Abstract
This study aimed to develop and optimize a β-cyclodextrin (β-CD)-based technique for extracting anthocyanins from Lycium ruthenicum Murr. and to establish a ultra-high-performance liquid chromatography-diode array detector (UPLC-DAD) method for their analysis. β-CD solutions produced higher extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum fruit than did pure water and aqueous hydroxypropyl-β-cyclodextrin (HPβ-CD) and ethanol and methanol solutions. Extraction at 50 °C for 30 min using 1.65% β-CD solution and a liquid/solid ratio of 15:1 produced the optimal extraction yield of L. ruthenicum anthocyanins. A UPLC-DAD method was developed for the determination of L. ruthenicum anthocyanins using an ethanol-based mobile phase, and the primary anthocyanins were identified using two-dimensional LC-MS/MS. Method validation showed that the developed method was accurate, stable, and reliable for the analysis of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum fruit. The present study showed that β-CD-based extraction coupled with UPLC-DAD analysis is an efficient and green method for the extraction and analysis of anthocyanins from L. ruthenicum fruit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lycium ruthenicum Murr. (Solanaceae), widely distributed in the Qinghai-Tibet Plateau, has been used as folk medicine for hundreds of years (Chen et al. 2018). L. ruthenicum fruit is a rich source of bioactive components, especially anthocyanins (Xia et al. 2015; Zhao 2016). Previous reports have demonstrated that anthocyanins from L. ruthenicum possess a variety of bioactivities, such as anti-oxidative (Zheng et al. 2011), anti-atherosclerosis (Lin et al. 2012), and memory-improving effects (Wu et al. 2017). Moreover, Tang et al. (2017) reported that petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside, the main anthocyanin in the fruit of L. ruthenicum, has potential radical scavenging activities and a protective effect on PC12 cells. Therefore, developing a method for analyzing the anthocyanins from L. ruthenicum fruit is desired.
The conventional methods of extracting anthocyanins from plant materials use a mixture of water and an organic solvent (e.g., methanol, ethanol, and acetone) (Barnes et al. 2009); however, organic solvents are associated with environmental pollution, can present toxicological concerns, and are relatively expensive (Gao et al. 2016). Currently, green techniques for extracting bioactive compounds are receiving more and more attention, and many researchers have been actively exploring green solvents as substitutes for traditional toxic and volatile organic solvents (Wang et al. 2017). Cyclodextrins are enzyme-modified starch derivatives produced industrially that can increase the stability, solubility, and bioavailability of bioactive compounds by forming inclusion complexes. As a result, cyclodextrins have been widely used in pharmaceutical, food, cosmetic, and environmental and agricultural fields (Fenyvesi et al. 2016). Recently, aqueous solutions of cyclodextrin have been investigated in the extraction of natural compounds from plant materials, such as resveratrol from Polygonum cuspidatum (Mantegna et al. 2012), flavonols from apple pomace (Parmar et al. 2015), and polyphenols from tea leaves (Cui et al. 2017). Cyclodextrins are considered an environmentally friendly additive for the rapid and effective extraction of bioactive compounds (Zhang et al. 2015; Gao et al. 2016). To the best of our knowledge, cyclodextrin-based techniques for the extraction of anthocyanins have not been investigated. Development of a green method for extracting anthocyanins would be a significant advance for the field.
Green chromatography is a branch of green analytical method that focuses on achieving environmentally friendly chromatography analysis (Płotka et al. 2013). Replacing toxic and hazardous solvents with the greener alternative ones is a significant subject in green chromatography, and many greener solutions have been used to improve analytical approaches such as ethanol, superheated water, supercritical fluid, and ionic liquid (Korany et al. 2017). Ethanol is a desirable solvent for green chromatography and has been used as mobile phase to analyze various compounds such as caffeine, statins, and anthocyanins (Sik 2012; Assassi et al. 2015; Sang et al. 2017a, b). As shown in Table S1, acetonitrile/methanol-based mobile phases and long analytical processes are frequently used for analyzing L. ruthenicum anthocyanins. UPLC technology and ethanol-based mobile phases have potential to improve the green property of the analytical approach of L. ruthenicum anthocyanins. As a result, development of a work-safe, rapid, and efficient analytical method for the determination of L. ruthenicum anthocyanins is of significance.
