Abstract
There are several underutilized and wild fruit species in Ethiopia that can be used as sources of human food consumption. This study aimed to examine total phenolic contents (TPC), total flavonoid contents (TFC), proximate, mineral contents, and the in vitro antioxidant potentials of the spina-christi (Ethiopian Kunkura/Gava) fruit as compared with Indian jujube (Ber fruits — Umaran, Gola, and Mundia) fruits. According to this study, Kunkura, Umaran, Gola, and Mundia fruits are important sources of minerals (Mg, K, Ca, Na, P, Fe, Zn, Mn, Cu, and Se) than common cereals and grains. Soxhlet methanolic extraction at 45 °C for about 4 h was used to extract the compounds from fruits. A new statistical method approach, known as GraphPad Prism software, was used for the estimation and evaluations of fruit extracts in terms of EC50 values for the first time. The antioxidant activity was measured by the commonly accepted assays of 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)]-diammonium salt (ABTS) while the EC50 values were ranged between 2.94 (Mundia, the minimum value) and 15.48 (Gola, the maximum value) mg/mL. The EC50 estimations of DPPH, FRAP, and ABTS free radical scavenging activities of the fruit extracts were performed using GraphPad Prism statistical model. The total phenolic contents found were 311.26 ± 0.63 mg GAE/100 g extract for Gola (minimum) and 354.71 ± 18.59 mg GAE/100 g extract for Umaran (maximum). The maximum and the minimum flavonoid contents were 151.69 ± 2.68 mg QE/100 g for Umaran, and 124.76 ± 0.07 mg QE/100 g for Kunkura, respectively. Therefore, these nutritionally rich fruits can be the potential raw material for the food industries involved in manufacturing of value-added products like jam, jelly, juice, additives, and other similar products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wild edible plants are plant species that are neither cultivated nor domesticated, but exist in their natural environments and are used as sources of food and medicine (Beluhan and Ranogajec 2011; Lulekal et al. 2011). Padulosi et al. (2009) indicated underutilized plants as species whose potential has not been fully realized. From time immemorial, wild plants have been serving as an important source of food and income for the poor and malnourished children during famine and difficult times. They have made a substantial addition to the human diet, especially during times of war, failure, or other causes of food shortages. Recent findings showed that these wild edible plants are rich in chemical composition like protein, fat, dietary fiber, carbohydrate and energy (Serçe et al. 2010; Liu et al. 2012; Tee et al. 2014; Pereira et al. 2019), mineral composition and vitamins (FAO and WHO 2001; Bhat et al. 2010; Kuras et al. 2020; Sibiya et al. 2020), and bioactive compounds/phytonutrients (Delgado et al. 2014; Fu et al. 2015). Even if wild/underutilized edible fruits contribute significant amounts of nutrients and bioactive compounds, these are not utilized effectively in Ethiopia as well as in India.
Although Ethiopian rural communities use these wild/underutilized edible plants as a means of survival especially during times of drought, famine, and other difficult times (Duguma 2020; Lulekal et al. 2011), there is no adequate research on the nutritional composition and health benefits of the plants. The extent of use of these wild/underutilized edible plants varies from region to region in the country due to lack of awareness, ecological differences, and cultural differences. Such a difference continues because of the lack of research and nutritional value awareness among societies. For instance, Godere (Colocasia esculenta) (Azene 2017), Yams (Dioscorea spp.) (Mulualem et al. 2018), and Anchote (Coccinia abyssinica) are the best examples, which are limited to the southern and some parts of the Oromia region, Ethiopia (Parmar et al. 2017). At the same time, some of the wild/underutilized plants in Ethiopia are widely available on the food table in other countries. A typical example in this regard is Physalis peruviana (Hassanien 2011; Ramadan 2011; Ahmed 2014), also known as golden berry in English and “awut” in Amharic. The same is true for Kunkura (Z. spina-christi), Z. jujube/jujuba. Dokma (Syzygium guineense), and Tiringo/Citron (Citrus medica), which are consumable in limited areas of the country.
The Ziziphus jujube and Ziziphus spina-christi fruit species belong to the Rhamnaceae family. These plants grow mostly in East Africa, some parts of Asia (India, China), and Australia (Gao et al. 2011; Singh et al. 2012; Du et al. 2013). Ziziphus species have more than 170 sub-species (Abdoul-azize 2016) including spina-christi (Kunkura/Gava — local name) and jujube (Umaran, Gola, Mundia — local name in India) species, which are important for human medicine (Asgarpanah 2012), nutrition, sources of unique flavor, antimicrobial treatments, and antioxidants (Adam 2019). There are more than 90 different jujube, Z. mauritiana Lam. (Z. jujuba L.) cultivars in India, which are adapted to warm climates (Pareek 2013; Doijode 2020). In India, the jujube fruit species are also called Ber fruits. The report of Rashwan et al. (2020) showed that jujube fruits contain very high amounts of bioactive compounds and, therefore, the fruit is consumed traditionally as functional food staffs in their wild states. In Italy and China, the jujube pulp was used for the preparation of some food products, such as jams, beverages, pickles, and jelly. In addition to these, the Z. spina-christi and jujube plant species are drought resistant; fruits are available in excess amounts and can be good sources of food in countries where these plant species grow, including in India and Ethiopia. As a result, studying nutrient composition and bioactive compounds is critical to increasing food supplements.
According to Pérez-Burillo et al. (2018), fruits are foods that contain plenty of vitamins, minerals, and bioactive phytochemicals like polyphenols. Even if these fruit species are the best sources of these nutrients and bioactive compounds, there are no reported studies in Ethiopia on nutritional composition, bioactive compounds, antioxidant potentials, and mineral contents of spina-christi fruits. Therefore, it is important to characterize the chemical composition and to produce a typical food product or an input for a food product as an additive from such fruits. These fruits can be the important sources of the industry as the major inputs, since these fruits are nutritionally rich and the major sources of phytochemicals or as for one of the ingredients like, for instance, making jam, jelly, juice, additives, and others. These are also important in supporting the global food basket in minimally processed and processed food industries. Among many of processing industries, these fruits can be used for the development of functional or enriched foods or in nutraceuticals, pharmaceuticals, canned juice production, and cosmetics industries (Wang et al. 2018; Marmouzi et al. 2019).
Even though Z. spina-christi and Z. jujube fruit species have been in use for many years by herdsmen (cowherd or shepherd, goatherd) in tropical countries like in India and Ethiopia during drought or starvation, there is great dietary/nutrient and phytochemical composition importance of the fruits being as a staple food like other commonly consumed fruits. Every part of the Z. spina-christi and jujube plant (leaves, stems, root, and ash of the wood) is known for several medicinal applications (Shahat et al. 2001) and is a widespread traditional medicine, known also as jujube (having different species like Umaran, Golla, and Mundia) (Marmouzi et al. 2019). The Z. jujube/spina-christi plant species has been used as both an edible fruit and medicine for a long time in India as well as in China (Wang et al. 2018; Marmouzi et al. 2019). Therefore, to investigate the chemical compositions of the fruits, total phenolic contents (TPC), total flavonoid contents (TFC), proximate, mineral contents, and the in vitro antioxidant potentials of the fruits need to be assessed. The antioxidant activities of any extracts are evaluated effectively by effective concentrations (EC50) (Wu and Ng 2008). EC50 values have previously been used to evaluate drug discoveries, drug performance, effects of fungicides on fungi and oomycete pathogens, dose–response relationship study of stimulus toxicity, environmental toxicants, and other similar activities and analysis (Huber and Koella 1993; Ewijk Jh 1993; Jiang and Kopp-schneider 2014; Noel et al. 2018). Nowadays, the applications are extending to functional/nutraceutical foods to see the health effects of the food or food products on humans (Chen et al. 2013; Suriyatem et al. 2017; Sridhar and Charles 2019).
