Abstract
Purpose of Review
Pulmonary vein (PV) isolation is the cornerstone of atrial fibrillation (AF) ablation. However, the long-term procedural outcome remains suboptimal and there is a frequent need for repeat ablation procedure, especially in patients with non-paroxysmal AF. The review article summarizes the rationales, recent evidences, and strategies of ablation of extra-PV sites and its clinical outcomes.
Recent Findings
It is a consensus that durable PV isolations are a definite therapy in patients with paroxysmal AF. In non-paroxysmal AF, many laboratories still believe that adequate substrate ablation outside PVs is definitely required. Empirical linear ablation is not recommended because of difficulty in achieving complete linear block, unless macro-reentry atrial tachycardia developed during procedure. Most of laboratories applied complex fractionated atrial electrogram (CFAE) ablation after PV isolation in non-paroxysmal AF, but the efficacy is limited in the long-term follow-up studies. A combined approach using CFAE, non-linear similarity, and phase mapping strategy to identify rotors or focal sources for substrate modification increases the ablation outcome, when compared to CFAE ablation alone. Provocative test with mapping of non-PV triggers is also recommended in all patients to improve long-term ablation success.
Summary
Ablation beyond PV isolation is important, especially in non-paroxysmal AF patients, to modify the diseased atrial substrate and eliminate the non-PV triggers, which in turn improve the ablation outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than a decade ago, catheter ablation of atrial fibrillation (AF) was introduced as a method for maintaining sinus rhythm [1, 2]. Percutaneous catheter ablation is now widely used as an interventional tool for non-pharmacological AF rhythm control, particularly in those who are refractory to anti-arrhythmic medications [3]. Because most of the triggers for paroxysmal AF come from the PVs, circumferential pulmonary vein (PV) isolation, with confirmation of both entrance and exit blocks, is the cornerstone of this procedure. The ablation for persistent AF is more challenging and is associated with a less favorable outcome [4, 5]. Although the study from STAR AF II revealed no reduction in the rate of recurrent AF when either linear ablation or ablation of complex fractionated atrial electrogram (CFAE) was performed in addition to PV isolation [6], most laboratories still perform additional substrate modification in those with non-paroxysmal AF. Nevertheless, the long-term procedural outcome remains suboptimal and there is a frequent need for repeat ablation procedures to improve the long-term freedom from AF.
Beyond PV Isolation: What Else do We Ablate?
There are two major proposed mechanisms of AF: Multiple random propagating wavelets and focal electrical discharges [3, 7,8,9]. Those mechanisms are responsible for initiation and perpetuation of AF. Therefore, in the updated 2017 HRS/EHRA/ECAS Consensus Document for ablation reinforced, the concept of developing AF requires a trigger and an anatomic or functional substrate capable of both initiation and perpetuation of AF [3]. When we do PV isolation, we seclude the trigger inside the PVs and block the potential reentry at the PV antrum. However, we do not deal with the random propagating wavelets in the atrium, and neither do rare but existing focal electrical discharges outside PV. What are the clinical significance of those mechanisms?
Random Propagating Reentry
Moe and colleagues proposed that multiple reentrant wavelet hypothesis as a mechanism of AF in 1959 [10]. Random reentry, different from regular reentry due to circus movement, could cause AF. AF was consisted of a critical number of randomly distributed reentrant wavelets. Those wavelets propagate through the atria with fractionations that result in self-perpetuating ”daughter wavelets”. In addition, the wavelets could collide and divide or changing in size and velocity. The hypothesis is now widely accepted and Alessie et al. further reported the experimental results of the hypothesis [11]. Multiple reentrant wavelets are separated by functional conduction block lines. In the clinical practice, some cases do not have AF termination after PV isolation but it terminates during linear or CFAE ablations. It could be that ablation blocks those reentrant wavelets, especially in the non-paroxysmal AF patients.
Focal Electrical Discharges
The studies from Scherf et al. supported the concept of rapid firing focus initiating AF [12]. By administrating aconitine on the atrium, both rapid, regular atrial rhythm and rapid irregular atrial rhythm could be initiated. Goto et al. and Azuma et al. reproduced the similar findings and found the mechanism was secondary to enhanced automaticity [13, 14]. Therefore, some patients developed triggers outside PVs. In our previous publication, the incidence of AF originating from the non-PV foci was around 20% [15]. The superior vena cava and left atrial free wall were the most common locations of non-PV triggers.
