Abstract
The aim of this study was to investigate the biochemical composition and antioxidant activity of extra virgin olive oils (EVOOs) produced from different olive cultivars in the two coastal areas of Turkey; Aegean and Mediterranean regions. Samples were divided into two groups according to the olive-growing region and were classified by soft independent modeling of class analogy (SIMCA) algorithm based on chemical composition of EVOOs. Oleic acid was determined as the major fatty acid in both regions and varied from 46.7 to 71.6%. β-Sitosterol was the main sterol compound, and trioleoylglycerol (OOO) was determined as the main triacylglycerol (TAG) in all samples with >32% ratio. Memecik cultivar (263.4 mg/kg oil) and Gemlik cultivar (279.5 mg/kg oil) had the highest α-tocopherol content in Aegean and Mediterranean regions, respectively. Olive oil produced from Gemlik cultivar had the highest total phenolic content and antioxidant capacity in all samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Food and Agriculture Organization has reported that Turkey is one of the important producer countries of olive oil with 206,300 tonnes per year (FAO 2018). A total of 88 Turkish olive varieties and 26 foreign olive cultivars were registered by the Ministry of Agriculture and Rural Affairs of Turkey in 1990 depending on some parameters such as their pomological and morphological parameters (Canözer 1991). The Aegean region is Turkey’s leading olive-growing region and an important area for olive production. Approximately 65% of olive oil has been produced in North and South Aegean regions, the main olive-growing regions in Turkey (Gokcebag et al. 2013), where Ayvalik and Memecik are the main cultivars of economic importance. The major olive variety of the Marmara region is Gemlik cultivar, and it has also been cultivated widely in other olive-growing regions in Turkey for two decades. Nizip Yağlık is the quite important and common domestic variety in the South Anatolia region (Canözer 1991). Moreover, the most important olive varieties cultivated in South Anatolia region especially in Hatay are Halhalı, Saurani, Hasebi, Karamani, Sariulak, and Gemlik.
In recent years, monocultivar virgin olive oils such as Edremit, Ayvalık, Aydın, and Mugla are produced in specific geographic areas; these olive oils are preferred by costumers because of high quality (Gokcebag et al. 2013) The biochemical characterization of olive oils from both Mediterranean and non-Mediterranean regions is essential for conducting a geographical characterization and comparison of quality parameters of the olive oil. There is lack of data about the biochemical characteristics of some major Turkish cultivars including North Aegean, South Aegean, and especially Mediterranean cultivars (Andjelkovic et al. 2009).
Olive oil contains high ratio of mono-saturated fatty acid (MUFA), and also, they are a good source of tocopherols, carotenoids, and phenols. In addition, Mediterranean diet suggests consuming olive oils in daily meal which contain high levels of mono-saturated fatty acids and health benefits (Lopez-Miranda et al. 2010). There is growing interest in authenticity and traceability of virgin olive oil for consumer, because adulteration is a serious problem in oil industry (Diraman et al. 2011; Gokcebag et al. 2013). Therefore, determination of classification is necessary for monocultivar oils. The chemical composition of extra virgin olive oil can be affected from growing area, climate, and producing techniques (Gokcebag et al. 2013; Ilyasoglu et al. 2010). The olive-growing coasts of Turkey, Aegean and Mediterranean regions, have cool and rainy winters and hot and moderately dry summers. According to the General Directorate of Meteorological Service, the Aegean coast receives an average value of 621mm of rainfall, and the Mediterranean coast receives an average value of 762.5 mm rainfall. The mean air temperature for Aegean coast was 15.6°C in 2011, whereas this value was 17.6°C for the Mediterranean region (Ulupınar et al. 2012).
Fatty acid profiles are extremely useful for the characterization and discrimination of an olive cultivar or its geographical location (Gokcebag et al. 2013). There are several studies on geographical characterization of virgin olive oils based on fatty acid profiles in Turkey ((Diraman et al. 2011; Gurdeniz et al. 2008; Ilyasoglu et al. 2010) and for the other Mediterranean countries. In recent years, multivariate statistical methods, such as SIMCA, a classification procedure based on principal component analysis (PCA), have been used extensively to classify and characterize virgin olive oils based on their geographical origins (Diraman et al. 2011). Multivariate statistical (SIMCA) evaluation of data is not an exact solution but is a very promising approach for the evaluation of analytical data as to the chemical characteristics of a virgin olive oil sample.