Therefore, the study aimed to investigate TPC, TFC, proximate, mineral contents, and the in vitro antioxidant potentials of the spina-christi (Ethiopian Kunkura/Gava) fruit as compared with Indian jujube (Ber fruits — Umaran, Gola and Mundia) fruits. At the same time the new statistical method approach, known as GraphPad prism software, was used for the estimation and evaluations of fruit extracts in terms of EC50 values.
Materials and Methods
Raw Material
Ziziphus spina-christi (locally known as Kunkura/Gava) was collected from North Wollo, Ethiopia, in November 2018 whereas the three Ziziphus jujube fruit species (locally known as Umaran, Gola, and Mundia) were collected from Haryana, India, in January 2018 in the morning sessions. The geographical coordinates of the specific study areas are Raya-Kobo, Ethiopia, located at longitude: 39°37′59.9916″E and latitude: 12°9′0″N and Sonipat, India, at longitude: 75°42′20.52″E and latitude: 29°9′1.26″N. All fruit samples were collected at the medium maturity stage based on their accessibilities or convenient sampling method (Etikan 2016). In jujube and spina-christi fruit species, there are three maturities (white maturity, half-red maturity, and full red maturity) stages, but the first two maturity stages are rich in phenolics (TPC, TFC, and total phenolic acids) and the latter are rich in sugars and anthocyanins (Wang et al. 2016). The unripened fruits were selected, sorted out, and removed from the rest (the ripened) of the fruits. All the collected fruit samples were stored in a clean perfect (zipped) polyethylene flexible plastic sealed container (without oxygen transfer/exchange) and transported to the laboratory. Then, in the laboratory, the fruits were washed with tap water followed by distilled water and stored in a fruit storage fresh zone room with their clean plastic bags. Throughout the whole study period, the room temperature was between 20 and 25 ℃.
Chemicals, Standards, and Equipment
All chemicals and reagents used in this study were of analytical grade and prepared by using high-purity deionized water obtained from a Milli-Q water purification system (Milli-Q Ultrapure Water SystemCorp., Bedford, USA). 2,2-diphenyl-1-picrylhydrazyl (DPPH, ≥ 97%), [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)]-diammonium salt (ABTS, ≥ 97%), 6-hydroxy-2, 5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, ≥ 97%), gallic acid (98%), 2,4, 6- tripyridylS-triazine (TPTZ), and quercetin (≥ 97%) were obtained from Sigma-Aldrich India. Microwave digestion (Milestone, Ethos Easy), Kjeldahl (Buchi India Pvt. Ltd.), Automated Distillation Apparatus (Buchi India Pvt. Ltd.), ICP-OES (Optima 7000 DV, Perkin Elmer, USA), and UV–VIS spectrophotometer (SHIMADZU, UV-2600, USA) were used for analysis.
Extraction and Preparation of Extract Solution
The collected Kunkura fruit samples were transported to Addis Ababa Science and Technology University (AASTU), Industrial Chemistry Laboratory. Then, the fruit samples were washed with tap water followed by distilled water, and the edible parts of the fruits were removed from the seeds, and the edible flesh parts were dried at room temperature without exposure to sunlight. The air-dried edible portions of Kunkura fruits were transported to the National Institute of Food Technology, Entrepreneurship, and Management (NIFTEM), Haryana, India. The Umaran, Gola, and Mundia fruit species were transported to NIFTEM and prepared similar to Kunkura fruit samples. After drying, each fruit sample was ground to powder, sieved with a 250-µm sieve, and stored in the refrigerator for further works.
The extraction for TPC, TFC, and antioxidant assay determination was performed according to the method stated by Alara et al. (2018) with some modifications. Accordingly, 10 g of the air-dried fruit samples was weighed on Whatman filter paper, rolled, stapled, and put into a Soxhlet extractor thimble. Using the optimized dried powder to methanol ratio (1:20 w/v), 200 mL methanol (boiling point 64.7 ℃) was transferred to the thimble to make it wet for fast extraction. Each of the powdered samples was extracted continuously for 4 h at 40 ℃ using a heating mantle. The extract solutions were allowed to cool to room temperature and dried using a rotary evaporator. The extracts were kept at 4 ℃ for further studies.
Proximate Analysis
Proximate composition determination was done according to AOAC official method (AOAC 2000), Chew et al. (2011), and Konczak and Roulle (2011). All proximate contents were measured in triplicate and method blanks were performed simultaneously in a similar manner with the samples. For moisture content (MC) determination, 5 g of fresh fruit sample was heated in a hot air oven at 105 ℃ for 4 h, cooled in desiccators, and weighted and the moisture content was calculated by the following formula (Eq. 1).
The ash content was determined by heating the samples dried for MC in a muffle furnace at 600 ℃ for 6 h and cooled in a desiccator and the weight of the ash was measured. Then, the ash content was calculated as follows (Eq. 2).
The crude fat content was determined by taking 1–5 g of fresh fruit samples and extracted with petroleum ether (boiling point 60–80 ℃) using the Soxhlet apparatus for 6 h. The weight of the flask plus the extracted residue was measured after drying using a rotary evaporator and cooling in desiccators. Then, the fat content was determined by subtracting the empty flask from the flask containing fat residue multiplied by 100 (Eq. 3).
where FC is the fat content, Fskresid is the flask containing fat residue, EFsk is the empty flask, and Wt of Sample is the weight of sample.
Crude protein was determined by Kjeldhal method by taking 1 g of the sample digested with 20 mL concentrated H2SO4 and Kjeldhal catalyst (4 parts of K2SO4 and one part of CuSO4) in a digestion block until it changes from greenish to clear and colorless solution. The blank control sample was digested parallel with the sample. After digestion, the clear sample solution was distilled in Kjeldhal distillation unit in the presence of 40% NaOH and 4% of boric acid in the receiver flask. The condensed ammonia was titrated against 0.2 N of H2SO4 after the addition of 3 drops of the mixture of methyl red and bromocrysol indicator. The concentration of nitrogen and protein were calculated as follows (Eq. 4):
where A is the volume (mL) of (0.2 N) H2SO4 used in sample titration, B is the volume (mL) of (0.1 N) HCl used in blank titration, and 14 is the atomic weight of nitrogen.
