeli P, Natale A. How to ablate long-standing persistent atrial fibrillation? Curr Opin Cardiol. 2013;28:26–35.
Gerstenfeld EP, Callans DJ, Dixit S, Zado E, Marchlinski FE. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol. 2003;14:685–90.
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano Jr RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Heart Rhythm Society Task Force on C and Surgical Ablation of Atrial F. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:632–96. e21
Raviele A, Natale A, Calkins H, Camm JA, Cappato R, Ann Chen S, Connolly SJ, Damiano Jr R, DEP R, Edgerton JR, Haissaguerre M, Hindricks G, Ho SY, Jalife J, Kirchhof P, Kottkamp H, Kuck KH, Marchlinski FE, Packer DL, Pappone C, Prystowsky E, Reddy VK, Themistoclakis S, Verma A, Wilber DJ, Willems S, Venice C. Venice Chart international consensus document on atrial fibrillation ablation: 2011 update. J Cardiovasc Electrophysiol. 2012;23:890–923.
Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS, Chen SA. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–83.
Goya M, Ouyang F, Ernst S, Volkmer M, Antz M, Kuck KH. Electroanatomic mapping and catheter ablation of breakthroughs from the right atrium to the superior vena cava in patients with atrial fibrillation. Circulation. 2002;106:1317–20.
Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74.
Arruda M, Mlcochova H, Prasad SK, Kilicaslan F, Saliba W, Patel D, Fahmy T, Morales LS, Schweikert R, Martin D, Burkhardt D, Cummings J, Bhargava M, Dresing T, Wazni O, Kanj M, Natale A. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18:1261–6.
Oral H, Ozaydin M, Chugh A, Scharf C, Tada H, Hall B, Cheung P, Pelosi F, Knight BP, Morady F. Role of the coronary sinus in maintenance of atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:1329–36.
Kim DT, Lai AC, Hwang C, Fan LT, Karagueuzian HS, Chen PS, Fishbein MC. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–7.
Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, Seward JB, Tajik AJ, Edwards WD. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112–5.
Horak D, Svec F, Kalal J, Adamyan A, Titova M, Trostenyuk N, Skuba N, Dan V, Voronkova O, Gumargalieva K. Biologically active thrombin-containing hydrogels based on poly (2-hydroxyethyl methacrylate) for endovascular occlusion. Polim Med. 1991;21:31–41.
Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, Gallinghouse GJ, Bailey SM, Zagrodzky JD, Santangeli P, Hao S, Hongo R, Beheiry S, Themistoclakis S, Bonso A, Rossillo A, Corrado A, Raviele A, Al-Ahmad A, Wang P, Cummings JE, Schweikert RA, Pelargonio G, Dello Russo A, Casella M, Santarelli P, Lewis WR, Natale A. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18.
Dhanunjaya R, Lakkireddy MR, Sridhar ARM, Pillarisetti J, Maybrook R, Kanmanthareddy A, Matthew Earnest VS, Atkins D, Bommana S, Nath J, Ferrell R, Dawn B. Left Atrial Appendage Ligation and Ablation for Persistent Atrial Fibrillation (LAALA-AF Registry). JACC. 2014;63:A390.
Panikker S, Jarman JW, Virmani R, Kutys R, Haldar S, Lim E, Butcher C, Khan H, Mantziari L, Nicol E, Foran JP, Markides V, Wong T. Left atrial appendage electrical isolation and concomitant device occlusion to treat persistent atrial fibrillation: a first-in-human safety, feasibility, and efficacy study. Circulation Arrhythmia and electrophysiology. 2016;9
•• Di Biase L, Burkhardt JD, Mohanty P, Mohanty S, Sanchez JE, Trivedi C, Güneş M, Gökoğlan Y, Gianni C, Horton RP, Themistoclakis S, Gallinghouse GJ, Bailey S, Zagrodzky JD, Hongo RH, Beheiry S, Santangeli P, Casella M, Dello Russo A, Al-Ahmad A, Hranitzky P, Lakkireddy D, Tondo C, Natale A. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol. 2016;68:1929–1940. This randomized clinical trial demonstrated the benefits of empiric LAA isolation in patients with LSPAF
Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–36.
Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–85.
Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J Am Coll Cardiol. 2013;62:138–47.
Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol. 2014;63:1761–8.
Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–9.
Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K, Mandapati R. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2015;8:554–61.
Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, Mandapati R, Ellenbogen KA, Shivkumar K. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart rhythm : the official journal of the Heart Rhythm Society. 2016;13:636–41.
Malcolme-Lawes LC, Lim PB, Wright I, Kojodjojo P, Koa-Wing M, Jamil-Copley S, Dehbi HM, Francis DP, Davies DW, Peters NS, Kanagaratnam P. Characterization of the left atrial neural network and its impact on autonomic modification procedures. Circulation Arrhythmia and electrophysiology. 2013;6:632–40.
Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–25.
Lo LW, Scherlag BJ, Chang HY, Lin YJ, Chen SA, Po SS. Paradoxical long-term proarrhythmic effects after ablating the “head station” ganglionated plexi of the vagal innervation to the heart. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:751–7.
Mao J, Yin X, Zhang Y, Yan Q, Dong J, Ma C, Liu X. Ablation of epicardial ganglionated plexi increases atrial vulnerability to arrhythmias in dogs. Circulation Arrhythmia and electrophysiology. 2014;7:711–7.
Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A, Schotten U. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol. 2014;
Michowitz Y, Nakahara S, Bourke T, Buch E, Vaseghi M, De Diego C, Wiener I, Mahajan A, Shivkumar K. Electrophysiological differences between the epicardium and the endocardium of the left atrium. Pacing and clinical electrophysiology : PACE. 2011;34:37–46.
Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97.
Edgerton Z, Perini AP, Horton R, Trivedi C, Santangeli P, Bai R, Gianni C, Mohanty S, Burkhardt JD, Gallinghouse GJ, Sanchez JE, Bailey S, Lane M, DI Biase L, Santoro F, Price J, Natale A. J Cardiovasc Electrophysiol. 2016;27:524–30.
Takahashi Y, O'Neill MD, Hocini M, Dubois R, Matsuo S, Knecht S, Mahapatra S, Lim KT, Jais P, Jonsson A, Sacher F, Sanders P, Rostock T, Bordachar P, Clementy J, Klein GJ, Haissaguerre M. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008;51:1003–10.
Rostock T, Steven D, Hoffmann B, Servatius H, Drewitz I, Sydow K, Mullerleile K, Ventura R, Wegscheider K, Meinertz T, Willems S. Chronic atrial fibrillation is a biatrial arrhythmia: data from catheter ablation of chronic atrial fibrillation aiming arrhythmia termination using a sequential ablation approach. Circulation Arrhythmia and electrophysiology. 2008;1:344–53.
Sacher F, Corcuff JB, Schraub P, Le Bouffos V, Georges A, Jones SO, Lafitte S, Bordachar P, Hocini M, Clementy J, Haissaguerre M, Bordenave L, Roudaut R, Jais P. Chronic atrial fibrillation ablation impact on endocrine and mechanical cardiac functions. Eur Heart J. 2008;29:1290–5.
Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–47.
He X, Zhou Y, Chen Y, Wu L, Huang Y, He J. Left atrial posterior wall isolation reduces the recurrence of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2016;46:267–74.
Elayi CS, Di Biase L, Bai R, Burkhardt JD, Mohanty P, Santangeli P, Sanchez J, Hongo R, Gallinghouse GJ, Horton R, Bailey S, Beheiry S, Natale A. Administration of isoproterenol and adenosine to guide supplemental ablation after pulmonary vein antrum isolation. J Cardiovasc Electrophysiol. 2013;24:1199–206.
Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, Vaid H, O’Donnell M, Laupacis A, Cote R, Sharma M, Blakely JA, Shuaib A, Hachinski V, Coutts SB, Sahlas DJ, Teal P, Yip S, Spence JD, Buck B, Verreault S, Casaubon LK, Penn A, Selchen D, Jin A, Howse D, Mehdiratta M, Boyle K, Aviv R, Kapral MK, Mamdani M, Investigators E and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–77.
Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, Investigators CA. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86.
Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506.
Anselmino M, Matta M, D'Ascenzo F, Bunch TJ, Schilling RJ, Hunter RJ, Pappone C, Neumann T, Noelker G, Fiala M, Bertaglia E, Frontera A, Duncan E, Nalliah C, Jais P, Weerasooriya R, Kalman JM, Gaita F. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014 ;7(6): 1011–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Di Biase is a consultant for Biosense Webster, Boston Scientific, and St Jude Medical.
Dr. Di Biase has received speaker honoraria/travel from Medtronic, Atricure, EPiEP, and Biotronik.
Dr. Natale received speaker honoraria from Boston Scientific, Biosense Webster, Medtronic, and St. Jude.
Dr. Romero has no disclosures.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Arrythmias
Rights and permissions
About this article
Cite this article
Romero, J., Avendano, R., Natale, A. et al. Ablation of Advanced Subtypes of Atrial Fibrillation: Highlighting the Art of When and When Not to Perform Additional Ablation. Curr Cardiovasc Risk Rep 11, 19 (2017). https://doi.org/10.1007/s12170-017-0544-7
Published:
DOI: https://doi.org/10.1007/s12170-017-0544-7