Although it has been reported that some information about the chemical characterization and chemometric classification of Turkish extra virgin olive oils (Diraman and Dibeklioglu 2009; Diraman et al. 2011) and domestic olive varieties (Diraman and Dibeklioglu 2009), there is limited information available on olive oils harvested from Mediterranean and South Anatolia regions of Turkey, to a certain degree for Aegean region. Limited information is found about the nature and/or concentrations of minor components and the chemical composition and antioxidant activity of the oils from the monocultivars grown in these regions (Gurdeniz et al. 2008).
This study was undertaken to investigate the chemical composition of 28 Turkish extra virgin olive oil samples by analyzing several parameters (e.g., fatty acids, sterol and TAG composition, antioxidant activity) as well as minor components (phenolic compounds, β-carotene and squalene, valuable parameters of the quality of the oil) and to classify the predominant olive cultivars by official methods and SIMCA statistical analysis to determine a reliable indicator for characterization, classification, and comparison of these olive oils.
Materials and Method
Samples
Commercial monovarietal extra virgin olive oils belonging to 2011 harvest season were supplied from the Zeytindostu Agency, which is a non-commercial national association. All twenty-eight oil samples from several olive varieties were collected from different parts of Turkey. The North Aegean olive oils were obtained from Ayvalık (n=11), and South Aegean olive oils from Memecik (n=2), Uslu r (n=1), Erkence (n=2), whereas Mediterranean olive oils were obtained from Nizip Yağlık (n=2), Saruani (n=1), Karamani (n=1), Halhalı (n=2), Gemlik (n=2), Sarıulak (n=1), Haşebi (n=1), and Mix (n=2). 1.5 L of each olive oil was divided into three dark brown bottles and stored at 4°C until analyzed. All olive samples were harvested during the period between October and December in 2011. EVOOs were produced by different processing systems, which are hydraulic system and two- and three-phase continuous systems. Location of cultivar, type of cultivar, and production zone (region) of each sample were recorded and shown in Table 1, where all the samples are named by their first three letters.
Chemicals
A fatty acid methyl ester (FAME) mixture, triacylglycerol (TAG) mixture, and sterol mixture were purchased from Supelco (Bellefonte, PA). All other chemicals and reagents for analysis were analytical or HPLC grade and purchased from Sigma-Aldrich (Steinheim, Germany).
Chemical Analysis
Determination of free fatty acid (FFA) value was carried out by BS EN ISO 660:2009 official methods (BS EN ISO 660:2009 2009) of analysis, and results were expressed as %oleic acid. The peroxide value (PV) was determined as described in the AOAC official method (965.33) (AOAC n.d). UV absorption characteristics (K232 and K270) were determined by the analytical methods described in the Regulation EC/1989/2003 (1989/2003). Absorbance of the solution at 232 and 270 nm was measured using a Jenway 6705 UV-visible spectrophotometer, with cyclohexane as the blank.
Fatty Acid Composition
Fatty acid composition of the samples was determined according to the method described in Turkish Food Codex Communique on Olive Oil and Pomace Oil (Commonique number 2010/36) (IOC 2015). FAMEs were analyzed using an Agilent 7820A (Agilent Technologies Inc., Palo Alto, USA) GC equipped with a capillary column (30m×0.25 mm i.d.×0.25 μm; Agilent 112-8837) and a flame ionization detector (FID) to identify and quantify the individual fatty acids in the olive oils. The injector and detector temperatures were maintained at 250 and 280 °C, respectively. The injection volume was 1 μL. The carrier gas hydrogen flow rate was 40 mL/min, and the split ratio was 1/50. Results were expressed as weight percentage by comparing retention time of FAMEs of samples with the retention times of standard FAME mixture.