The content of protein was estimated by the formula: \({\varvec{p}}{\varvec{r}}{\varvec{o}}{\varvec{t}}{\varvec{e}}{\varvec{i}}{\varvec{n}}\left(\boldsymbol{\%}\right)=\boldsymbol{\%}{\varvec{N}}\times 6.25\), where 6.25 is the protein conversion factor.
Carbohydrate was calculated by difference from the formula: Carbohydrate (%) = 100 − [M.C. − Ash − Protein − Fat − dietary fiber] (Wisker and Feldheim 1990).
Mineral and Heavy Metal (Elemental) Contents
All glassware and polyethylene sample bottles were washed with 5 N nitric acid and de-mineralized water after soaking for 3 days with detergents. Mineral and heavy metal analyses were performed according to FDA 2010 and other related methods (Bhat et al. 2010; William 2010; Konczak and Roulle 2011; Karasakal 2021) for microwave digestion and ICP-OES elemental analysis. Samples were digested with concentrated nitric acid in a microwave digestion apparatus. In detail, 0.5 g of dried fruit sample was placed in TFM vessel in safety hood and the mixture of HNO3 and H2O2 (8:2) was transferred into the vessel. The mixture was digested at 280 ℃ and 850 Watt microwave power. The analysis was performed in triplicate for each sample. Method blanks were digested similarly with the sample. Limit of detection (LOD) and limit of quantitation (LOQ) were calculated to be LOD (for method — MDL) = \(\overline{X}\) + 3Sblank and LOQ = \(\overline{X}\) + 10Sblank, respectively, where Sblank is the standard deviations of the blank (n = 7) and 3 and 10 are the Student t-test values at 95% statistical confidence level (Miller and Miller 2010).
An ICP-OES was used for the measurement of Fe, Mn, Zn, P, Se, Cr, Cu, Na, K, Ca, Mg, and Pb. Standard calibration curves were prepared for all minerals and heavy minerals on the same day of analysis. This instrument was set with a shear gas system, adjustable and quick-change torch cassette, cyclonic Spray Chamber, and two high-performance solid-state detectors (SCD). The ICP OES operating conditions were as follows: purge gas flow rate was high, delay time was 19 s, plasma condition was varying depending on each element, the sample flow rate was 1.5 mL/min, and flush time was 5 s.
Determination of Total Phenolic, and Total Flavonoid Contents and the Free Radical Scavenging Potential
Total Phenolic Content
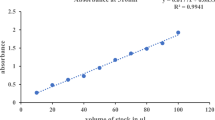
The TPC of the four fruit extracts was determined by UV–VIS spectrophotometer using Folin–Ciocalteu reagent method (Alara et al. 2018). Briefly, 1 mL of extract at a concentration of 1 g/L was mixed with 200 μL of Folin–Ciocalteu reagent, which was diluted with methanol (1:10 ratio, respectively). Then, after 5 min 0.6 mL of 0.2 mM Na2CO3 solution was thoroughly mixed with the prepared mixture. The mixture solution was left for 130 min and absorbance was measured at 765 nm using a UV–VIS spectrophotometer. Finally, the concentration of TPC in the fruit extract was calculated from the gallic acid standard calibration curve (ranging from 50 to 400 mg/L, mg GAE/g dw Eq. 5) with absorbance at 765 nm. The control samples were performed using methanol instead of sample extracts with the same treatments as a sample.
where C is the concentration obtained from calibration curve, V is the final volume, m is the mass of the fruit powder, and DF is the dilution factor.
Total Flavonoid Content (TFC)
For TFC determination, an aliquot of 400 μL of 1 g/L extract solution was thoroughly mixed with 400 μL of 2% AlCl3 solution and allowed to stand for 30 min in the dark at room temperature. The absorbance of the clear-blue solution was measured at 417 nm using a UV–VIS spectrophotometer. Then, the concentration of TFC in the extract was calculated from the standard calibration curve (mg QE/g dw, following Eq. 6) of quercetin ranging from 50 to 500 mg/L (Alara et al. 2018).
where C is the concentration obtained from the calibration curve, V is the final volume, m is the mass of the fruit powder, and DF is the dilution factor.
Scavenging Activity of 2,2-diphenyl-1-picrylhydrazyl Radical (DPPH)
In the presence of an antioxidant, which can donate an electron to DPPH, the purple color typical of free DPPH radical decays. The antioxidant scavenging activities of the fruit extracts were determined based on the scavenging effect on the stable DPPH free radical activity. Briefly, the DPPH stock solution was prepared by dissolving 1.9716 mg DPPH in methanol in a 50 mL brown volumetric flask and kept in the refrigerator till use. The absorbance was adjusted to 1.1 ± 0.05 by adding methanol or stock solution of DPPH in each day of measurement. In 15 mL amber vials, 2850 μL DPPH solution was added into 150 μL of each diluted fruit extract or trolox series standards (0.025 and 0.9 mM). After shaking properly, the mixture was kept in the dark at room temperature for 30 min and the absorbance was measured at 517 nm against a blank. Methanol was used as a method blank. The following equation (Eq. 7) was used to determine the percentage of the radical scavenging activity of each extract (Ilahy et al. 2011; Thaipong et al. 2006)).
where Ac is the absorbance of the control/blank and Af is the absorbance of the fruit extract.
Scavenging Effect of the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)]-diammonium salt (ABTS)
ABTS radical cation (ABTS•+) was generated by dissolving equal proportions of the reaction of a 7 mM ABTS (360.234 mg of ABTS in methanol in 100 mL volumetric flask) solution and 2.45 mM potassium persulfate (66.23 mg was dissolved with distilled water in 100 mL volumetric flask) solution. The mixture solution was stored in the dark at room temperature for 12 h before use. The ABTS•+ solution was diluted with methanol or ABTS•+ solution to adjust to an absorbance of 1.0 ± 0.02 at 734 nm. After the addition of 150 μL of fruit extract or trolox (standard) to 10 mL size amber vials, 2850 μL of ABTS•+ solution was added and the absorbance was measured at 734 nm after 4 min in the darkroom. The trolox standard calibration curve was constructed within 0.05–0.90 mM ranges and the percentage of the radical scavenging activity of each extract was calculated by the following equation (Eq. 8) (Thaipong et al. 2006).
where Ac is the absorbance of the control/blank and Af is the absorbance of the fruit extract.
Ferric Reducing Antioxidant Power Assay (FRAP)
The FRAP was performed using a method formerly described by Thaipong et al. (2006) with slight modifications. The fresh FRAP reagent was prepared in 500 mL brown bottles by mixing 250 mL of acetate buffer (300 mM pH 3.6), 50 mL of 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) (10 mM), and 50 mL of FeCl3.6H2O (50 mM). After the addition of 150 μL of fruit extract or trolox (standard) to 10 mL size amber vials, 2850 μL of FRAP solution was added. Then, the mixture solution was heated at 37 ℃ and allowed to react for a few minutes and the UV–VIS spectrophotometer measurement was performed at 593 nm. The trolox standard was used to draw a standard curve (0.025 and 0.7 mM) and is expressed as mM TEAC/g dry mass.
