Abstract
Cotoneaster, a member of the Rosaceae family comprises around 300 species globally and has attained a significant place in traditional medicine. Although polyphenols are known to be prevalent in most Cotoneaster species, very little is known about their specific content and antioxidant activity. Therefore, the current study aimed to evaluate the content of total phenolics, total flavonoids, and antioxidant activity of six Cotoneaster species viz, Cotoneaster integerrimus, Cotoneaster affinis, Cotoneaster nummularia, Cotoneaster horizontalis, Cotoneaster rosea, and Cotoneaster microphylla growing in Kashmir Himalaya. The folin-Ciocalteu method and aluminium chloride method were used to evaluate the total phenolics and total flavonoids respectively. Also, the antioxidant properties of the methanolic extracts were assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Reducing power assays. The results revealed noteworthy antioxidant activity and total phenolic content. The fruit extracts of C. rosea and C. nummularia had the highest content of phenolic and flavonoids (297.05 0.63 f mg/g GAE and 30.47 0.22 d mg/g QE, respectively). The highest antioxidant activity observed by the DPPH was found in the fruit extract of C. rosea (IC50 = 3.45 ± 0.01). Moreover, Antioxidant activity and total phenolic content were shown to be significantly correlated. This study underscores the potential therapeutic value of these Cotoneaster species and positions them as a valuable source of potent natural antioxidants for pharmaceutical and nutraceutical applications. However, further research is required to evaluate the toxicity and other biological properties of these crude extracts, along with the identification of different compounds present within them.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Free radicals are highly reactive and unstable chemical entities with unpaired electrons. Free radicals of significant biological importance are oxygen-reactive species, including carboxy radicals, hydroxy radicals, superoxide radicals, hydrogen peroxide, and singlet oxygen (Schöneich 1999). These free radicals are formed in the human body and can intrude into important macromolecules, thereby causing cell damage, heart disease, cancer, and aging (Finkel and Holbrook 2000). In addition to endogenous defensive mechanisms, nutritional antioxidants, primarily plant phenols, play a crucial role in shielding live cells from pro-oxidants (Pandey and Rizvi 2009). As per the reports, synthetic antioxidants, including butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), are harmful to humans (Kahl and Kappus 1993). People seek out natural antioxidants used in pharmaceuticals, food, and cosmetics as alternatives to synthetic antioxidants that may be hazardous and toxic (Liu and Yang 2018). The use of natural antioxidants can aid in improving the stability of meals, nutrients, and medications, while also enhancing the body’s capacity to fight inflammation, allergies, and tumors due to the polyphenolic properties of these antioxidants (Moure et al. 2001).
Antioxidants are chemical substances that occur naturally in foods and the human body (Mititelu et al. 2020; Sharifi-Rad et al. 2020). These chemicals are critical in reducing cell damage brought on by oxidative degradation because of free radical production (Salehi et al. 2020). In recent years, scientists have begun to investigate the potential of plants as sources of antioxidants and substances that have the ability to treat diseases with few adverse consequences (Teo 2021). Sarkar et al. (2022) discussed the potential of plant-derived agents in treating various diseases and the use of phenolic-rich extracts as a substitute for synthetic antioxidants, which are becoming increasingly popular and approved over synthetic antioxidants because they appear less harmful and more effective. Among the many plants that have attracted scientists’ curiosity, the Cotoneaster genus appears to be a potential candidate for further study.