Sterol Composition
Sterol content was determined according to a modified procedure explained by Verleyen et al. (2002). To prepare the unsaponifiable matter, 1.5 g oil was weighed in a flask, and 2 mL of internal standard (0.1 % cholesterol in chloroform) was added. The sample was mixed with 10 M KOH; prepared in 50 mL methanol, in flasks; and shaken at 30 °C for 24 h. After incubation, 10 mL distilled water, 1 mL ethanol, and 20 mL hexane were added. The flask was shaken vigorously in a separatory funnel, and after separation, the hexane layer was collected. The other layer (including the aqueous/alcohol phase) was extracted twice more with 20 mL hexane. The combined hexane extracts were dried over anhydrous Na2SO4. The solvent was completely removed under vacuum. The residue was re-dissolved in 0.5 mL pyridine and 1 mL N,O-bis-(trimethylsilyl)-trifluoroacetamide containing 1% trimethylchlorosilane solution and derivatized at 80 °C for 30 min. The samples were analyzed using an Agilent 7890 A gas chromatograph equipped with a capillary column and a flame ionization detector at 360 °C. The oven temperature of the GC was initially held at 285 °C for 35 min and then increased to 310 °C, with an increase of 10 °C/min, and kept for 10 min. The carrier gas was helium and the flow rate was 0.5 mL/ min. The sterol composition was identified by comparing retention times of sterols in samples with the standard sterol mixture. The internal standard method was used for quantification.
Triacylglycerol (TAG) Composition
The triacylglycerol (TAG) composition of the oils was determined according to the official method in the Turkish Food Codex Communique on Olive Oil and Pomace Oil (Communique number 2010/36) (IOC 2015). 0.2 g oil was weighed and dissolved in 20-mL n-heptane and then transferred into vials. Triacylglycerol composition was analyzed with an Agilent 7820 A gas chromatograph equipped with a capillary column and flame ionization detector at 360 °C. The oven temperature was initially 285 °C for 35 min and then increased at 10 °C/min to 310 °C and kept for 10 min. The carrier gas was helium and the flow rate was 0.5 mL/min. Triacylglycerol compositions were identified by comparing their retention times with those of the standard TAG mixture.
Total Phenolic Content (TPC) and Phenolic Fractions
TPC was determined according to the Folin-Ciocalteu method as described by Gutfinger et al. with minor modifications (Gutfinger 1981). 100μL of methanolic extract of oil was added into a test tube containing 1.5 mL Folin-Ciocalteu reagent (1×10-fold diluted). The tubes were thoroughly mixed, and then 1.2 mL of 7.5% sodium carbonate was added and mixed again. After 1h of incubation at room temperature, the absorbance of mixture was measured at 765 nm. The results were expressed as μg gallic acid equivalents (GAE)/g oil.
Phenolic fractions were determined according to Baiano et al. (2013). The methanolic extract was filtered through a 0.45-μm PTFE membrane filter into the vial and injected into an Agilent HPLC system (Agilent, Kyoto, Japan). A gradient solution including two solvents, (A) 2% acetic acid in water and (B) methanol:acetonitrile (1:1, v/v) at constant flow rate of 1 mL/min, was used to achieve the separation of phenolic compounds. The gradient program of solvent was the following: 100 to 70 % A in 30 min, 70 to 50 % A in 15 min, 50 to 40 % A in 10 min, 40 to 0 % A in 10 min, 0 % for 5 min, and 0 to 100 % A in 5 min. The determination compounds were carried out at 280 nm and 320 nm.
Tocopherol Content
Tocopherol isomers of the oils were determined according to the method described by Uluata and Ozdemir (Uluata and Ozdemir 2012). A HPLC system (Agilent, Kyoto, Japan) equipped with Inertsil ODS-3 normal phase column (250 mm × 4.6 mm, 5 μm) and SPD-M20A photodiode array detector was used for the identification of tocopherol isomers. Separation of tocopherols was based on isocratic elution with n-hexane:isopropanol (96:4%) at 1 mL/min. The eluate was monitored at 292 nm by using a photodiode array detector (SPD-M20A). The compounds were identified by comparing their retention times and the UV spectra with the authentic standards. Tocopherols were quantified based on the peak areas compared with the external standards. Tocopherol analysis was performed in triplicate for the single samples of each variety, and the average values were reported.