In the present study, a β-cyclodextrin (β-CD)-based extraction methodology was developed and optimized to recover petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum, and a green ultra-high-performance liquid chromatography-diode array detector (UPLC-DAD) method was established for determination of L. ruthenicum anthocyanins.
Materials and Methods
Plant Material and Reagents
L. ruthenicum dry fruits were purchased from a local market in Qing Hai, China. The materials were ground and sieved (40 mesh). The powder was stored at − 20 °C prior to extraction. Cyanidin-3-O-glucoside (purity ≥ 98%) was bought from Chengdu Must Bio-Technology Co., Ltd. (Chengdu, China). HPLC grade formic acid and trifluoroacetic acid were purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). HPLC grade ethanol was purchased from Fisher Chemicals Co., Ltd. (USA). β-CD and hydroxypropyl-β-cyclodextrin (HPβ-CD) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). All other chemicals and reagents were of analytical grade, and deionized water was used in all experiments.
Extraction of Anthocyanins
Finely milled material (1 g) and 15 mL of extraction solvent, namely, pure water, aqueous ethanol and methanol solutions (50 and 70%; v/v), and aqueous β-CD and HPβ-CD solutions (1, 2, 3, 4, and 5%; m/v) all with 0.1% HCl (v/v), were added to a 50-mL flask. The extractions were conducted at 55 °C for 60 min using an ultrasonic processor (JY92-IIDN, NingBo Scientz Biotechnology Co., Ltd., Zhejiang, China) coupled with an ultrasound probe (Φ 6 mm; 300 W; 25 kHz). The extract was centrifuged at 5000g for 20 min, and 1 mL of the supernatant was diluted with 2 mL of ethanol (0.1% HCl, v/v). The mixture was centrifuged at 5000g for 20 min and used for UPLC-DAD analysis. The extraction parameters, including extraction solvent, β-CD concentration (%, w/v), extraction temperature (°C), extraction time (min), and liquid/solid ratio (g/mL), were optimized using single-factor experiments and response surface methodology (Khazaei et al. 2016; Fattahi and Rahimi 2016) (Table 1).
UPLC-DAD Analysis of Anthocyanins
An Ultimate 3000 UHPLC system (Thermo Fisher Scientific Co., Ltd., USA) equipped with a binary gradient pump, an auto-sampler, a diode array detector, a thermostatically controlled column oven with a six-way valve, and a Chromeleon Chemstation software was used to analyze the extracted anthocyanins. An analytical ZORBAX Eclipse Plus C18 column (100 × 3 mm, 1.8 μm particle size, Agilent Technologies Co., Ltd., USA) was used. The mobile phase consisted of ethanol with trifluoroacetic acid (0.1%, v/v), and a gradient elution was performed as follows: 15–25% ethanol from 0 to 10 min and 25–29% ethanol from 10 to 12 min. The injection volume was 5 μL, the detection wavelength was 520 nm, the flow rate was 0.5 mL/min, and the column temperature was 35 °C. Total anthocyanins could be approximated by the sum of the peak areas of the UPLC-DAD chromatogram. Cyanidin-3-O-glucoside was used as a standard to quantify the anthocyanins, and the extraction yield of anthocyanins were expressed as mg cyanidin-3-O-glucoside equivalent/g L. ruthenicum dry fruit.
HPLC-MS Analysis of Anthocyanins
An Agilent 1290 HPLC coupled to an Agilent 6460C triple quadrupole mass spectrometer with an AJS electrospray ionization source (Agilent Technologies Co., Ltd., USA) was used to identify the anthocyanins. The mobile phase was a mixture of 10% ethanol and 90% aqueous formic acid (0.1%, v/v), the flow rate was 0.4 mL/min, the injection volume was 20 μL, the capillary voltage was 3500 V, the sheath gas temperature was 350 °C, the sheath gas flow rate was 12.0 L/min, the nebulizer pressure was 45 psi, the drying gas temperature was 300 °C, the drying gas flow rate was 6.0 L/min, and the scan range was 100–1200 m/z in the positive mode.
Statistical Analysis
A Design-Expert.V8.0.6.1 software was used to design and analyze response surface optimization. Other statistical analyses were performed using Microsoft Office 2007 and Graph-Pad.Prism.v.5.0. One-way analysis of variance was used to determine the statistical significance; p < 0.05 was considered significant.