One of the most commercially significant plant families in the world is the Rosaceae family, which includes the genus Cotoneaster and consists of approximately 300 species worldwide, including mostly woody (shrubs, rarely small trees) species (Popoviciu et al. 2020), with the highest concentration of taxa in the Himalayas and West China (Bartish et al. 2001). Since Cotoneaster produces extracts that are used as antitumor, expectorant, antioxidant, hepatoprotective, diuretic, cardioprotective, antiviral, and antispasmodic agents, it has been used in traditional medicine (Cakilcioglu and Turkoglu 2010). Some of the species in this study have also been used in traditional and local medicines. The Cotoneaster affinis fruits are used to improve digestion and cure abdominal pain in Pakistan (Shah and Hussain 2012). The leaves and fruits of Cotoneaster integerrimus are used to treat diabetes mellitus, coughs, fevers, and digestive issues (Zengin et al. 2014). The Cotoneaster nummularia/ manna fruits are used to treat neonatal jaundice in Iran (Fakhri et al. 2016). The plant parts of Cotoneaster microphyllus are used to treat irregular menstruation, bile malfunctioning diseases, expectorant, astringent, stomach-ache, haemostatic, and a remedy for cuts, wounds, and diarrhoea (Haq et al. 2011; Swati et al. 2018). There may not always be scientific support for many traditional practices, which are sometimes founded on cultural beliefs and historical events. The bioactive substances and possible health advantages linked to the use of Cotoneaster species must therefore be validated and understood through additional research. The presence of considerable content of total phenolics in the plant species may significantly contribute to the antioxidant properties and thus can serve as a rich source of natural antioxidants for pharmaceutical and nutraceutical applications. Although polyphenols constitute the majority of Cotoneaster species, very little is known about their presence and antioxidant activity (Zengin et al. 2014). According to Zengin et al. (2014), plant tissues comprising leaves and fruits from Cotoneaster species, other than those under study, can be predicted to have a considerably higher phenolic content. Therefore, the selection criteria of the species included in this study were influenced by a combination of factors including documented historical uses, and the existence of preliminary scientific evidence that suggests bioactivity. Our study does not rely exclusively on traditional practices; rather it adheres to modern pharmacological bioprospecting concepts, which emphasizes the significance of investigating many plant species for their unrealized medicinal potential. Our approach could be biased toward bioprospecting, but it aims to strike a balance between traditional wisdom and cutting-edge scientific investigations. Additionally, some species mentioned in this study have not yet been studied. Furthermore, to the best of our knowledge, this is the first study of its kind on the genus Cotoneaster from the Kashmir region of India. Based on the above knowledge gap, the goal of this study was to evaluate and compare the content of total phenolics, total flavonoids, and antioxidant activity of the leaves and fruits of six Cotoneaster species viz, Cotoneaster integerrimus, Cotoneaster affinis, Cotoneaster nummularia, Cotoneaster horizontalis, Cotoneaster rosea, and Cotoneaster microphylla. we believe this study will provide scientific insights that corroborate, enhance, or reinterpret their traditional significance and ultimately, will contribute to a broader understanding of the bioactive potential of these Cotoneaster species and their potential applications in modern health contexts.
Materials and methods
Chemicals and reagents
2,2-diphenyl-1-picrylhydrazyl (DPPH), Potassium ferricyanide, Quercetin, Gallic acid, FC reagent, Sodium carbonate, Phosphate buffer, Aluminium chloride, Sodium acetate, Methanol, Trichloro acetic acid, Ferric chloride, Ascorbic acid.
Plant material
Leaves and fruits of six species of Genus Cotoneaster viz, C. integerrimus, C.affinis, C.nummularia, C. horizontalis, C. rosea, and C. microphylla were collected from the different localities of Kashmir Himalayas in September 2022. The different localities and their geo-coordinates are given in Table 1. The identification and validation of the target species were done at the Center of Plant Taxonomy, Department of Botany, University of Kashmir. The specimens of each species were submitted to the KASH herbarium of the Department of Botany.
Preparation of the plant extracts
The collected fruits and leaves of all six species were washed and cleaned with a tissue towel. Pyrenes of mature pomes of various individuals from all six species were manually removed and the pulp of the fruit was ground in a mortar using liquid nitrogen to get a fine powder whereas the collected leaves of all species were dried in the shade and were grounded to a fine powder using a mortar and pestle. One gram (1 g) of pulverized material was extracted in 20 ml of methanol, left overnight on a shaker, and centrifuged for 10 min at 10,000 rpm. The collected supernatant was filtered through Whatman filter paper (No. 1) and used in different biochemical assays.
Estimation of total flavonoid content (TFC)
TFC was calculated using an aluminium chloride method (Bag et al. 2015). Briefly, 50 µL of the plant extract was taken, and the volume was made up to 1 ml using methanol. To this 100 µL of 10% aluminum chloride was added and shaken well. After 5 min 100 µL of sodium acetate (1 M) and distilled water (2.8 ml) were added and shaken thoroughly. The mixture was then incubated at room temperature in the dark for 1 h. The absorbance was measured at 510 nm using a UV-1900 l spectrophotometer. Without the extract, 1 ml of methanol and all the reagents were used as blank. Quercetin was used as a standard. A standard calibration (Fig. 1) curve was created using quercetin solutions (1 mg/ml) at various concentrations. mg equivalent of quercetin (QE) per gram of extract was used to determine the overall flavonoid content. The experiment was performed in triplicate.