ABTS Assay
The radical scavenging capacity of the oils was determined by 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid (ABTS) method (Re et al. 1999). ABTS radical cation (ABTS.+) stock solution was prepared by reacting 7.0 mM ABTS stock solution with 2.45 mM (final concentration) and potassium persulfate in the dark for 16 h. The solution was diluted with ethanol by adjusting the absorbance to 0.700 ± 0.020 at 765 nm. 100 μL of the diluted oil samples in ethanol and 2.9 mL of diluted ABTS+ solution were added. The solution was agitated with a vortex mixer for 20 s. The absorbance was measured after 6 min at 765 nm. The results were expressed as μg Trolox equivalent/100 g oil.
DPPH Assay
DPPH (α, α-diphenyl-β-picrylhydrazyl) activity was determined according to the method mentioned by Bondet et al. (1997). Freshly prepared DPPH solution was dissolved in a small volume of ethyl acetate and then diluted with ethyl acetate by adjusting the absorbance to 0.700 ± 0.020 at 520 nm. 20 mg oil was weighed in a test tube, and then 80 μL ethyl acetate and 2.9 mL DPPH free radical solution were added. The sample was agitated with a vortex mixer for 20 s. After 30 min of incubation in darkness at room temperature, absorbance was measured at 520 nm against ethyl acetate. Trolox was used as a standard, and the results were expressed as μg Trolox equivalent/100 g oil.
ORAC Assay
Oxygen radical absorbance capacity (ORAC) was determined according to the method described by Huang et al. (2002) and Uluata et al. (2016). 0.5 g of oil sample was dissolved in 20-mL acetone. An aliquot of sample mixture was properly diluted with 7 % randomly methylated β-cyclodextrin (RMCD) solvent (w/v) prepared in 50 % acetone-water mixture (v/v) and then shaken at room temperature for 1 h on an orbital shaker at 400 rpm. ORAC analysis was carried out by a BioTek Synergy HT spectrophotometer (BioTek Instruments,Winooski, VT). All reagents were prepared with 75 mM phosphate buffer (pH 7.4), with the exception of samples and Trolox standards, which were prepared in 7 % RCMD solution. In the final assay mixture (0.4 mL total volume), fluorescence (FL) (6.3x10−8 M) was used as a target of free radical attack, and 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) (1.28x10−2 M) was used as a peroxyl radical generator. Trolox was used as the control standard and 7 % RMCD was used as a blank. Final results were calculated using the differences of area under the FL decay curves between the blank and a sample. The results were expressed as μmol Trolox equivalent/100 g oil.
Squalene, Chlorophyll-a, and β-Carotene Determination
Squalene content was determined according to the procedure explained in sterol determination (Verleyen et al. 2002). After preparation of unsaponifiable matter, the samples were analyzed with a GC system. Squalene content of oils was determined by comparing retention time of test samples with squalene standard solution. The internal standard method was used for quantification. Chlorophyll-a and β-carotene contents of oils were obtained by directly reading on Lovibond tintometer (PFX 195 V 3.2, Amesbury, UK).
Statistical Analysis
A Minitab statistics program version 17 (Minitab Inc.) was used to evaluate statistical analysis of the obtained data. The differences among groups were obtained by using one-way ANOVA and Tukey’s post hoc test for multiple comparisons with statistical significance at a 95% confidence level (p<0.05). SIMCA, a classification procedure based on principal component analysis (PCA), was used to cluster oil samples based on their location sources. SIMCA’s discriminating power plot was used to identify important compounds associated with the sample classifications. If the interclass distances were above 3, classes were considered as significantly different from each other.
The data obtained from instrumental analysis were analyzed using multivariate statistical analysis software (Pirouette® version 4.0, Infometrix Inc., Woodville, WA, USA). HPLC and GC chromatograms were not transformed to resolve peak overlap and eliminate baseline shifts. The probability threshold was set as 0.95 for all prediction models.