Based on those evidences, extra-PV atrial sites are important because it might harbor some triggers and also provide substrates for maintaining AF. It is therefore critical to identify which patient requires additional ablation and where we should target beyond PV isolation.
Beyond PV Isolation: How Consensus Tells Us?
Among all updated AF guideline, including AHA/ACC, European Society of Cardiology, and HRS Consensus Document [3, 16, 17], catheter ablation of AF is a class I (paroxysmal) or IIa (persistent) indication in symptomatic AF refractory or intolerant to at least one class 1 or 3 anti-arrhythmic medication. PV isolation is the cornerstone in the patients with either paroxysmal or persistent AF. Unfortunately, due to the substantial recurrence rate observed in patients with PV isolation alone, continued efforts are underway to identify additional strategies to improve the long-term outcome. The steps after PV isolation are ill-defined, and there is no consensus on the optimal strategy in these patients. In general, there are four methods that are more definitive and recommended in the Consensus Document for ablation not targeting PVs: Linear, CFAE, non-PV triggers, and ganglionated plexi (GP) ablations [3]. Recently, some literatures also reported that rotor ablation could be an alternative to modify the atrial substrate and improve the ablation outcome [18,19,20,21,22,23].
Ablation Approaches Not Targeting the PVs
Linear Ablation Strategy
Linear ablation is one of the most widely used strategies in conjunction with PV isolation after the prospective randomized study conducted by Willems and colleagues [24]. Additional left atrial linear lesions increase the success rate significantly after PV isolation, compared with PV isolation alone in patients with persistent AF. The most commonly targeted linear lesion sets are left atrial roof and mitral isthmus lines. Iesake et al. reported that PV isolation followed by biatrial predetermined linear ablations for substrate modification is feasible, and AF termination happened in 51% of the patients with an AF free rate of 74% after 1.7 procedures in a 1.5-year follow-up [25, 26]. Later, the same group reported that AF termination during linear ablation is the sole predictor of arrhythmia freedom at a 5-year follow-up data [27]. Pak and Kim proposed that linear ablation at left atrial anterior wall resulted in a better clinical outcome in persistent AF patients [28].
Unfortunately, linear lesion failed to show benefits in the patients with paroxysmal AF. An updated meta-analysis of randomized controlled trials published that additional linear ablation did not exhibit any benefits in terms of sinus rhythm maintenance following a single procedure but increased the mean procedural, fluoroscopy and radiofrequency application times [29]. Linear ablation is also considered as a double-edged sword because proarrhythmic atrial tachycardias can be created secondary to an incomplete block line. Some literatures also showed conflicting results of linear ablation in persistent AF population. Morady and colleagues reported that during a repeat procedure, up to 90% of atrial tachycardias after AF ablation (including linear ablation in the first procedure) were reentrant, and the mitral isthmus, roof, and septum accounted for 75% of the ablation targets for the macro-reentrant arial tachycardia [30]. In the study by Sawhney and Feld, more patients developed left atrial flutter after PV isolation plus linear ablations, compared to segmental PV isolation alone [31]. In our recent publication, among the patients who received multiple AF ablation procedures, the incidence of atypical flutter or atrial tachycardia was around 30% or higher after a second procedure [32].
From the above evidences, linear ablation itself may be helpful in eliminating AF sources in the beginning; lack of durable and incomplete lesions during follow-ups are proarrhythmic and even complete lines can also promote reentry. We need to reconsider the risk-benefit ratio of such ablation strategy. It should be reserved for those with macro-reentry atrial tachycardia developed after PV isolation during first or recurrent procedure and should not be applied in pure paroxysmal AF cases.