Results and Discussion
UPLC-DAD Separation and Peak Identification
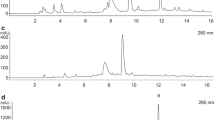
A UPLC-DAD method was developed to analyze L. ruthenicum anthocyanins using an ethanol-based mobile phase, and ten anthocyanin peaks could be detected within 12 min (Fig. 1a). Peaks 2 (11.90%), 3 (2.28%), 6 (2.44%), and 7 (79.66%) accounted for 96.28% of the total anthocyanins in L. ruthenicum fruit. The trifluoroacetic acid solution is not recommended for use in HPLC-MS analysis because it can damage or pollute the ion source and suppress ionization. As a result, the anthocyanins were tentatively identified using two-dimensional HPLC-MS. The four target peaks were isolated using a six-way valve, and the interval ranges of peaks 2, 3, 6, and 7 were 2.3–3.0, 3.3–3.8, 6.0–6.5, and 6.8–7.8 min, respectively (Fig. 1b–e). Following separation, the four fractions were directly transferred to HPLC-MS. Peaks 2 and 3 exhibited similar fragmentation patterns (MS+ = m/z 1095; MS/MS = m/z 933, 771, 479, 317) (Fig. S1), and they were determined to be petunidin-3-O-(glucosyl-trans-p-coumaroyl)-rutinoside-5-O-glucoside and petunidin-3-O-(glucosyl-cis-p-coumaroyl)-rutinoside-5-O-glucoside, respectively (Jin et al. 2015a, b). Previous studies demonstrated that petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside was the main anthocyanin in L. ruthenicum. In addition, based on the MS data (MS+ = m/z 933; MS/MS = m/z 771, 479, 317) (Fig. S1), the main peak (peak 6) was identified as petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside. Peak 7 exhibited a similar fragmentation pattern (MS+ = m/z 933; MS/MS = m/z 771, 479, 317) to that of peak 6 (Fig. S1) and was identified as petunidin-3-O-(cis-p-coumaroyl)-rutinoside-5-O-glucoside.
Method Validation
Linearity and Limits of Detection and Quantification
Cyanidin-3-O-glucoside standard solutions (0.02, 0.10, 0.20, 0.30, 0.40, and 0.50 mg/mL) were used to prepare a calibration curve. The calibration curve was given by Y (peak area) = 654.92 × X (cyanidin-3-O-glucoside concentration) + 0.9855 (R2 = 0.9999). The limits of detection (signal-to-noise ratio of 3) and quantitation (signal-to-noise ratio of 10) were 0.000117 and 0.000390 mg/mL, respectively.
Precision, Repeatability, and Stability
The intra- and inter-day precisions of the UPLC-DAD method were investigated by analyzing a sample in triplicate on two consecutive days. The repeatability was evaluated by analyzing six replicate samples. Stability was determined by analyzing a sample 0, 1, 2, 3, 4, 5, and 6 h after preparation. As shown in Table 2, the relative standard deviations (RSDs) of intra- and inter-day precision, repeatability, and stability were within the recommended value of 5%, implying that the developed method was valid and efficient for determining petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins in L. ruthenicum fruit.
Optimization of Extraction for Anthocyanins
Effects of Extraction Solvents on Anthocyanins
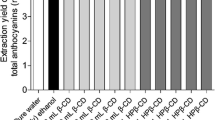
The effects of extraction solvent on the extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum fruit are shown in Fig. 2. The extraction yield of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside was higher with 2, 3, 4, and 1% β-CD solutions and 1% HPβ-CD solution than it was with 50% methanol. Water gave the lowest extraction yield (Fig. 2a). Additionally, β-CD solutions provided higher extraction yields of total anthocyanins than those with other extraction solvents, and the lowest extraction yield was obtained with water (Fig. 2b). Water with added cyclodextrins gave better extraction results than pure water for both petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum fruit. Cui et al. (2017) suggested that the hydrophobic cavity of the cyclodextrins provided a microenvironment for phenolic compounds to form inclusion complexes once they escaped the plant matter, thus increasing the concentration difference between plant tissue and water improving the dissolution rate. The extraction yield of L. ruthenicum anthocyanins initially increased and then decreased as the concentration of β-CD increased from 1 to 5% (Fig. 2). This observation is in good agreement with a previous study that found that the extraction yield of target compounds was not directly proportional to the β-CD concentration (Parmar et al. 2015). Additionally, Gao et al. (2016) suggested that a high concentration of cyclodextrins could decrease the extraction yield of target compounds due to its high viscosity. However, Rajha et al. (2015) reported that β-CD concentration had a linear positive effect on polyphenol extraction from vine shoots and suggested that the observations could arise from the fact that cyclodextrins formed inclusion complexes with hydrophobic polyphenols which increases their water solubility. Results that differed from ours were also reported by Cui et al. (2017). They found that the extraction yield of target compounds increased with increasing β-CD concentration, and they suggested that more β-CD-target compound complexes were formed with increasing β-CD concentration leading to greater amounts of target compounds being extracted with each increase in β-CD content.