Results and Discussion
Chemical Parameters of EVOOs
Free fatty acidity, UV absorption parameters (K232 and K270), and peroxide value (PV) were shown in supplementary materials. All these values are important quality factors and generally used to classify olive oils’ category. The EU regulation established a value of FFA< 0.8% for extra virgin olive oil (Regulation EC/1989/2003). FFA values of all samples were below the limit (0.8 oleic acid %) except Halhalı, Haşebi, and Savandere (Memecik type) samples with 0.95, 0.95, and 0.98 oleic acid %, respectively. This could be due to improper fruit harvesting, transport, and storage condition. The EU regulations establish a value of K232<2.5 and K270<0.25 for extra virgin olive oils (Regulations EC/1989/2003). UV absorption parameters (K232 and K270) of all samples were lower than the EC regulations’ limits. In all samples, PV ranged from 4.2 to 10.9 mequiv O2/kg oil, and PV of all samples was lower than legal limit (PV<20 mequiv O2/kg oil, EC/1989/2003). All the results of chemical parameters for EVOO samples, except some FFA values, meet the International Olive Council (IOC) certification criteria for EVOO (IOC 2015)
Fatty Acid Compositions
Fatty acid composition has a wide range depending on genetic and environmental factors and has been used in the qualitative assessment of olive oil (Criado et al. 2008; Lanza et al. 1998). Olive oil has a rich content of monounsaturated fatty acids such as oleic acid, which are important with regard to nutrition and human health (Lanza et al. 1998). Fatty acid compositions of the oils were summarized in Tables 2 and 3. There were variations and significant differences among the fatty acid profiles of the Mediterranean and Aegean oil samples according to Tukey’s test (p<0.05). Oleic acid was the primary and dominant fatty acid in each of the samples with >46.7% ratio. Linoleic and palmitic acids were the other major fatty acids, whereas palmitoleic and linolenic acids were minor fatty acids. Oleic acid content of EVOOs belonging to Aegean region ranged from 52.8 to 71.2%, whereas this value was between 46.7 and 71.6% for Mediterranean region samples. The differences between the fatty acid compositions of olive oils grown in the two regions may be due to the region’s climate, soil characteristics, and precipitation rate. The oleic acid composition range of our findings for Aegean samples was quite similar to the findings of Dag et al. (61.9–71.40%) (2015), Diraman et al. (62.9–76.9%) (2010), and Yorulmaz et al. (60.1–76.2%) (2014). Ayvalık samples (Kbk and Ayv) were quite rich in oleic acid content with the level of 71.1 and 71.2%, respectively, whereas Ayvalık organic sample (Glk) had the lowest oleic acid content (52.8%). Oleic acid content of Memecik sample (Çin) (69.7%) was higher than other Memecik samples (Sav) (56.8%). Linoleic acid content of Ayvalık cultivars ranged from 8.5 to 17.8%. Ayvalık organic cultivated in Çanakkale (Glk) had the highest linoleic acid content (17.8%), whereas Ayvalık sample (Kbk) and Uslu cultivar (Akh) had the lowest linoleic acid content with 8.5 and 8.6% ratio, respectively. Linoleic acid content of Uslu samples (Akh) (13.0%) and Erkence samples (Mor) (13.4%) showed no statistically significant difference as in oleic acid content. The linoleic acid content range for Aegean olive oils was compatible with the findings of Dag (8.8–15.1%), Yorulmaz (8.3–16.3%), and Diraman (8.1–17.1%). Palmitic acid was the major saturated fatty acid in all olive oils and ranged between 12.9 and 22.3%; palmitic acid amount of Ayvalık and Memecik samples varied from 13.5 to 20.2% and 13.2 to 19.9 %, respectively. The mean values for palmitic acid content of Aegean olive oils were somewhat higher than the findings of Yorulmaz (10.9–17.4%), Diraman (9.6–18.9%), and Dag (12.5–15.6%) (Dag et al. 2015; Diraman et al. 2010; Yorulmaz et al. 2014). The content of linolenic acid in Aegean samples was less than 1.3%.