CFAE Ablation Strategy
Areas with CFAEs have been reported to potentially represent AF substrate. It has become targeted sites for AF ablation and is recommended for non-paroxysmal AF cases in the HRS Consensus Document [3]. Similar to linear ablation, CFAE ablation in addition to PV isolation does not provide additional benefit to sinus rhythm maintenance in paroxysmal AF patients [33]. Nearly 50% of Task Force members routinely apply CFAE-based ablation as part of the strategy during non-paroxysmal AF ablation. Although the true mechanism of CFAEs detected during ablation is not yet fully understood, our previous study demonstrated that different activation patterns existed in the repetitive and continuous fractionated CFAEs [34]. Non-PV ectopies are also found to be located at the same locations as the CFAEs, and targeting those CFAEs can effectively eliminate AF [35]. Twenty-five percent of CFAEs in left atrium and 57% of the CFAEs in right atrium are related to non-PV triggers after PV isolation. Similar finding was also reported by Natales and colleagues that non-PV triggers inducing AF after PV isolation were associated with stable or transient CFAE in more than 70% of cases in long-standing persistent AF [36]. The beneficial effect achieved by CFAE ablation reflexes elimination of non-PV triggers. In addition, the study from Oklahoma and Chen’s labs also revealed that the intrinsic cardiac autonomic activity is related to the fractionated atrial electrograms, ablation of the GP can attenuate CFAE activities [37, 38].
However, there are some controversies of CFAE ablations, including the end point of CFAE ablation, and how extensive amount of the ablation required. Therefore, how to differentiate active from passive CFAE has been investigated in some studies. Singh et al. reported that by using ibutilide to organize the atrial activity and facilitate AF termination during ablation while minimizing the ablation lesion set [39]. Narayan et al. used the monophasic action potential to map the active CFAE and localized the CFAE to true rapid AF sites [40]. Our recent publication demonstrated that in the patients with persistent AF who failed to achieve AF termination after PVI, targeting continuous CFAE (fractionated interval < 60 ms) could be considered as an initial ablation strategy because of the lower incidence of recurrent atrial flutter and better reverse remodeling of the left atrium, better outcome with freedom from any atrial arrhythmia after two procedures [41].
Although CFAE ablation has been widely accepted for more than a decade, the exact mechanism of CFAE has not been fully understood, nor the scientific basis of CFAE ablation is not universally accepted. The prospectively randomized trial from STAR AF II failed to demonstrate the favorable outcome of CFAE ablation in persistent AF patients [6]. Careful selection of the patients, avoiding empirical extensive defragmentation and complete lesion creation while CFAE ablation are recommended in order to prevent the proarrhythmic side effect.
Non-PV Trigger Ablation Strategy
The importance of non-PV ectopic foci initiating AF has been demonstrated in multiple literatures, including triggers from superior vena cava, left atrial free wall, crista terminalis, coronary sinus ostium, ligament of Marshall, left atrial appendage, and interatrial septum [3, 15, 35, 42,43,44,45,46,47,48,49]. In our laboratory, we found the incidence is higher (45%) in patients with long-standing persistent AF patient who received catheter ablation (unpublished data). Those with non-PV triggers also have a higher recurrence rate after catheter ablation [15, 45]. Figure 1 shows the incidence of non-PV triggers in patients received multiple ablation procedures. As the number of the procedure sessions increases, there is an increasing incidence of triggers from non-PV foci. The presence of non-PV trigger is also an independent predictor of AF recurrence during a long-term (4-year) follow-up study [15]. A study from the University of Pennsylvania showed that non-PV trigger ablation for long-standing persistent AF in addition to PV isolation provides a good long-term AF control in over 70% of patients with infrequent proarrhythmic atrial flutter after ablation [46]. This ablation approach also improves the maintenance of sinus rhythm and reverses disease progression. Later, the same group published that transformation from long-standing persistent to paroxysmal AF after initial ablation may be a step toward long-term freedom from recurrence arrhythmia [47].
Percentages of pulmonary vein (PV) and non-PV atrial fibrillation (AF) triggers at each ablation sessions in patient who received more than 2 AF ablation procedures. As the number of procedure sessions increases, there is an increase incidence of triggers from non-PV foci (reproduced with permission from: Lo LW, et al. J Cardiovasc Electrophysiol. 2015;26:1048–56, with permission of John Wiley and Sons) [32]
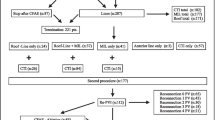
There are no standard provocation test and mapping technique of the non-PV triggers. Therefore, the reported incidence of non-PV triggers varied in different laboratories. It is also disputed whether the provocative non-PV triggers are related to clinical ectopies that initiate AF. Table 1 shows the provocative test used in our laboratory. Nevertheless, most of the laboratories still believe that non-PV triggers are important and ablation of those ectopies is required if detected or induced during the procedure. During sinus rhythm, mapping of non-PV triggers can be evaluated by the endocardial activation sequence of the high right atium, His-bundle, and coronary sinus [48]. Figure 2 is the algorithm showing the scheme we used for assessing the origin of the non-PV ectopic activity initiating AF. If patients remained AF after PV isolation, CFAE ablation is possible to eliminate non-PV triggers, as described in the previous paragraph [35].