Estimation of total phenolic content (TPC)
The TPC of the methanolic extracts of plant parts was calculated using the Folin-Ciocalteu method (Singleton and Rossi 1965). Briefly, 1 ml of plant extract was taken, and 5 ml of FC reagent (2 N, 1 FC:10 distilled water) was added and mixed thoroughly. Subsequently, 4 ml of 20% sodium carbonate was added and thoroughly mixed for 5 min. After one hour of incubation at room temperature in the dark, the absorbance of the mixture was measured at 760 nm. For comparison, gallic acid was selected, and the data were expressed as the milligram equivalents of gallic acid per gram of extract. The experiment was performed in triplicate.
Antioxidant properties
DPPH radical scavenging activity
DPPH assay was performed to evaluate the potential of the plant extracts to scavenge free radicals (Chu et al. 2000). The DPPH solution (0.004%) was prepared using methanol. Test tubes were filled with various concentrations (1.5–30 µl) of the plant extract and made up to 1 ml using methanol, followed by the addition of 2 ml of DPPH solution. The test tubes were covered with aluminum foil to shield them from light and then left at room temperature for 30 min in the dark. Using a spectrophotometer (UV-1900 l), the absorbance was measured at 517 nm following incubation. DPPH was used as a control, without any extract, and methanol was taken as a blank. The following equation was used to determine the percentage of DPPH free radical-scavenging activity:
All concentrations in the experiment were measured in triplicate. Furthermore, the experiment was performed in triplicate. A calibration curve was plotted for percentage scavenging activity, providing the equation \(y=mx+c\) from which the IC50 value was calculated.
Reducing power assay
The reducing power of the samples was tested according to the method of (Dorman and Hiltunen 2004). Plant extracts and ascorbic acid standard of different concentrations were made up to 1 ml using methanol, and mixed well with potassium ferricyanide (2.5 ml, 1%) and phosphate buffer (2.5 ml, P.H. 6.6, 0.2 M). The mixture was kept at 50ºC in a water bath for 20 min. Trichloroacetic acid (2.5 ml, 10%) was then added. The mixture was centrifuged at 3000 rpm for 5 min. The supernatant (2.5 ml) was combined with 2.5 ml of distilled water and 0.5 ml of ferric chloride, and absorbance was measured at 700 nm. Similar steps were performed to prepare the controls but without the extracts. The increase in the reducing power of the mixture was denoted by the increase in absorbance.
Statistical analysis
Execution of the analysis of variance (ANOVA) was performed and the significant differences between the mean values were calculated by Duncan's post-hoc analysis (P < 0.05) using SPSS v23.0 (IBM SPSS Statistics 2015). The correlation between variables was analysed using ‘R’ software (R Core Team 2022).
Results and discussion
Total phenolics
The TPC in methanolic extracts of fruits and leaves of different species was calculated using the standard curve equation obtained from Fig. 2. (\(y = 0.0071x+ 0.2446\), where \({R}^{2}\) = 0.9987) using gallic acid and expressed as milligram equivalents of gallic acid per gram of extract. The results of the TPC are shown in Table 2. The TPC analysis revealed significant variations between different species of Cotoneaster both in terms of leaf and fruit. In terms of fruit extracts the highest TPC was observed in C. rosea (297.05 ± 0.63f mg/g GAE), followed by C. affinis (241.46 ± 1.06e mg/g GAE). The lowest TPC was recorded for the C. integerrimus (97.14 ± 0.80a mg/g GAE). With respect to leaves, the highest TPC was found in C. integerrimus (277.14 ± 0.58f mg/g GAE), followed by C. microphylla (270.47 ± 0.37e mg/g GAE). The lowest TPC content was found in the leaves of C. horizontalis (22.68 ± 0.21a mg/g GAE). This is in accordance with the study of Yoo et al. (2019) for C. wilsonii with estimates of TPC = 214.65 ± 2.43 for methanolic stem extracts, and 220.95 ± 0.50 for methanolic leaf extracts, whereas for the fruit methanolic extracts, the TPC was relatively low (60.71 ± 2.03). Similarly, Zengin et al. (2014) reported different polarity extracts ranging from 8.11 to 266.39 mgGAE/g for C. nummularia. The antioxidant capabilities of any plant are directly correlated with its phenolic content. Phenolic compounds can scavenge free radicals, and operate as hydrogen donors, and reducing agents (Wojdyło et al. 2007). The presence of considerable content of total phenolics in the leaves and fruits of these species may significantly contribute to the antioxidant properties and thus can serve as a rich source of natural antioxidants for pharmaceutical and nutraceutical applications. Additionally, the species C. integerrimus, C. microphylla, C. nummularia, and C. affinis may have been utilized in conventional medications as a result of these properties. The total phenolic content of the various species tested in this study varied, which may be due to variances in the environmental conditions and genetic makeup of the species (Doshi et al. 2006).