Effective Concentration (EC50)
The EC50 estimations of DPPH, FRAP, and ABTS free radical scavenging activities of the fruit extracts were done using GraphPad Prism statistical model. This statistical model was used following the first approach by Chen et al. (2013) and later by Sridhar and Charles (2019). The EC50 estimation was performed using non-linear Log (inhibitor) versus normalized response–variable slope curve fitting model. This model is estimating EC50 values within narrow ranges of maximum and minimum points using the following formula adopted from Chen et al. (2013) and later by Sridhar and Charles (2019) (Eqs. 9 and 10):
where Y is representing the response activities of fruit extracts against in vitro antioxidants, X is the concentrations of fruit extracts, and Hillslope is a parameter to describe the steepness of the curve.
where bottom asymptote is the baseline, top asymptote is the response (inhibition of the activity of fruit extracts), Hillslope is the parameter to describe the steepness of the curve, xb is the concentration of the sample at the inflection point, and S is the asymmetry of the curve.
Statistical Analysis
All experiments were performed in triplicate and the results were expressed as the means ± standard deviation (SD). The data were analyzed (one-way ANOVA, Tukey’s HSD, post hoc descriptive statistics) using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows version 26). All the graphs in this study were analyzed by Excel Windows 10, GraphPad Prism 8.0.2.263, and OriginPro. 9.0 32 and 64 Bit.
Results and Discussion
Proximate Analysis
The proximate compositions of all four fruit samples namely Kunkura, Umaran, Gola, and Mundia fruits are presented in Appendix Table 5. The moisture content was the lowest (81.77 ± 5.74%) in Mundia fruit while the maximum (88.22 ± 0.10%) was found in Umaran on a wet basis (based on the fresh fruits). The lowest fat content was found (0.81 ± 0.02%) in Kunkura fruit while the highest (8.84 ± 0.16%) was in Gola fruit among others. After drying at room temperature, the moisture contents of Kunkura, Umaran, Gola, and Mundia fruits were determined and found to be 9.47 ± 1.41, 10.03 ± 0.83, 9.79 ± 0.17, and 11.72 ± 0.056%, respectively. The maximum (targeted) moisture content of dry fruits to ensure safe storage for a long time is 20%; therefore, the obtained results have lower water activities (Afolabi 2014). These MCs (after drying at room temperature for 1 week) of all fruits were in line with the previously reported results from Oman (13.30 ± 0.16%) (Singh et al. 2012) and Morocco (13.96 ± 0.22%) (El Maaiden et al. 2020), but higher than the reported data from Gusau, Zanfara, Nigeria (3.13 ± 0.67%) (Abubakar 2021). However, the reported data from China (Li et al. 2007) jujube species (18.99 ± 1.23, 20.98 ± 1.12, 17.38 ± 1.21, 21.09 ± 1.39 and 22.52 ± 1.43%) were higher than the current moisture contents. The protein content was highest (1.32 ± 0.04%) in Kunkura fruit and lowest (0.55 ± 0.04%) in Umaran fruit as shown in Table 5. The carbohydrate and dry matter (DM) contents (calculated by differences) were ranged from 6.47 ± 3.63 to 10.65 ± 1.96% and 11.78 ± 0.10 to 18.89 ± 3.60%. The highest carbohydrate and DM contents were obtained from Kunkura fruit while lower contents were found in other fruit species. There were no significant differences between fruit types for DM and carbohydrate contents. The values found in this study are similar to the reported values in the literature for Kunkura fruit (Osman and Ahmed 2009) and jujube fruits (Pareek 2013). Generally, there are significant differences (α = 0.05) in all samples within and between groups in all parameters. There were no significant differences (p > 0.05) among Umaran, Gola, and Mundia for both MC and ash contents. There are significant differences between the mean values of MC, ash, carbohydrate, and dry contents in Kunkura fruit samples compared with other fruit samples (Table 5). In general, all analyzed fruits had similar or closer proximate values as compared with Chinese jujube cultivars reported by Li et al. 2007). The analysis results of this study indicated that these underutilized fruits are rich in nutrient compositions.
Mineral and Trace Elemental Content Analysis
Potassium (K) is the most abundant mineral in all fruits (Fig. 1). Kunkura fruit was the lowest in K (4151.78 ± 1.64 mg/kg), and Umaran fruit was the highest (4924.75 ± 3.05 mg/kg). Generally, the highest K content was detected in all fruit samples compared with the other elemental contents. There were significant differences (at p value ≤ 0.05) between each pair of all fruits for K. Potassium levels in all fruits exceed the maximum safe limit for processed food set by FAO/WHO (550–1400 mg/kg) (FAO and WHO 2004). All fruits have high amounts of Ca (1162.80 ± 2.51–2212.50 ± 0.43 mg/kg) (Fig. 1). The contents of Na (106.03 ± 0.05 mg/kg), P (1051.37 ± 2.28 mg/kg), and Pb (BDL — below detection limit) in Kunkura fruits were lower than the reference standard limits set by WHO/FAO (Fig. 1). This study revealed that wild/underutilized edible fruits, commonly used since ancient times, can serve as a good alternative to improve the daily human intake of minerals. Similar high results were reported in 11 selected Southern Africa wild edible fruits (6902–14,271 mg/kg) by Sibiya et al. (2020).
Fe and Zn contents in all fruits were found to be high and sufficient for human nutrient balances (FAO and WHO 2004). The content of Zn (27.19 ± 0.07 mg/kg) in Umaran was much higher than the other fruit species. All the fruit species are good sources of functional and antioxidant mineral Zn for human consumptions. According to WHO/FAO report, Zn has a very important function in the protection of body tissues against oxidative stress and is helpful for antioxidant purposes in the human body. Mn (41.38 ± 0.25 mg/kg) content in Umaran fruit sample is much higher than the other Ethiopian and Indian fruit species (4.70 ± 0.05–13.39 ± 1.10 mg/kg). The levels of Cu, Pb, and Cr in all fruits were found to be above the safe limits of WHO/FAO/European Commissions; especially in Mundia fruit, Cr (17.73 ± 0.61 mg/kg) content was significantly high (Fig. 1). As a result, according to the current study, these minerals (Cu, Pb, and Cr) may have harmful effects on the consumers at these sampling points. Generally, Cr content was the lowest in Gola fruit followed by Kunkura fruit. Cu was the lowest in both Gola and Mundia fruits, but the highest in Kunkura and Umaran fruits.
Se content in Kunkura fruit was found to be 0.33 ± 0.04 mg/kg, dry weight basis, which is lower in comparison with world standards set by WHO/FAO. But Se was not detected in all other fruit species (Umaran, Gola, and Mundia). According to WHO/FAO report, selenium has a very important function in the protection of body tissues against oxidative stress, maintenance of defenses against infection, and modulation of growth and development (FAO and WHO 2004).
The method validation and quality control were performed using reference standards and blank parallel with the sample. The method was validated and the recoveries of the elements were within the accepted ranges (89 − 107%). The relative percent differences of matrix spike and matrix spike-dup were below 7.3% indicating that the method used was fairly precise.
Significant differences (p > 0.05) were not observed between Gola and Mundia for Na and Cu contents and between Gola and Kunkura for Cr content. However, all other elements significantly vary among all fruits. Additionally, there were significant differences (p ≤ 0.05) between groups and within groups for all fruit species (Fig. 1).