Algorithm showing scheme used in our laboratory for assessing the origin of the non-PV ectopic activity initiating AF. AFCL atrial fibrillation cycle length, CS coronary sinus, CSd distal portion of coronary sinus, CSp proximal potion of coronary sinus, CT crista terminalis, HIS His-bundle area, HRA high right atrium, LA left atrium, LOM ligament of marshall, L-PV left pulmonary vein, RA right atrium; R-PV right pulmonary vein, SVC superior vena cava (reproduced with permission from: Higa S, et al. Heart Rhythm. 2006;3:1386–90, with permission from Elsevier) [48]
GP Ablation Strategy
Cardiac autonomic nervous system is considered as a modulator for initiation and maintenance of AF. The intrinsic cardiac autonomic system (GP) is located epicardially at the junction of PV to left atrium [50]. There are four major GPs in the left atrium, including anterior right GP, inferior right GP, superior left GP, and inferior left GP. High-frequency stimulation can identify the location of the GP, and ablation targeting those GPs had been applied in some laboratories. In a prospective randomized trial, GP ablation in addition to PV isolation confers a significantly higher success rate compared to PVI or GP ablation alone in patients with paroxysmal AF [51]. Similar data has also been found in patients with persistent and long-standing persistent AFs; Pokushalov et al. reported that PV isolation plus GP ablation resulted in a superior clinical result with less ablation-related left atrial flutter and reduced AF recurrence compared to PV isolation plus linear ablation after a 3-year follow-up [52].
On the contrary, AFACT study showed that GP ablation during the thoracoscopic surgery for advance AF has no detectable effect on AF recurrence but causes more major bleeding, sinus node dysfunction, and pacemaker implantation [53]. But, denervation of cardiac autonomic nervous system in this study was executed epicardially; the mechanism and end point for denervation might be different from that in percutaneous transcatheter approach. Therefore, further researches are required to clarify the effect of GP ablation in AF patients.
Rotor Ablation Strategy
Rotor is a phase singularity; it means spiral waves radiate at a high speed into the surrounding tissues [7]. This concept was first observed by Jalife and colleagues and then supported by the evidence from optical mapping in isolated animal heart preparation [7, 54, 55]. There are two forces from a rotor; one is a rotational force with a curvature, and the other is a divergence force with peripheral fibrillatory conduction. Recent studies have reported a successful rotor identification by phase mapping of simultaneous recordings using a multi-electrode mapping catheter in clinical practice [20, 21, 23]. Focal impulse and rotor modulation (FIRM) mapping was first reported in 2011 to systematically and reproducibly identify localized drivers in human AF. Using a 64-pole basket catheter in the left atrium, Narayan et al. proposed a panoramic contact mapping, incorporating a phase analysis, repolarization, conduction dynamics, and oscillation in the AF rate, and hypothesized that AF may be sustained by electrical rotors and focal impulses [20]. Ablation of such sources has been shown to improve ablation outcome compared with conventional ablation alone. In the extended follow-up of the CONFIRM trial, Narayan et al. claimed rotors or focal sources were observed in 97.7% of the patients during AF. After more than 2 years of follow-up with 1.2 ablation procedures, 78% of the patients maintained freedom from AF [21]. However, a study from UCLA group then reported that using the FIRM technique to guide AF ablation and found that rotor sites did not exhibit quantitative atrial electrogram characteristics expected from rotor and did not differ from the surrounding tissue; AF termination or organization was only observed in 17% of the patients [56]. After a 1.5-year follow-up, only 37% of patients were free from documented recurrent AF, indicating a poor efficacy of the FIRM ablation outcome [57]. FIRM-identified rotor ablation is also found to be not effective in preventing recurrence of atrial tachyarrhythmia in other prospective multi-center study [58].