Total flavonoids
The TFC in methanolic extracts of fruits and leaves of different species was calculated using the standard curve equation obtained from Fig. 1. (\(y = 0.0177x+ 0.0853\), where \({R}^{2}\) = 0.9941) using Quercetin and expressed as milligram equivalents of quercetin per gram of extract. The results of the TFC are shown in Table 2. In the context of fruits, the highest TFC was observed in C. nummularia (30.47 ± 0.22d mg/g QE), followed by C. integerrimus (13.37 ± 0.20c mg/g QE). The lowest content was recorded for C. rosea and C. horizontalis (0.32 ± 0.22a and 0.35 ± 0.22a mg/g QE respectively). In terms of leaf extracts the highest TFC content was noted in C. microphylla (9.94 ± 0.25d mg/g QE), followed by C. integerrimus (9.51 ± 0.14d mg/g QE). The lowest content was recorded for C. rosea (2.31 ± 0.26a mg/g QE). We found differences between the results of our TFC assessment and the earlier findings that had been published in the literature (Popoviciu et al. 2020). In particular, the TFC values obtained from C. horizontalis fruit extracts in our investigation were significantly lower (0.35 ± 0.22a mg/g QE) than Popoviciu’s findings, who recorded a TFC range of 4.457–10.94 mg/g for the C. horizontalis. (Mohamed et al. 2012) also noted a TFC value of 6.8 mg/g for the same species. However, the values for the C. microphyllus (5.55 ± 0.19b mg/g QE) were in accordance with the findings of Popoviciu who reported a TFC range of 3.44 – 10.33 mg/g for the same species. These differences in TFC between our study and the previous reports could potentially be attributed to a range of factors, such as environmental conditions, variation in sample collection, and analysis techniques. In addition, it is worth mentioning that there is a dearth of comprehensive literature available pertaining to these Cotoneaster species underscoring the need for meticulous investigation.
Antioxidant activity
The antioxidant capacities of methanol extracts of the fruits and leaves of Cotoneaster species were evaluated using a free radical scavenging assay (DPPH) and a reducing power assay. The IC50 values were used to determine the free radical scavenging activities. The extract concentrations of both leaves and fruits required to neutralize 50% of the DPPH radicals are expressed in terms of IC50 values and are listed in Table 3. The extracts showed a concentration-dependent increase in their ability to scavenge free radicals. Ascorbic acid was used as standard. The IC50 value of ascorbic acid was 2.32 ± 0.02. In the context of fruits, the highest radical scavenging activity was noted in C. rosea with an IC50 value of 3.45 ± 0.01a, followed by C. affinis (IC50 4.48 ± 0.1b). the lowest radical scavenging activity was noted in C. integerrimus (IC50 18.15 ± 0.13f). In the case of leaf extracts the highest radical scavenging activity was observed in C. integerrimus with an IC50 of 4.15 ± 0.02a, followed by C. microphylla (IC50 5.01 ± 0.32b), and C. nummularia (IC50 5.17 ± 0.10b). the lowest radical scavenging activity in leaf extracts was noted in C. horizontalis with an IC50 of 30.73 ± 0.07e. The ability of the isolated fruit and leaf samples to scavenge DPPH free radicals at various concentrations was remarkable. For most of the species tested, the IC50 value for fruits and leaves was found to be less than 10, indicating a remarkably high level of antioxidant activity. Notably, the fruits of C. rosea, leaves of C. integerrimus, and leaves of C. affinis showed almost the same results as the ascorbic acid standard. The results of this study suggest that these species have a high level of antioxidant potency, indicating their potential use as sources of natural antioxidants. The discrepancies in IC50 values between these species show the unique antioxidant chemical profiles of each species and most of the antioxidant activity in plants is because of the phenols (Mansouri et al. 2005). Remarkably, our findings align well with the overall pattern of total phenolic content observed in these Cotoneaster species and the total phenolic content suggests that phenolic compounds might indeed be the principal contributors to the observed antioxidant potential.