Estimated EDI of Minerals and Heavy Minerals
The estimated daily intake (EDI) of twelve minerals (Pb, Cr, Mn, Cu, Fe, Zn, Se, Ca, Mg, P, Na, and K) was calculated (Rahman et al. 2020) according to the mean concentration of each metal in each fruit and the respective consumption rates (Table 1). The EDI and maximum tolerable daily intake (MTDI, not reported by WHO/FAO for all minerals) of the studied minerals from consumption of fruits are shown in Table 1. Total daily intakes of some of the elements, which have the available MTDI from WHO/FAO in mg/day, are below the set values for all fruits. In all fruits, mean values of EDI decreased in the following order with the same fashion: K > Ca > P > Mg > Na > Fe > Mn > Zn > Cu > Cr ≈ Pb > Se (Table 1).
Total Phenolic, and Total Flavonoid Contents and In Vitro Antioxidant Analysis
The TPC results varied from 311.26 ± 0.63 mg GAE/100 g extract of Gola fruit, 332.42 ± 3.76 mg/100 g (Mundia), 344.84 ± 9.25 mg/100 g (Kunkura) to 354.71 ± 18.59 mg GAE/100 g extract of Umaran fruit (Table 2). The fruits had the highest contents of TFC in Umaran fruit (151.69 ± 2.68 mg QE/100 g), and the lowest was in Kunkura fruit (124.76 ± 0.07 mg QE/100 g). The TPC on the methanolic extracts of four fruits were found in the increasing order of Gola > Mundia > Umaran > Kunkura, whereas the contents of TFC were in the order of Kunkura > Gola > Umaran > Mundia (Table 2). These variations may be due to Indian and Ethiopian geographical differences in general and climate differences such as attitude, soil type, and climate of the specific sampling areas in particular (Arruda et al. 2017). In the case of TPC, there were no statistically significant differences between Umaran and Kunkura, and Gola and Mundia, but there were significant differences between Umaran and Gola, Umaran and Mundia, Kunkura and Gola, and Kunkura and Mundia. Similarly, there were significant differences between Umaran and Gola, Umaran and Mundia, Umaran and Kunkura, Gola and Mundia, and Gola and Kunkura for TFC tests. In another way, between Mundia and Kunkura was not significantly varied.
The FRAP values were 279.95 ± 31.77 (Gola), 518.45 ± 31.57 (Mundia), 679.54 ± 2.71 (Umaran), and 760.44 ± 31.24 (Kunkura) mM trolox equivalent for antioxidant capacity (TEAC)/100 g dw in methanolic extracts at maximum inhibition (> 90% RSA, Table 2). The antioxidant capacity evaluated by ABTS radical scavenging assay were 129.09 ± 1.27 (Mundia), 151.77 ± 2.8 (Umaran), 151.77 ± 1.26 (Gola), and 153.85 ± 2.54 mM TEAC/100 g dw (Kunkura). In the DPPH assay, the antioxidant capacity were 109.59 ± 5.21 (Mundia), 113.82 ± 1.81 (Umaran), 119.53 ± 4.47 (Gola), and 133.89 ± 3.86 (Kunkura) mM TEAC/100 g dw. The findings of this study were higher than the report of Pareek (2013) (34.2–117.3 mmol/100 g), Li et al. (2007) (34.2 ± 45.8–117.3 ± 112 mmol/100 g), and Wang et al. (2016) (0.1–4.5 mmol/100 g) of Chinese jujube fruit.
The statistical significant tests (p ≤ 0.05) were performed among fruit samples and types of assays used (Table 2). There were statistically significant differences at p ≤ 0.05 value between Umaran and Kunkura, Gola and Kunkura, and Mundia and Kunkura, but there were no significant differences between Umaran and Gola, and Umaran and Mundia in DPPH assay. In ABTS assay, there were significant differences (p ≤ 0.05) between Umaran and Mundia, Gola and Mundia, and Mundia and Kunkura (p > 0.05), whereas there were no significant differences observed between Umaran and Gola, Umaran and Kunkura, and Gola and Kunkura. There were significant differences between Umaran and Gola, Umaran and Mundia, Gola in FRAP assay. However, there were no significant differences between Umaran and Kunkura, and between Mundia and Kunkura for FRAP assay.
All of the fruit extracts were found to be capable of scavenging DPPH, ABTS, and FRAP radicals in a concentration-dependent manner, which shows that all of the fruit extracts have strong scavenging activity with respect to TEAC (Table 2). A similar study has been reported by Gao et al. (2011) in which DPPH (3794.5 ± 428.9 mmol TEAC/100 g much higher than this study values), ABTS (1.7 ± 0.3 mmol TEAC/100 g — which is much lower than this study values), and FRAP (626.0 ± 92.0 mmol TEAC/100 g — higher than this study values) assays have been assessed. In addition, the Oman spina-christi fruits were evaluated against these antioxidant potentials at a single point evaluation and the inhibition was from 87 to 98%. These study values were investigated (averagely from 5 to 99%) at different levels of fruit (at least 7 levels of concentration) and standard concentrations (9 points calibration). Not much comparison was made on these species, especially on Kunkura fruit, due to the lack of reported data in Ethiopia. According to these experimental results and previously reported data (Gao et al. 2011), jujube and spina-Christi fruit species are generally recognized as an outstanding source of biologically active compounds related to both nutritional and functional/nutraceutical values.
Effective Concentration (EC50)
The estimation of EC50 values of DPPH, ABTS, and FRAP radical scavenging activities of four fruit methanolic extracts was calculated using GraphPad Prism software model and is presented in Table 3. The Soxhlet methanol extract of all fruits had the highest inhibitory activity against DPPH, ABTS, and FRAP radicals as compared with EC50 estimation values of TEAC (Table 3). The tests were performed using 8 points of extract concentrations by decreasing the concentrations of each fruit extract (40, 20, 15, 8, 4, 0.8, 0.4, 0.2 mg/g or mg/mL of extracts of each fruit). The scavenging activities of fruit extracts against EC50 values of DPPH were found to be 2.94 mg/mL (Mundia), 3.09 mg/mL (Umaran), 3.56 mg/mL (Gola), and 3.68 mg/mL (Umaran) with a decreasing order. The EC50 values of fruits against ABTS were found to be 3.26 mg/mL (Mundia), 5.71 mg/mL (Umaran), 6.07 mg/mL (Kunkura), and 7.75 mg/mL (Gola). In the case of FRAP test, the EC50 values of fruits were found to be 11.89 mg/mL (Umaran), 13.28 mg/mL (Mundia), 15.33 mg/mL (Kunkura), and 15.48 mg/mL (Gola) with a decreasing order. The highest EC50 values were recorded against DPPH scavenging activities as compared with ABTS and FRAP methods (Table 3).