Due to inconsistent results of FIRM ablation, rotor ablation has been evaluated by using other mapping catheters or methodologies. Ghoraani et al. used the 20-pole circular mapping catheter to identify the localized rotational activity and found that low-voltage CFAE associated with rotational activity is importance for arrhythmia maintenance [59]. Kalman and colleagues used epicardial high-density mapping plaque to identify the wave front activation intra-operatively and reported less than 10% of transient rotational circuits during AF [60]. Bourdeaux group used an array of 252 body surface electrodes and non-contrast computed tomography scan to obtain an accurate biatrial geometry. They reported that in the early months, persistent AF is predominantly maintained by unstable reentry drivers with meandering and periodic occurrence [61]. It is also different from Narayan’s temporary stable rotors. In our laboratory, we used the non-linear analysis to evaluate the fibrillatory electrogram similarity and combined with phase mapping technique to identify the small-radius reentry [22, 23, 62]. Figure 3 shows the scheme of the non-linear similarity analysis and phase mapping methodology. By using this method, we found that an average of 2.6 ± 0.9 high similarity index region in each chamber, rotor, and focal sources was found in around two thirds of the patients. This mapping technique can predict a freedom from AF recurrence after 18 months of follow-up [23].
Schematic presentation of the nonlinear similarity mapping and phase mapping for rotor identification. a Shows the bipolar fibrillation electrogram obtained from multiple electrode mapping catheter and was first band-pass filtered (10 to 300 Hz) for preprocessing. b Shows the associated envelope of the filtered signals was subsequently obtained by the order-statistic filter, which could effectively attenuate noise and far-field contamination to highlight the local activation wave (LAW). C Indicates the multiple electrode mapping catheter facilitates characterization of wave front propagation by real-time phase mapping derived from the reconstructed envelop function. Yellow arrows indicate the direction of the wave front propagation. d Shows the similarity index quantified based on the temporal and spatial consistency of the morphological repetitiveness of LAW. e Shows the rotors were identified in the high similarity index region with aids of real-time 3D display of the similarly index/phase mapping (reproduced with permission from: Lin YJ, et al. J Am Coll Cardiol EP. 2016;2:667–78. with permission from Elsevier) [23]
There is no consensus on the rotor ablation in AF patients because of inconsistent outcomes based on current evidences. We need more literatures to confirm the best methods and tools in identifying the rotor and focal sources. Rotor mapping and ablation may be of particular benefit in patients with persistent AF, and maybe a patient-tailored therapy is the best approach to reduce unnecessary ablation lesions. Multicenter randomized controlled studies are ongoing to better define the role of rotor ablation in this population.
Voltage Map-Guided Ablation Strategy
Atrial fibrosis and its border zones are considered an important substrate for focal and reentry activity involved in the initiation and perpetuation of AF [3, 63]. It can be identified from cardiac MRI with delayed enhancement or 3D mapping system with bipolar low-voltage electrogram (<0.5 mV). In general, the low-voltage zones (LVZs) can be demonstrated in approximately every third patients with persistent AF and less often in patients with paroxysmal AF [18]. Those pre-existing LVZs have been shown to be related to arrhythmia recurrence after catheter ablation and may lead to a rapid firing secondary to local automaticity or micro-reentry [18, 63]. Hindricks and colleagues first described this method and found that additional voltage-based substrate modification had a comparable 1-year outcome when compared with the patients with normal voltage undergoing PV isolation alone [18]. Another study from Jadidi et al. also found that ablation within border zones of LVZ in addition to PV isolation is more effective than conventional PVI-only strategy for persistent AF [64]. The study also found that PV isolation only seems to be sufficient to treat patients with left atria LVZs of <10%. Study from a China group revealed that sinus rhythm LVZs were presented in 70% of the patients with persistent AF. Selective electrophysiologically guided substrate modification during sinus rhythm after PV isolation is clinically more effective than the stepwise approach for persistent AF with less post-procedural proarrhythmic atrial tachycardia [65].
However, similar to rotor ablation, this strategy was only reported in limited laboratories, and we required more data to confirm the long-term outcome. In addition, in patients with diffuse LVZ (strawberry left atrium), it is not reasonable and is difficult to define how extensive the ablation lesions should be applied.