In our study, 2% β-CD solution resulted in a higher extraction yield of L. ruthenicum anthocyanins than water (p < 0.05), 50% ethanol (p < 0.05), 70% ethanol (p > 0.05), 50% methanol (p > 0.05), 70% methanol (p > 0.05), and 1% HPβ-CD solution (p > 0.05). Previous reports suggested that cyclodextrin solutions are selective for the extraction of certain compounds (Mantegna et al. 2012; López-Miranda et al. 2016; Cui et al. 2017). As a result, the effects of cyclodextrin solutions on anthocyanin compositions were investigated using UPLC-DAD, and the representative chromatograms of anthocyanins extracted from L. ruthenicum using various extraction solutions are shown in Fig. S2. The results demonstrated that cyclodextrin solutions had little effect on the composition of the isolated anthocyanins. Therefore, β-CD solutions were selected for further response surface methodology optimization.
Fitting the Model for Predicting Anthocyanins
The ANOVA of response surface methodology is summarized in Table 3. The models were significant (p < 0.05), and the lack of fit of each response was insignificant (p > 0.05). The coefficients of determination (R2) of the models were more than 96% for both petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside (R2 = 0.9733) and the total anthocyanins (R2 = 0.9632), which indicated excellent fit between experimental and predicted values. In addition, the coefficients of variation were 2.64 and 2.45% for petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins, respectively. These results indicated that the two mathematical models (Eqs. (1) and (2)) were statistically acceptable and adequate to predict the extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins, respectively:
where Y1 is the extraction yield of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside, Y2 is the extraction yield of total anthocyanins, X1 is the extraction temperature, X2 is β-CD concentration, and X3 is extraction time.
Response Surface Optimization of Extraction for Anthocyanins
Table 3 shows the effects of extraction parameters on the extraction yield of anthocyanins. Figure 3 shows the three-dimensional response surface plots of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanin extraction, which are the graphical representations of the regression equations (Eq. (1) and (2)) and facilitated the analysis of the relationships between independent and dependent variables (Sang et al. 2017a). Extraction temperature had a significant effect (p < 0.0001) on the extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins (Table 3). As shown in Fig. 3, the extraction yield of anthocyanins initially increased with increasing extraction temperature and then decreased. Rising extraction temperature correlated to a higher extraction yield of anthocyanins; however, excessive extraction temperature accelerated anthocyanin degradation. Extraction time also had a significant impact on the extraction yield of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside (p = 0.0091) and total anthocyanins (p = 0.0154) from L. ruthenicum (Table 3). Increasing extraction time decreased the extraction yield of L. ruthenicum anthocyanins, especially at higher extraction temperatures (Fig. 3). When the β-CD concentration was increased from 1 to 3%, the extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins increased up to a maximum and then decreased; however, β-CD concentration was not a significant parameter (p > 0.05) (Table 3). The optimal extraction conditions for petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside (10.05 mg/g) were predicted to be as follows: extraction temperature of 49.39 °C, β-CD concentration of 1.70%, and extraction time of 30 min. In addition, the optimal extraction conditions for total anthocyanins (12.46 mg/g) were predicted to be as follows: extraction temperature of 49.85 °C, β-CD concentration of 1.6%, and extraction time of 30 min. To maximize the extraction yield of the anthocyanins from L. ruthenicum, a simultaneous optimization was performed using Design-Expert.V8.0.6.1 software. The predicted optimal extraction conditions of L. ruthenicum anthocyanins were an extraction temperature of 50 °C, a β-CD concentration of 1.65%, and an extraction time of 30 min; 10.11 ± 0.13 mg/g of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and 12.43 ± 0.27 mg/g of total anthocyanins were extracted from L. ruthenicum fruit.