The lowest EC50 values (Table 3) revealed that higher antioxidant activities were observed in all fruits, which were an indication of the potential of the fruits for healthy consumption. There were significant differences among all fruits for both DPPH and FRAP antioxidant tests whereas in the case of ABTS test, there were significant differences among Umaran, Gola, and Mundia as well as among Kunkura, Gola, and Mundia. But there was no significant difference between Umaran and Kunkura fruits for ABTS test. There is no similar study and comparison for these fruit extracts in the case of antioxidant EC50 values as far as our knowledge is concerned. There was also no similar study and comparison for these fruit extracts in the case of antioxidant %RSA values with different concentrations.
The designed model proposed by Chen et al. (2013) and Sridhar and Charles (2019) to estimate EC50 values of bioactive compounds extracted from fruits (Fig. 2) was effective in this experiment. As shown in Fig. 2, EC50 were evaluated from multiple experiments (7–9 points) and which was a simple and robust method to estimate the effective concentrations of fruit antioxidant compounds. From normalized sigmoid curve (Fig. 2) from the concentration–response curve of fruit extracts, the fruit extracts showed strong association between concentration and percentage inhibition against DPPH, ABTS, and FRAP radicals. The obtained normalized sigmoid curve (Fig. 2) for all fruit samples was similar to the reported sigmoid curve by Sridhar and Charles (2019) for Kyoho grape extracts in DPPH and ABTS assays.
In general, the optimum values of proximate, mineral, high TPC, TFC, and antioxidant contents of the fruits can be supported by some reported studies such as by Shahat et al. (2001) and Marmouzi et al. (2019) for the whole plant parts and Wang et al. (2018) and Marmouzi et al. (2019) for the fruits. The fruits also showed unique flavor and high nutritional value, high mineral contents, and high antioxidant capacity (Wang et al. 2016; Shi et al. 2018; El Maaiden et al. 2020). High amounts of major bioactive compounds such as flavonoids, alkaloids, saponins, and terpenoids, for instance, contents can be from 297 to 4078 mg/100 g (Abdoul-azize 2016). That is why there were maximum chemical contents, antioxidant potentials, and elemental (mineral) contents recorded in these four studied fruits. The present study had shown that the fruit species of Ziziphus (Z. spina-christi and Z. jujube fruit species) are sources of important phytochemicals, antioxidants, and minerals. The high mineral contents and antioxidant activities in the fruits suggest that they can be a good source of minerals and bioactive compounds and can be used as functional, health-enhancing foodstuffs, particularly for the prevention of diseases as supported by a previous study on Chinese jujuba/jujube (Chen et al. 2017). Further characterizations of the fruits, food product developments, and the quantifications of individual phenolic, flavonoids, organic acids, anthocyanins, pectin content, and the other components fractionation and further identification/analysis should be performed.
Principal Component Analysis
Principal component analysis (PCA) is one of the most commonly used chemometric methods of multivariate analysis technique for the discriminations and differentiation of groups of samples or species (Nowicka et al. 2019). The PCA was run for the minerals and antioxidants of four Ziziphus fruit species to look at the main data structures and association or variances among Umaran, Gola, Mundia, and Kunkura plant species, and the relationship between data and samples. In this study, PCA was run for the data obtained from fruits with different geographical origins after quantitative analysis of minerals using ICP-OES and antioxidants using a UV–VIS spectrophotometer. The PCA was performed using Virmax with Kaiser normalization–rotation and rotated component matrix methods with eigenvalues > 1 (KMO) (Table 4, Fig. 3).
Principal component analysis of minerals and antioxidants (TPC, TFC, DPPH, ABTS, and FRAP assays) for the species of Ziziphus (Umaran, Gola, Mundia, and Kunkura); association or variation of the four plant species. Those PCAs with eigenvalues greater than one were selected for the given data, and correlations between the plant fruits were calculated using statistical package v26 between
Two PCAs’ results divided the analyzed fruits into three distinct clusters (PC1, PC2, and PC3) explaining the associations and variances of minerals, antioxidants, and fruits. The first PC (PC1) of minerals explains 58.24% of the overall variations. The second (PC2) and the third (PC3) explain 27.61% and 14.15% variations among the studied minerals, respectively. The cumulative variance of PC1 and PC2 explained 84.24% of the overall variances of the fruits. That means the PC1 is clearly identified with Umaran (PC1), Gola and Mundia (PC2), and Kunkura (PC3) for fruit species and Na, K, Ca, Mg, P, Fe, Zn, and Pb in one cluster (PC1), Se in the second cluster (PC2), and Cr the third cluster (PC3). The factors that most contributed (strong positive association) to PC1, PC2, and PC3 (in the positive sides) were Na, Mg, Mn, Fe, P, Zn, K, Cu, and Ca (in the decreasing order), Pb, and Cr, respectively. Apart from this, on the negative side that the most contributed factor is Se, but there are no strong negative factors contributed to PC1 and PC3. Therefore, PC1 is the major contributor to the associations or variances of fruits in this study. On the other hand, the first PC (PC1) of antioxidants explains 45.56% of the overall variations. The second (PC2) and the third (PC3) explain 30.04% and 24.41% variations among the studied antioxidants, respectively. The cumulative variance of PC1 and PC2 explained 75.59% of the overall variances of the fruits. According to these analyses, the main data structure for the associations and variations of Umaran, Gola, Mundia, and Kunkura fruits is PCA of minerals. The PCA results (Fig. 3, Table 4) also showed that minerals and antioxidants have significant contributions to the strong associations of Ethiopian and Indian Ziziphus fruit species. Accordingly, there was a significant correlation between fruit species and their antioxidant and mineral contents. Therefore, it is clearly shown that the common feature for the Umaran, Gola, Mundia, and Kunkura fruits was the high contents of minerals and antioxidants. In addition, the PCA model indicated that the varieties of these fruits could be characterized by minerals and antioxidants and it was possible to discriminate the different Ziziphus species according to the geographical origin of the plants.