Results from other previous studies seem to be difficult to contrast because of the other factors used during the experiments. However, the DDPH test was used to examine the antioxidant activity of 70% methanol extract fractions of three species of Cotoneaster's leaves cultivated in Poland (Kicel et al. 2018). According to the authors, the IC50 values ranged from 3.19 to 27.88 μg/mL for the C. bullatus ethyl acetate fraction and C. integerrimus water residue thereby showing a strong scavenging effect. In addition, (Krzemińska et al. 2022) investigated the antioxidant potential of several leaf extracts from C. hsingshangensis and C. hissaricus using DPPH assay. The C. hsingshangensis ethyl acetate fraction showed the highest DPPH scavenging activity (IC50 = 2.08 ± 0.03 μg/mL), followed by the butanol fraction (IC50 = 3.43 ± 0.02 μg/mL) and the diethyl ether fraction (IC50 = 4.15 ± 0.05 μg/mL). The water fraction exhibited the lowest activity. (IC50 = 32.37 ± 0.19 μg/mL) and crude extract of C. hissaricus (IC50 = 21.73 ± 0.13 μg/mL).
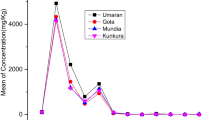
From the reducing power assay, as shown in Figs. 3 and 4, as the sample concentration increased, the absorbance increased for both the leaves and fruits, indicating their antioxidant activity. The highest reducing power was found in the fruits of C. rosea, followed by those of C. affinis. Their effect was found to be higher than that of ascorbic acid used as a standard when treated from 40 to 120 µg/ml, whereas the lowest was found in the leaf samples of C. horizontalis. Yoo et al. (2019) studied different parts of C. wilsonii and obtained similar results for the tested extracts and the positive control ascorbic acid. Antioxidant activity and reducing power are related, and reducing power may be a useful indicator of antioxidant activity. Reducing power compounds donate electrons to oxidize intermediary compounds of lipid peroxidation processes, in order for them to serve as both primary and secondary antioxidants, this helps to prevent damage to cells and tissues caused by oxidative stress (Chanda and Dave 2009).
Linear regression analysis (P < 0.01) was conducted between the TPC, TFC, reducing power, and DPPH assays for all tested fruit and leaf samples. As shown in Fig. 5, the antioxidant activities of the tested species showed a strong correlation with TPC levels. The phenols and flavonoids containing hydrogen-donating groups cause the methanolic DPPH solution to get reduced as a result of the generation of non-radicals (Mensor et al. 2001). However, this dependence was not found in the case of TFC, which was probably caused by the relatively lower amount of TFC. The results of the DPPH and reducing power assays were the same. The DPPH and reducing power assays also showed a strong correlation with each other, which implies that Cotoneaster extracts might have the potential to offer antioxidant benefits across different experimental approaches. They use both fundamental reaction pathways and function as direct quenchers of ROS, as well as reducing agents. Also, it might be said that the amount of TPC reflected in GAE is a significant indicator of antioxidant capacity. This could be used to screen plant extracts as natural sources of antioxidants. However, it is noteworthy that the negative signs in the relation between DPPH and TPC levels are because the data of the DPPH assays (radical scavenging activity) have been taken in terms of IC50 value. The lower IC50 value indicates higher radical scavenging activity and vice-versa. Our findings support those of Olszewska et al. (2010) and Sravani and Padma (2011). However, it is necessary to conduct a more thorough investigation of the compounds contained in Cotoneaster samples and their chemical nature.