According to the results of the Mediterranean region samples, Mix 2 and Nizip yağlık samples had the highest oleic acid content (71.6 and 70.5%, respectively), whereas Halhalı sample (Hl2) had the lowest oleic acid content (46.7%). There was a statistically significant difference (p<0.05) in Nizip yağlık cultivars cultivated in different locations, Gaziantep and Antalya. Gemlik cultivar (Nzg) had higher oleic acid content (66.3 %) than Gemlik cultivar (Mtg) cultivated in Mut/Mersin (64.3%). The oleic acid composition range of our findings for Mediterranean samples was quite compatible to findings of Dag (62.69–70.06%) who investigated fatty acid composition of olive oil of nine varieties cultivated in Mediterranean and South Anatolia regions of Turkey (Dag et al. 2015). Palmitic acid value of Mediterranean samples varied from 12.9 to 22.3%. Halhalı samples (Hl2) had the highest linoleic acid content (17.0%), whereas Mix 2 had the lowest linoleic acid content (6.8%). Linolenic acid content of all the samples was less than 1%. There are significant differences in the fatty acid composition of olive oil belonging to the same cultivar but harvested from different region. The fatty acid composition results of samples are generally in agreement with the findings of the other studies in the literature about Turkish olive oils (Andjelkovic et al. 2009; Dag et al. 2015; Diraman et al. 2010; Yorulmaz et al. 2014). These results also indicated that the oleic acid content of Ayvalık and Nizip Yağlık samples was higher than Tunisian olive oils (Chetoui, Jarboui, Ain Jarboua, Neb Jmel, Rekhami, Reregui) (Haddada et al. 2008), Spanish cultivar (Bodocal), Italian cultivar (Ascolana), and Greece cultivar (Koroneike) (Benito et al. 2010).
Sterol Compositions
Sterols are mainly constituents of the unsaponifiable fractions of olive oil found in almost all fats and oils. Sterol composition is an important nutritional and authenticity parameter (Uluata and Ozdemir 2012). The sterol compositions of Aegean and Mediterranean olive oil samples were given in supplementary material. Mainly detected sterols were β-sitosterol, ∆5-avenostrol, and stigmasterol in all samples. Brassicasterol and campesterol were determined in trace amounts. β-Sitosterol was the main sterol in all samples, and its content ranged from 66.6 to 86.6% for Aegean region and ranged from 63.5 to 80.8% for Mediterranean region. Ayvalık sample (Kbk) had the highest β-sitosterol content, as in oleic acid content. β-Sitosterol content of Memecik (Sav) (81.3%) was higher than that of Memecik (Çin) (78.8%). The second major sterol in all the samples of Aegean region was ∆5-avenostrol, which varied from 4.9 to 20.7 %. Ayvalık samples from Havran (Hav) had the highest ∆5-avenostrol content, whereas Ayvalık cultivar located in Kabakum (Kbk) had the lowest value. The sterol composition range of major sterols, β-sitosterol and ∆5-avenostrol, of six different olive oils (73.68–89.83% and 6.49–23.64%) studied by Dag et al. (2015) and Yorulmaz et al. (2014) had a wide range and were in agreement with our findings. The percentage of β-sitosterol content of Mediterranean samples varied from 63.5 to 80.8 %. β-Sitosterol content of Gemlik cultivar (80.3%) cultivated in Mut/Mersin (Mtg) was higher than that of Gemlik cultivar (77.7%) cultivated in Nizip/Gaziantep (Nzg). There was no significant differences between ∆5-avenostrol contents of Ayvalık located in Mut (Mya), Haşebi (Hsb), Halhalı (Hal), and Karamani (Kar) cultivars (13.8, 13.7, 13.6, and 13.2%, respectively). The amount of campesterol was lower than the legal maximum value of 4 % in all EVOO samples.