Conclusion
The wild edible spina-christi and jujube fruits are rich sources of various minerals, some nutrients, and antioxidant bioactive compounds. The fruits have significant antioxidant activities against various in vitro antioxidant scavenging activities. The GraphPad Prism statistical model was effective for the estimations of EC50 values for all fruits. Moreover, the studied fruit species can be used as an easily accessible source of natural antioxidants. The consumption of these fruits may also play a vital role in fulfilling nutritional and mineral requirements and preventing various diseases, and aging. But further individual bioactive components studies are required to confirm if these fruit species can be used as functional/nutraceutical food supplements.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdoul-azize S (2016) Potential Benefits of jujube (Zizyphus Lotus L.) Bioactive compounds for nutrition and health. J Nutr Metab 2016:1–13. https://doi.org/10.1155/2016/2867470
Abubakar YS (2021) Proximate and selected mineral elements analysis of Nigerian Ziziphus spina-christi (L.) willd edible fruit pulp. Earthline J Chem Sci 5(1):231–240. https://doi.org/10.34198/ejcs.5121.231240
Adam IAAF (2019) Wild fruits: Composition, nutritional value and products. Springer, First
Afolabi IS (2014) Moisture migration and bulk nutrients interaction in a drying food systems: a review. Food Nutr Sci 05:692–714. https://doi.org/10.4236/fns.2014.58080
Ahmed LA (2014) Renoprotective effect of Egyptian cape gooseberry fruit (Physalis peruviana L.) against acute renal injury in rats. Sci World J 2014:1–7. https://doi.org/10.1155/2014/273870
Alara OR, Abdurahman NH, Ukaegbu CI (2018) Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J Appl Res Med Aromat Plants 11:12–17. https://doi.org/10.1016/j.jarmap.2018.07.003
AOAC (2000) Association of Official Analytical Chemists. In: Analytical Methods. pp 94–100
Arruda HS, Pereira GA, Pastore GM (2017) Optimization of extraction parameters of total phenolics from Annona crassiflora Mart. (Araticum) fruits using response surface methodology. Food Anal Methods 10:100–110. https://doi.org/10.1007/s12161-016-0554-y
Asgarpanah J (2012) Phytochemistry and pharmacologic properties of Ziziphus spina-christi (L.) Willd. African J Pharm Pharmacol 6:2332–2339. https://doi.org/10.5897/ajpp12.509
Azene H (2017) Nutritional composition and effects of cultural processing on anti-nutritional factors and mineral bioavailability of Colocasia esculenta (Godere) grown in Wolaita Zone, Ethiopia. J Food Nutr Sci 5:147. https://doi.org/10.11648/j.jfns.20170504.12
Beluhan S, Ranogajec A (2011) Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem 124:1076–1082. https://doi.org/10.1016/j.foodchem.2010.07.081
Bhat R, Kiran K, Arun AB, Karim AA (2010) Determination of mineral composition and heavy metal content of some nutraceutically valued plant products. Food Anal Methods 3:181–187. https://doi.org/10.1007/s12161-009-9107-y
Chen Z, Bertin R, Froldi G (2013) EC50 estimation of antioxidant activity in DPPH Å assay using several statistical programs. Food Chem 138:414–420. https://doi.org/10.1016/j.foodchem.2012.11.001
Chen J, Liu X, Li Z et al (2017) A review of dietary Ziziphus jujuba Fruit (jujube): developing health food supplements for brain protection. Evidence-Based Complement Altern Med 2017:1–10. https://doi.org/10.1155/2017/3019568
Chew LY, Prasad KN, Amin I et al (2011) Nutritional composition and antioxidant properties of Canarium odontophyllum Miq. (dabai) fruits. J Food Compos Anal 24:670–677. https://doi.org/10.1016/j.jfca.2011.01.006
Delgado T, Marinero P, Asensio-S-Manzanera MC et al (2014) Antioxidant activity of twenty wild Spanish Thymus mastichina L. populations and its relation with their chemical composition. LWT - Food Sci Technol 57:412–418. https://doi.org/10.1016/j.lwt.2013.12.041
Doijode S d. (2020) Indian jujube: Ziziphus mauritiana Lam. Seed Storage Hortic. Crop. 135–138
Du L, Gao Q, Ji X et al (2013) Comparison of flavonoids, phenolic acids, and antioxidant activity of explosion-puffed and sun-dried jujubes (Ziziphus jujuba Mill.). J Agric Food Chem 61:11840–11847. https://doi.org/10.1021/jf401744c
Duguma HT (2020) Wild edible plant nutritional contribution and consumer perception in ethiopia. Int J Food Sci 2020:1–16. https://doi.org/10.1155/2020/2958623
El Maaiden E, El Kharrassi Y, Lamaoui M et al (2020) Variation in minerals, polyphenolics and antioxidant activity of pulp, seed and almond of different ziziphus species grown in Morocco. Brazilian J Food Technol 23:1–7. https://doi.org/10.1590/1981-6723.20619
Etikan I (2016) Comparison of convenience sampling and purposive sampling. Am J Theor Appl Sta 5:1. https://doi.org/10.11648/j.ajtas.20160501.11
FAO, WHO (2001) Human vitamin and mineral requirements. Hum. Vitam. Miner. Requir. 303
FAO, WHO (2004) Vitamin and mineral requirements in human nutrition, 2nd editio
FDA (2010) Food and drug administration, elemental analysis manual for food and related products, version 1, pp 1–25
Fu Y, Zhou X, Chen S et al (2015) Chemical composition and antioxidant activity of Chinese wild raspberry (Rubus hirsutus Thunb.). LWT - Food Sci Technol 60:1262–1268. https://doi.org/10.1016/j.lwt.2014.09.002
Gao QH, Te WuP, Liu JR et al (2011) Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci Hortic (amsterdam) 130:67–72. https://doi.org/10.1016/j.scienta.2011.06.005
Hassanien MFR (2011) Physalis peruviana: a rich source of bioactive phytochemicals for functional foods and pharmaceuticals. Food Rev Int 27:259–273. https://doi.org/10.1080/87559129.2011.563391
Huber W, Koella JC (1993) A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55:257–261. https://doi.org/10.1016/0001-706X(93)90083-N
Ilahy R, Hdider C, Lenucci MS et al (2011) Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J Food Compos Anal 24:588–595. https://doi.org/10.1016/j.jfca.2010.11.003
Jiang X, Kopp-schneider A (2014) Summarizing EC50 estimates from multiple dose-response experiments: A comparison of a meta-analysis strategy to a mixed-effects model approach. Biometrical J 56:493–512. https://doi.org/10.1002/bimj.201300123
Karasakal A (2021) Determination of major, minor, and toxic elements in tropical fruits by ICP-OES after different microwave acid digestion methods. Food Anal Methods 14:344–360. https://doi.org/10.1007/s12161-020-01884-3
Konczak I, Roulle P (2011) Nutritional properties of commercially grown native Australian fruits: lipophilic antioxidants and minerals. Food Res Int 44:2339–2344. https://doi.org/10.1016/j.foodres.2011.02.023
Kuras MJ, Zielińska-Pisklak M, Duszyńska J, Jabłońska J (2020) Determination of the elemental composition and antioxidant properties of dates (Phoenix dactyliferia) originated from different regions. J Food Sci Technol 57:2828–2839. https://doi.org/10.1007/s13197-020-04314-8
Li JW, Fan LP, Ding SD, Ding XL (2007) Nutritional composition of five cultivars of Chinese jujube. Food Chem 103:454–460. https://doi.org/10.1016/j.foodchem.2006.08.016
Liu YT, Sun J, Luo ZY et al (2012) Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem Toxicol 50:1238–1244. https://doi.org/10.1016/j.fct.2012.01.023
Lulekal E, Asfaw Z, Kelbessa E, Damme P (2011) Wild edible plants in Ethiopia: a review on their potential to combat food insecurity. Afrika Focus 24:71–121. https://doi.org/10.21825/af.v24i2.4998