Conclusion
This study demonstrates the TPC, TFC, and antioxidant activities of six Cotoneaster species growing in the Kashmir Himalaya. Most of the samples showed a significantly high TPC and antioxidant activity. The fruits of C. rosea and leaves of C. integerrimus showed a remarkable high TPC and antioxidant activity. The highest TFC values were shown by the fruits of C. nummularia. The antioxidant activity of leaves and fruits positively correlate with their TPC values. The TPC, TFC, and antioxidant activity of the various species tested in this study varied, which may be due to variances in the environmental conditions and genetic makeup of the species. The remarkable antioxidant activity of these species lends credence to the notion that their products could be valuable sources of potent natural antioxidants that can be used in the diet, medications, cosmetics, and other applications where antioxidants are needed. However, further research is required to evaluate the crude extracts of these species and identify different compounds present in them. Additionally, their toxicity and other biological properties for potential use in the pharmaceutical and nutraceutical sectors need to be evaluated.
Data availability
The data will be available on request.
References
Bag GC, Devi PG, Bhaigyabati TH (2015) Assessment of Total Flavonoid Content and Antioxidant Activity of Methanolic Rhizome extract of three Hedychium species of Manipur valley. Int J Pharm Sci Rev Res 30(1):154–159
Bartish IV, Hylmö B, Nybom H (2001) RAPD analysis of interspecific relationships in presumably apomictic Cotoneaster species. Euphytica 120:273–280
Cakilcioglu U, Turkoglu I (2010) An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J Ethnopharmacol 132(1):165–175
Chanda S, Dave R (2009) In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr J Microbiol Res 3(13): 981–996
Chu YH, Chang CL, Hsu HF (2000) Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric 80(5):561–566. https://doi.org/10.1002/(SICI)1097-0010(200004)80:5%3c561:AID-JSFA574%3e3.0.CO;2
Dorman HJD, Hiltunen R (2004) Fe (III) reductive and free radical-scavenging properties of summer savory (Satureja hortensis L.) extract and subfractions. Food Chem 88(2):193–199. https://doi.org/10.1016/j.foodchem.2003.12.039
Doshi P, Adsule P, Banerjee K (2006) Phenolic composition and antioxidant activity in grapevine parts and berries (Vitis vinifera L.) cv. Kishmish Chornyi (Shared Seedless) during maturation. Int J Food Sci Technol 41(SUPPL. 1):1–9. https://doi.org/10.1111/j.1365-2621.2006.01214.x
Fakhri M, Azadbakht M, Yousefi SS, Mousavinasab SN, Farhadi R, Azadbakht M (2016) Medicinal plants for treatment of neonatal jaundice by community of attars (traditional healers) of several urban areas in Mazandaran province, northern of Iran. Br J Med Med Res 1(11):1–3
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress, and the biology of ageing. Nature 408(6809):239–247
Haq F, Ahmad H, Alam M (2011) Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. J. Med. Plant Res 5(1):39–48
IBM Spss Statistics (2015) IBM, SPSS Statistics for windows (23). IBM Corp, Armonk
Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift Für Lebensmittel-Untersuchung Und Forschung 196:329–338
Kicel A, Kolodziejczyk-Czepas J, Owczarek A, Marchelak A, Sopinska M, Ciszewski P, Nowak P, Olszewska MA (2018) Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules. https://doi.org/10.3390/molecules23102472
Krzemińska B, Dybowski MP, Klimek K, Typek R, Miazga-Karska M, dos Santos Szewczyk K (2022) The anti-acne potential and chemical composition of two cultivated Cotoneaster Species. Cells. https://doi.org/10.3390/cells11030367
Liu Z, Yang, (2018) Antisolvent precipitation for the preparation of high polymeric procyanidin nanoparticles under ultrasonication and evaluation of their antioxidant activity in vitro. Ultrason Sonochem 43:208–218. https://doi.org/10.1016/j.ultsonch.2018.01.019
Mansouri A, Embarek G, Kokkalou E, Kefalas P (2005) Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem 89(3):411–420. https://doi.org/10.1016/j.foodchem.2004.02.051
Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15(2):127–130. https://doi.org/10.1002/ptr.687
Mititelu RR, Pădureanu R, Băcănoiu M, Pădureanu V, Docea AO, Calina D, Barbulescu AL, Buga AM (2020) Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines. https://doi.org/10.3390/BIOMEDICINES8050125
Mohamed SA, Sokkar NM, El-Gindi O, Ali ZY, Alfishawy IA (2012) Phytoconstituents investigation, anti-diabetic and anti-dyslipidemic activities of Cotoneaster horizontalis. Life Sci J 9(2):394–403
Moure A, Cruz JM, Franco D, Domı́nguez JM, Sineiro J, Domı́nguez H, Núñez MJ, Parajó JC (2001) Natural antioxidants from residual sources. Food Chem 72(2):145–171
Olszewska MA, Nowak S, Michel P, Banaszczak P, Kicel A (2010) Assessment of the content of phenolics and antioxidant action of inflorescences and leaves of selected species from the genus sorbus sensu stricto. Molecules 15(12):8769–8783. https://doi.org/10.3390/molecules15128769
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 1(2):270–278
Popoviciu DR, Negreanu-Pirjol T, Motelica L, Stefan B, Pirjol N (2020) Carotenoids, Flavonoids, Total Phenolic Compounds and Antioxidant Activity of Two Creeping Cotoneaster Species Fruits Extracts. Rev. Chim, 71(3): 136–142. https://doi.org/10.37358/Rev
R Core Team (2022) R: a language and environment for statistical computing (4.1.2). R Foundation for Statistical Computing Vienna, Austria. https://www.r-project.org/. Accessed 5 Jan 2023
Salehi B, Sharifi-Rad J, Cappellini F, Reiner Z, Zorzan D, Imran M, Sener B, Kilic M, El-Shazly M, Fahmy NM, Al-Sayed E, Martorell M, Tonelli C, Petroni K, Docea AO, Calina D, Maroyi A (2020) The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Front Pharmacol 11:1300. https://doi.org/10.3389/fphar.2020.01300
Sarkar C, Chaudhary P, Jamaddar S, Janmeda P, Mondal M, Mubarak MS, Islam MT (2022) Redox activity of flavonoids: impact on human health, therapeutics, and chemical safety. Chem Res Toxicol 35(2):140–162. https://doi.org/10.1021/ACS.CHEMRESTOX.1C00348
Schöneich C (1999) Reactive oxygen species and biological aging: a mechanistic approach. Exp Gerontol 34(1):19–34
Shah SM, Hussain F (2012) Ethnomedicinal plant wealth of Mastuj valley, Hindukush range, district Chitral, Pakistan. J Med Plant Res 6(26):4328–4337
Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini E, Peluso I, Prakash Mishra A (2020) Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol 11:694
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 3:144–158
Sravani T, Paarakh P (2011) Evaluation of anthelmintic activity of Hedychium spicatum Buch. Int J Res Pharm Sci 2(1):66–68
Swati S, Manjula R, Kattupalli S, Vennela Y, Tanuja K (2018) A phyto pharmacological review on Cotoneaster microphyllus species. J Pharm Sci Res 10(9):2166–2168
Teo WL (2021) Diagnostic and management considerations for “maskne” in the era of COVID-19. J Am Acad Dermato 84(2):520–521. https://doi.org/10.1016/j.jaad.2020.09.063
Wojdyło A, Oszmiański J, Czemerys R (2007) Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 105(3):940–949. https://doi.org/10.1016/j.foodchem.2007.04.038
Yoo NH, Kim HK, Song JM, Lee CO, Park JH, Park BJ, Choi YB, Baek YS, Hwang YJ, Kim MJ (2019) Biological Activities and Separation of Active Substance of Extract and Fractions from Cotoneaster wilsonii Nakai Leaf. Korean J Med Crop Sci 27(6):412–418
Zengin G, Uysal A, Gunes E, Aktumsek A (2014) Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): a potential source for functional food ingredients and drug formulations. PLoS ONE 9(11):e113527
Acknowledgements
We are thankful to the University grants commission (UGC), Government of India for providing a JRF fellowship to the corresponding author (Award letter no. 191620210617). All authors contributed to the conception and design of the study. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Javid, W., Nabi, G. & Wani, A.A. Assessment of total phenolics, flavonoids, and antioxidant properties within the genus Cotoneaster in Kashmir Himalayas. Vegetos (2023). https://doi.org/10.1007/s42535-023-00744-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-023-00744-9