Optimization of the Liquid/Solid Ratio
The effects of the liquid/solid ratio (10:1, 15:1, 20:1, and 25:1; mL/g) on the extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum were investigated. In this series of experiments, the other extraction parameters were fixed at an extraction temperature of 50 °C, a β-CD concentration of 1.65%, and an extraction time of 30 min. As shown in Fig. S3, the extraction yield of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins increased substantially with increasing liquid/solid ratio from 10:1 to 15:1 (p < 0.05) and then decreased slightly (p > 0.05). As a result, a 15:1 liquid/solid ratio was selected for β-CD-based extractions of anthocyanins from L. ruthenicum fruit. In the present study, the extraction yield of total anthocyanins from L. ruthenicum fruit was 12.43 mg/g under the optimal extraction conditions. As shown in Table S3, the extraction yield of total anthocyanins from L. ruthenicum fruit ranged from 2.15 to 88.74 mg/g in previous studies, which may be because of various sample origin, plucking time, and extraction considerations. According to the present results, β-CD solutions produced higher extraction yields of anthocyanins L. ruthenicum fruit than did traditional solvents (pure water and ethanol and methanol solutions) under the same extraction parameters. Therefore, β-CD-based extraction method has great potential for anthocyanin recovery.
Conclusions
In this study, a green approach for the extraction and determination of anthocyanins from L. ruthenicum fruit was developed using a β-CD-based extraction coupled with UPLC-DAD analysis. The effects of the extraction parameters on the extraction yields of petunidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside and total anthocyanins from L. ruthenicum fruit were investigated and optimized using single-factor experiments and response surface methodology. Extraction at 50 °C for 30 min using 1.65% β-CD solution and a liquid/solid ratio of 15:1 gave the optimal extraction yield of L. ruthenicum anthocyanins. A green, rapid, and reliable UPLC-DAD method was developed and validated to analyze L. ruthenicum anthocyanins using an ethanol-based mobile phase. The current β-CD-based UPLC-DAD method provides a green approach for the extraction and analysis of anthocyanins from plant materials.
References
Assassi AL, Roy C, Perovitch P, Auzerie J, Hamon T, Gaudin K (2015) Green analytical method development for statin analysis. J Chromatogr A 1380:104–111
Barnes JS, Nguyen HP, Shen S, Schug KA (2009) General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J Chromatogr A 1216:4728–4735
Chen S, Zeng Z, Hu N, Bai B, Wang H, Suo Y (2018) Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem 242:1–8
Cui L, Liu Y, Liu T, Yuan Y, Yue T, Cai R, Wang Z (2017) Extraction of epigallocatechin gallate and epicatechin Gallate from tea leaves using β-cyclodextrin. J Food Sci 82:394–400
Fattahi M, Rahimi R (2016) Optimization of extraction parameters of phenolic antioxidants from leaves of Capparis spinosa using response surface methodology. Food Anal Methods 9:2321–2334
Fenyvesi É, Vikmon M, Szente L (2016) Cyclodextrins in food technology and human nutrition: benefits and limitations. Crit Rev Food Sci Nutr 56:1981–2004
Gao F, Zhou T, Hu Y, Lan L, Heyden YV, Crommen J, Lu G, Fan G (2016) Cyclodextrin-based ultrasonic-assisted microwave extraction and HPLC-PDA-ESI-ITMSn separation and identification of hydrophilic and hydrophobic components of Polygonum cuspidatum: a green, rapid and effective process. Ind Crop Prod 80:59–69
Jin H, Liu Y, Guo Z, Yang F, Wang J, Li X, Peng X, Liang X (2015a) High-performance liquid chromatography separation of cis–trans anthocyanin isomers from wild Lycium ruthenicum Murr. employing a mixed-mode reversed-phase/strong anion-exchange stationary phase. J Agric Food Chem 63:500–508