Marmouzi I, Kharbach M, El M et al (2019) Industrial crops & products antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind Crop Prod 132:134–139. https://doi.org/10.1016/j.indcrop.2019.02.007
Miller JN, Miller JC (2010) Statistics and chemometrics for analytical chemistry, 6th edn. Pearson
Mulualem T, Mekbib F, Hussein S, Gebre E (2018) Analysis of biochemical composition of yams (Dioscorea spp.) landraces from southwest Ethiopia. Agrotechnology 07:1–8. https://doi.org/10.4172/2168-9881.1000177
Noel ZA, Wang J, Chilvers MI, Sciences M (2018) Significant influence of EC 50 estimation by model choice and EC 50 type. Plant Dis 102:708–714. https://doi.org/10.1094/PDIS-06-17-0873-SR
Nowicka P, Wojdyło A, Laskowski P (2019) Principal component analysis (PCA) of physicochemical compounds’ content in different cultivars of peach fruits, including qualification and quantification of sugars and organic acids by HPLC. Eur Food Res Technol 245:929–938. https://doi.org/10.1007/s00217-019-03233-z
Osman MA, Ahmed MA (2009) Chemical and proximate composition of (Zizyphus spina‐christi) nabag fruit. Nutr Food Sci 39:70–75. https://doi.org/10.1108/00346650910930842
Padulosi S, Hodgkin T, Williams JT, Haq N (2009) Underutilized crops: trends, challenges and opportunities in the 21st century. In: Managing plant genetic diversity. Proceedings of an international conference, Kuala Lumpur, Malaysia, 12–16 June 2000
Pareek S (2013) Nutritional composition of jujube fruit. Emirates J Food Agric 25:463–470. https://doi.org/10.9755/ejfa.v25i6.15552
Parmar A, Gebre B, Legesse A et al (2017) Nutritional comparison of white and red Coccinia abyssinica (Lam.) Cong. Accessions: an under-utilised edible tuber of the Ethiopian highlands. Foods 6:71. https://doi.org/10.3390/foods6080071
Pereira APA, Angolini CFF, Paulino BN et al (2019) A comprehensive characterization of Solanum lycocarpum St. Hill and Solanum oocarpum Sendtn: Chemical composition and antioxidant properties. Food Res Int 124:61–69. https://doi.org/10.1016/j.foodres.2018.09.054
Pérez-Burillo S, Oliveras MJ, Quesada J et al (2018) Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res Int 105:461–472. https://doi.org/10.1016/j.foodres.2017.11.022
Rahman M, Islam MA, Zaved MM (2020) Assessment of essential and potentially toxic elements and possible health risks in Hylocereus undatus and Punica granatum. Biol Trace Elem Res 197:1–7. https://doi.org/10.1007/s12011-020-02072-4
Ramadan MF (2011) Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): an overview. Food Res Int 44:1830–1836. https://doi.org/10.1016/j.foodres.2010.12.042
Rashwan AK, Karim N, Shishir MRI et al (2020) jujube fruit: a potential nutritious fruit for the development of functional food products. J Funct Foods 75:104205. https://doi.org/10.1016/j.jff.2020.104205
Serçe S, Özgen M, Torun AA, Ercişli S (2010) Chemical composition, antioxidant activities and total phenolic content of Arbutus andrachne L. (Fam. Ericaceae) (the Greek strawberry tree) fruits from Turkey. J Food Compos Anal 23:619–623. https://doi.org/10.1016/j.jfca.2009.12.007
Shahat AA, Pieters L, Apers S et al (2001) Chemical and biological investigations on Zizyphus spina-christi L. Phyther Res 15:593–597. https://doi.org/10.1002/ptr.883
Shi Q, Zhang Z, Su J, Zhou J, Li X (2018) Comparative analysis of pigments, phenolics, and antioxidant activity of Chinese jujube ( Ziziphus jujuba Mill.) during fruit development. Molecules 23:1–14. https://doi.org/10.3390/molecules23081917
Sibiya NP, Kayitesi E, Moteetee A (2020) Mineral composition of selected indigenous wild southern African fruits. South African J Bot 132:87–94. https://doi.org/10.1016/j.sajb.2020.04.014
Singh V, Guizani N, Essa MM et al (2012) In vitro antioxidant activities of Ziziphus spina-christi Fruits (red date) grown in Oman. Biotechnology 11:209–216. https://doi.org/10.3923/biotech.2012.209.216
Sridhar K, Charles AL (2019) In vitro antioxidant activity of Kyoho grape extracts in DPPH % and ABTS % assays: estimation methods for EC50 using advanced statistical programs. Food Chem 275:41–49. https://doi.org/10.1016/j.foodchem.2018.09.040
Suriyatem R, Auras RA, Intipunya P, Rachtanapun P (2017) Predictive mathematical modeling for EC50 calculation of antioxidant activity and antibacterial ability of Thai bee products. J Appl Pharm Sci 7:122–133. https://doi.org/10.7324/JAPS.2017.70917
Tee LH, Yang B, Nagendra KP et al (2014) Nutritional compositions and bioactivities of Dacryodes species: A review. Food Chem 165:247–255. https://doi.org/10.1016/j.foodchem.2014.05.084
Thaipong K, Boonprakob U, Crosby K et al (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Van EwijkJh P (1993) Calculation of the EC50 and its confidence interval when subtoxic stimulus is present. Ecotoxicol Environ Saf 25:25–32. https://doi.org/10.1006/eesa.1993.1003
Wang B, Huang Q, Venkitasamy C et al (2016) Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT 66:56–62. https://doi.org/10.1016/j.lwt.2015.10.005
Wang L, Fu H, Wang W et al (2018) Analysis of reducing sugars, organic acids and minerals in 15 cultivars of jujube (Ziziphus jujuba mill.) fruits in China. J Food Compos Anal 73:10–16. https://doi.org/10.1016/j.jfca.2018.07.008
William, Mindak Scott D (2010) Elemental analysis manual for food and related products
Wisker E, Feldheim W (1990) Metabolizable energy of diets low or high in dietary fiber from fruits and vegetables when consumed by humans. J Nutr 120:1331–1337. https://doi.org/10.1093/jn/120.11.1331
Wu SJ, Ng LT (2008) Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT - Food Sci Technol 41:323–330. https://doi.org/10.1016/j.lwt.2007.03.003
Acknowledgements
The authors would like to acknowledge the administration and staff of the National Institute of Food Technology Entrepreneurship and Management (NIFTEM), India, for their continuous support and follow-up during this laboratory work. We also want to give thanks to Addis Ababa Science and Technology University (AASTU) for this collaboration and support.
Funding
This research work was funded by Department of Science and Technology, Government of India, under an Indo-Ethiopian bilateral project (Grant number: DST/INT/Ethiopia/Biotechnology/2017). The authors are thankful for the financial support provided by Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This research did not require ethical approval.
Consent to Participate
Informed consent not applicable.
Conflict of Interest
Tilahun Belayneh declares that he has no conflict of interest. Sosina Gebremichael declares that she has no conflict of interest. Ajay Chinchkar declares that he has no conflict of interest. Tarekegn Berhanu declares that he has no conflict of interest. Anurag Singh declares that he has no conflict of interest. Ashutosh Upadhyay declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Belayneh, T., Gebremichael, S., Chinchkar, A.V. et al. Comparative Study on Chemical Composition and Antioxidant Properties (GraphPad Prism Approach) of Wild Ethiopian Z. spina-christi and Indian Z. jujube Fruit Species. Food Anal. Methods 15, 2224–2237 (2022). https://doi.org/10.1007/s12161-022-02274-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-022-02274-7