Patient-Tailored Ablation Strategy beyond PV Isolation
It is of no doubt that a durable PV isolation is the cornerstone of ablation in patients with paroxysmal AF. Routine substrate modification is not recommended in these patients [33], except macro-reentrant atrial tachycardia developed during ablation and requiring linear lesion sets. Targeting the non-PV triggers is suggested if detected or induced during or after PV isolation. In patients with non-paroxysmal AF, how to select and perform substrate ablation on top of PV isolation is still disputed. Table 2 summarized the ablation strategy beyond PV isolation. Based on the available evidences, we performed CFAE maps and identified the location of continuous CFAE after PV isolation [41]. Then, regional analysis is suggested by using a high-density multi-electrode mapping catheter (i.e., circular catheter or Penta-ray™ catheter), followed by a real-time phase mapping using non-linear method at the continuous CFAE sites [22, 23, 62]. Areas with a similarity index higher than 0.57 are selected for quantification of the rotor curvature and divergence forces. Rotor ablation is applied after identification of these sites. If AF still persisted, right atrial CFAE mapping with rotor identification and ablation are then performed. If AF still persisted after biatrial substrate modification, electrical cardioversion is given to restore sinus rhythm. Searching for non-PV triggers is recommended after restoration to sinus rhythm by AF procedural termination or electrical cardioversion [32, 35]. Activation mapping is recommended at any step if AF transformed to an organized atrial tachycardia.
Conclusions
Although a durable PV isolation is the most important step during catheter ablation of AF, a portion of patients still require additional lesion sets to eliminate AF sources and increase the success rate, especially in those with non-paroxysmal AF. Empirical linear ablation is not recommended because of the difficulty in achieving complete linear block. It is recommended only when macro-reentry atrial tachycardia developed during the procedure. Most laboratories applied CFAE ablation after PV isolation in non-paroxysmal AF, but the efficacy is limited in the long-term follow-up study [6]. We recommended a combined approach using CFAE, non-linear similarity, and phase mapping strategy to identify rotors or focal sources for substrate modification. In addition, application of provocative test with mapping of non-PV triggers are also important to increase the ablation outcome in both paroxysmal and non-paroxysmal AF patients. Success rate can be further improved if those foci are adequately detected and eliminated.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Haissaguerre M, Jais P, Shah DC, Takashashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–86.
•• Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. Heart rhythm society task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017; doi:10.1016/j.hrthm.2017.05.012. This article provided the updated consensus of atrial fibrillation ablation in clinical practice
Parkash R, Verma A, Tang AS. Persistent atrial fibrillation: current approach and controversies. Curr Opin Cardiol. 2010;25:1–7.
Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010;7:835–46.
• Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. This study demonstrated the importance of pulmonary vein isolation, even in the patients with persistent atrial fibrillation, but also opened the dispute whether additional ablation beyond pulmonary vein is required or not
Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–16.
Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26.
Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325.
Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70.
Allessie MA, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of Moe's multiple wavelet hypothesis of atrial fibrillation. In: Zipes DJ, Jalife J, editors. Cardiac electrophysiology and arrhythmias. New York: Grune & Stratton; 1985. p. 265–75.
Scherf D. Studies on auricular tachycardia caused by aconitine administration. Proc Expt Biol Med. 1947;64:233–9.
Goto M, Sakamoto Y, Imanaga I. Aconitine-induced fibrillation of the different muscle tissues of the heart and the action of acetylcholine. In: Sano T, Matsuda K, Mizuhira B, editors. Electrophysiology and Ul-trastructure of the heart. New York: Grune & Stratton; 1967. p. 190–209.
Azuma K, Iwane H, Ibukiyama C, Watabe Y, Shih-Mura H, Iwaoka M, et al. Experimental studies on aconitine-induced atrial fibrillation with microelectrodes. Isr J Med Sci. 1969;8:177–92.
Chang HY, Lo LW, Lin YJ, Chang SL, Hu YF, Li CH, et al. Long-term outcome of catheter ablation in patients with atrial fibrillation originating from nonpulmonary vein ectopy. J Cardiovasc Electrophysiol. 2013;24:250–8.
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–104.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962.
Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–33.
Kottkamp H, Bender R, Berg J. Catheter ablation of atrial fibrillation: how to modify the substrate? J Am Coll Cardiol. 2015;65:196–206.
Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol. 2012;60:628–36.
Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation). J Am Coll Cardiol. 2014;63:1761–8.
Lo LW, Lin YJ, Chang SL, Hu YF, Chung FP, Chen SA. Pearls and pitfalls in catheter ablaton of persistent atrial fibrillation. Circ J. 2016;80:306–13.
• Lin YJ, Lo MT, Chang SL, Lo LW, Hu YF, Chao TF, et al. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. J Am Coll Cardiol EP. 2016;2:667–78. This manuscript describe a new substrate modification method in patients with persistent atrial fibrillation, guided by nonlinear phase mapping methods for rotor detection
Willems S, Klemm H, Rostock T, Brandstrup B, Ventura R, Steven D, et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J. 2006;27:2871–8.
Miyazaki S, Taniguchi H, Komatsu Y, Uchiyama T, Kusa S, Nakamura H, et al. Sequential biatrial linear defragmentation approach for persistent atrial fibrillation. Heart Rhythm. 2013;10:338–46.
Miyazaki S, Taniguchi H, Kusa S, Uchiyama T, Nakamura H, Hachiya H, et al. Impact of atrial fibrillation termination site and termination mode in catheter ablation on arrhythmia recurrence. Circ J. 2014;78:78–84.
Miyazaki S, Taniguchi H, Kusa S, Nakamura H, Hachiya H, Hirao K, et al. Five-year follow-up outcome after catheter ablation of persistent atrial fibrillation using a sequential biatrial linear defragmentation approach: what does atrial fibrillation termination during the procedure imply? Heart Rhythm. 2017;14:34–40.
Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011;8:199–206.
Hu X, Jiang J, Ma Y, Tang A. Is there still a rle for additional linear ablation in addition to pulmonary vein isolation in patents with paroxysmal atrial fibrillation? An updated meta-analysis of randomized controlled trials. Int J Cardiol. 2016;209:266–74.
Chae S, Oral H, Good E, Dey S, Wimmer A, Crawford T, et al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;50:1781–7.
Sawhney N, Anousheh R, Chen W, Feld GK. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:243–8.
Lo LW, Lin YJ, Chang SL, Hu YF, Chao TF, Chung FP, et al. Predictors and characteristics of multiple (more than 2) catheter ablation procedures for atrial fibrillation. J Cardiovasc Elecrophysiol. 2015;26:1048–56.
Li WJ, Bai YY, Zhang HY, Tang RB, Miao CL, Sang CH, et al. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a meta-analysis. Circ Arrhythm Electrophysiol. 2011;4:143–8.
Lo LW, Higa S, Lin YJ, Chang SL, Tuan TC, Hu YF, et al. The novel electrophysiology of complex fractionated atrial electrograms: insight from noncontact unipolar electrograms. J Cardiovasc Electrophysiol. 2010;21:640–8.
Lo LW, Lin YJ, Tsao HM, Chang SL, Hu YF, Tsai WC, et al. Characteristics of complex fractionated electrograms in nonpulmonary vein ectopy initiating atrial fibrillation/atrial tachycardia. J Cardiovasc Electrophysiol. 2009;20:1305–12.
Elayi CS, Di Biase L, Bai R, Burhardt JD, Mohanty P, Sanchez J, et al. Identifying the relationship between the non-PV triggers and the critical CFAE sites post-PVAI to curtail the extent of atrial ablation in longstanding persistent AF. J Cardiovasc Electrophsyiol. 2011;22:1199–205.
Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–23.
Lu Z, Scherlag BJ, Lin J, Niu G, Ghias M, Jackman WM, et al. Autonomic mechanism for complex fractionated atrial electrograms: evidence by fast fourier transform analysis. J Cardiovasc Electrophysiol. 2008;19:835–42.
Singh SM, D'Avila A, Kim SJ, Houghtaling C, Dukkipati SR, Reddy VY. Intraprocedural use of ibutilide to organize and guide ablation of complex fractionated atrial electrograms: preliminary assessment of a modified step-wise approach to ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:608–16.
Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011;8:244–53.
Lin YJ, Chang SL, Lo LW, Hu YF, Chong E, Chao TF, et al. A prospective and randomized comparison of limited versus extensive atrial substrate modification after circumferential pulmonary vein isolation in nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:803–12.
Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–83.
Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74.
Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–8.
Chang HY, Lo LW, Lin YJ, Chang SL, Hu YF, Feng AN, et al. Long-term outcome of catheter ablation in patients with atrial fibrillation originating from the superior vena cava. J Cardiovasc Electrophysiol. 2012;23:955–61.
Lin D, Frankel DS, Zado ES, Gerstenfeld E, Dixit S, Callans DJ, et al. Pulmonary vein antral isolation and nonpulmonary vein trigger ablation without additional substrate modification for treating longstanding persistent atrial fibrilation. J Cardiovasc Electrophysiol. 2012;23:806–13.
Liang JJ, Elafros MA, Muser D, Pathak RK, Santangeli P, Zado ES, et al. Pulmonary vein antral isolation and nonpulmonar vein trigger ablation are sufficient to achieve favorable long-term outcomes including transformation to paroxysmal arrhythmias in patients with persistent and long-staning persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e004239.
Higa S, Tai CT, Chen SA. Catheter ablation of atrial fibrillation originating from extrapulmonary vein areas. Taipei approach. Heart Rhythm. 2006;3:1386–90.
Allamsetty S, Lo LW, Lin YJ, Chang SL, Chung FP, Hu YF, et al. Impact of aortic encroachment to left atrium on non-pulmonary vein triggers of atrial fibrillation. Int J Cardiol. 2017;227:650–5.
Lo LW, Chiou CW, Lin YJ, Lee SH, Chen SA. Neural mechanism of atrial fibrillation: insight from global high density frequency mapping. J Cardiovasc Electrophysiol. 2011;22:1049–56.
Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–25.
Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013;10:1280–6.
Driessen AH, Berger WR, Krul SP, van den Berg NW, Neefs J, Piersma FR, et al. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol. 2016;68:1155–65.
Davidenko JM, Kent PF, Chialvo DR, Michaels DC, Jalife J. Sustained vortex-like waves in normal isolated ventricular muscle. Proc Natl Acad Sci U S A. 1990;87:8785–9.
Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–8.
Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K, et al. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:554–61.
Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm. 2016;13:636–41.
Gianni C, Mohanty S, Di Biase L, Metz T, Trivedi C, Gökoğlan Y, et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm. 2016;13:830–5.
Ghoraani B, Dalvi R, Gizurarson S, Das M, Ha A, Suszko A, et al. Localized rotational activation in the left atrium during human atrial fibrillation: relationship to complex fractionated atrial electrograms and low-voltage zones. Heart Rhythm. 2013;10:1830–8.
Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, et al. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97.
Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–8.
Lin YJ, Lo MT, Lin C, Chang SL, Lo LW, Hu YF, et al. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:851–8.
Higa S, Tai CT, Lin YJ, Liu TY, Lee PC, Huang JL, et al. Focal atrial tachycardia: new insight from noncontact mapping and catheter ablation. Circulation. 2004;109:84–91.
Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, et al. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9:e002962.
Yang G, Yang B, Wei Y, Chang F, Ju W, Chen H, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2016;9:e003382.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Li-Wei Lo, Yenn-Jiang Lin, Shih-Lin Chang, Yu-Feng Hu, Fa-Po Chung, and Shih-Ann Chen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
The present work was supported by the Taipei Veterans General Hospital (V102B-002, V102E7-003, V103C-042, V103C-126, V103E7-002, VGHUST103-G1-3-1, V104C-131, V104E7-003, VA105C-60), Ministry of Science and Technology (NSC 101-2911-I-008-001, NSC 102-2325-B-010-005, MOST 103-2314-B-075-062-MY3, MOST 104-2314-B-075-065-MY2), and Research Foundation of Cardiovascular Medicine (RFCM 100-02-011, 101-01-001, 104-01-009-01).
Additional information
This article is part of the Topical Collection on Invasive Electrophysiology and Pacing
Rights and permissions
About this article
Cite this article
Lo, LW., Lin, YJ., Chang, SL. et al. Beyond Pulmonary Vein Isolation: the Role of Additional Sites in Catheter Ablation of Atrial Fibrillation. Curr Cardiol Rep 19, 86 (2017). https://doi.org/10.1007/s11886-017-0884-4
Published:
DOI: https://doi.org/10.1007/s11886-017-0884-4