Jin H, Liu Y, Yang F, Wang J, Fu D, Zhang X, Peng X, Liang X (2015b) Characterization of anthocyanins in wild Lycium ruthenicum Murray by HPLC-DAD/QTOF-MS/MS. Anal Methods 7:4947–4956
Khazaei KM, Jafari SM, Ghorbani M, Kakhki AH, Sarfarazi M (2016) Optimization of anthocyanin extraction from saffron petals with response surface methodology. Food Anal Methods 8:1993–2001
Korany MA, Mahgoub H, Haggag RS, Ragab MAA, Elmallah OA (2017) Green chemistry: analytical and chromatography. J Liq Chromatogr R T 40:839–852
Lin L, Li J, Lv H, Ma Y, Qian Y (2012) Effect of Lycium ruthenicum anthocyanins on atherosclerosis in mice. China J Chin Materia Medica 37:1460–1466
López-Miranda S, Serrano-Martínez A, Hernández-Sánchez P, Guardiola L, Pérez-Sánchez H, Fortea I, Gabaldón JA, Núñez-Delicado E (2016) Use of cyclodextrins to recover catechin and epicatechin from red grape pomace. Food Chem 203:379–385
Mantegna S, Binello A, Boffa L, Giorgis M, Cena C, Cravotto G (2012) A one-pot ultrasound-assisted water extraction/cyclodextrin encapsulation of resveratrol from Polygonum cuspidatum. Food Chem 130:746–750
Parmar I, Sharma S, Rupasinghe HPV (2015) Optimization of β-cyclodextrin-based flavonol extraction from apple pomace using response surface methodology. J Food Sci Technol 52:2202–2210
Płotka J, Tobiszewski M, Sulej AM, Kupska M, Górecki T, Namiesnik J (2013) Green chromatography. J Chromatogr A 1307:1–20
Rajha HN, Chacar S, Afif C, Vorobiev E, Louka N, Maroun RG (2015) β-Cyclodextrin-assisted extraction of polyphenols from vine shoot cultivars. J Agric Food Chem 63:3387–3393
Sang J, Dang K, Ma Q, Li B, Huang Y, Li C (2017a) Partition behaviors of different polar anthocyanins in aqueous two-phase systems and extraction of anthocyanins from Nitraria tangutorun Bobr. and Lycium ruthenicum Murr. Food Anal Methods. https://doi.org/10.1007/s12161-017-1071-3
Sang J, Sang J, Ma Q, Hou X, Li C (2017b) Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem 218:386–395
Sik B (2012) Development and validation of a green high performance liquid chromatographic method for the determination of some artificial sweeteners and caffeine in soft drinks. Food Anal Methods 5:1443–1452
Tang J, Yan Y, Ran L, Mi J, Sun Y, Lu L, Gao Y, Zeng X, Cao Y (2017) Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J Funct Foods 30:97–107
Wang T, Jiao J, Gai Q, Wang P, Guo N, Niu L, Fu Y (2017) Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J Pharmaceut Biomed Anal 145:339–345
Wu X, Li X, Jia S, Gao Z, Lu Z, Dai X, Sun Y (2017) The enhance memory and antioxidant activities of Lycium ruthenicum Murray anthocyanin extracts in a rat model of Aβ 42 induced dementia modern. Modern Food Sci Technol 33:1–6
Xia Y, Mo R, Qu W, Liu W (2015) Research progress in chemical constituents of Lycium ruthenicum Murr. Prog Pharm Sci 39:351–356
Zhang H, Liu Y, Wang M, Wang Y, Deng Y, Cui M, Ren X, Qi A (2015) One-pot β-cyclodextrin-assisted extraction of active ingredients from Xue–Zhi–Ning basing its encapsulated ability. Carbohyd Polym 132:437–443
Zhao X (2016) A review on bioactives from Lycium rethenicum murr. J Food Sci Biotechnol 35:561–568
Zheng J, Ding C, Wang L, Li G, Shi J, Li H, Wang H, Suo Y (2011) Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem 126:859–865
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yao Zhang declares that she has no conflict of interest. Fang-fang Chen declares that she has no conflict of interest. Jun Sang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Electronic Supplementary Material
ESM 1
(DOCX 144 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Chen, Ff. & Sang, J. Green Approach for Sample Preparation and Determination of Anthocyanins from Lycium ruthenicum Murr. Using a β-Cyclodextrin-Based Extraction Method Coupled with UPLC-DAD Analysis. Food Anal. Methods 11, 2141–2148 (2018). https://doi.org/10.1007/s12161-018-1191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1191-4