TAG Compositions
TAG is an important indicator to determine quality and purity of olive oils (Yorulmaz et al. 2014). In this study, mainly ten TAGs were identified, and individual TAG compounds of samples are shown in Tables 4 and 5. The main five TAGs were OOO, POO, LOO, PLO, and SOO, whereas LOL and POP were secondary TAGs. However, LLL, PPL, and POS were present in low percentage with <3% ratio. OOO was the major TAG, and its content ranged between 43.9 (Çine) and 30.5% (Glk2). TAG content of Ayvalık samples collected from different locations ranged from 41.6 to 30.4%. OOO content of Memecik (Sav) (43.9%) was higher than that of Memecik (Çin) (41.9%). OOO content of Erkence cultivars was similar to each other (41.6 and 40.5 %). Yorulmaz et al. (2014) have already studied on TAG composition of Aegean olive oils, and our findings are in agreement of their findings although OOO content is somewhat higher than their values (24.72–39.94%). In Mediterranean region, Mix 2 had highest OOO content (41.8 %), whereas its PLO content had lowest percentage (6.1%). Gemlik cultivar (Nzg) and Karamani cultivar (Kar) had the lowest OOO content (31.8 and 31.9%). OOO contents of Nizip yağlık (Nzy), Sarıulak (Sru), Haşebi (Hsb), Halhalı (Hal), and Saruani (Srn) samples were similar to each other’s. There was no significant differences (p<0.05) among these samples. POO was the second major TAG, and POO content of Ayvalık samples varied from 29.9 to 26.0%. Ayvalık cultivars Ayv and Kbk had the highest POO content (29.9 and 29.0 %), whereas Ayvalık cultivar (Adt) had the lowest POO content (26.0 %). POO content of Memecik samples was 25.0 and 26.2%. Nizip yağlık sample (Nzy) had the highest POO content (30.1%), whereas Karamani (Kar) had the lowest POO content (25.4%) in Mediterranean region. LLL level of all samples did not exceed 0.5 % except of Saurani (Srn) and Karamani (Kar) samples where maximum limit was determined according to EUC regulation for olive oils. These results indicated that TAG composition of olive oils showed great variability among cultivars, as described for fatty acid composition.
Total Phenolic Content (TPC) and Phenolic Fractions
In the evaluation of EVOO quality, phenolic compounds are one of the most important parameters, because phenols widely contribute to oil sensory properties and aroma, which are bitterness and astringency, and prevent it from oxidation (Ilyasoglu et al. 2010). In the present study, the TPC of Aegean and Mediterranean region samples were shown in Tables 6 and 7. In Aegean region, TPC ranged from 62.1 to 504.3 μg gallic acid/g oil, whereas TPC of Mediterranean region ranged from 64.8 to 1064.5 μg gallic acid/g oil, and Gemlik/Nizip (Nzg) had the highest total phenolic content with 1064.5 mg/kg oil. Thus, values for Gemlik cultivar was higher than Spanish, Tunisian, and Greece olive oil (Benito et al. 2010; Haddada et al. 2008). In this study, EVOOs obtained from Gemlik cultivar from both southern and northern locations showed higher phenolic content (Ilyasoglu et al. 2010).
Individual phenolic fractions were determined by RP-HPLC, and results were shown in supplementary material. Hydroxytyrosol and tyrosol were detected as the main phenols in olive oil. p-Coumaric, syringic, and ferulic acids were detected at trace amounts. In Mediterranean region, hydroxytyrosol amount of EVOOs ranged from 0.44 to 28.37 mg/kg, and tyrosol amount was detected between 0.12 and 15.82 mg/kg. Nizip-Gemlik/Gaziantep (Nzg) had highest amount of both hydroxytyrosol and tyrosol. Within Aegean samples, tyrosol amount ranged from 0.32 to 41.21 mg/kg, and hydroxytyrosol amount ranged from 0.15 to 34.43 mg/kg. Erkence/Mordağan has the highest tyrosol value. There is no statistically significant difference in tyrosol values of Ayvalık cultivar located in Kabakum (Kbk), Karaağaç (Kae), and Gülpınar (Glk). However, hydroxytyrosol is the main phenolic in olive oil; Karaağaç, Kabakum (Kbk), and Erkence (Mor) cultivars were richer in tyrosol. According to the findings of Kesen et al. (2014), tyrosol amount of Nizip yaglık and Kilis yaglık samples was higher than their hydroxytyrosol content and ranged between 12.45–18.73 and 13.39–15.84 mg/kg, respectively (Kesen et al. 2014). Contrary to these findings, we obtained higher hydroxytyrosol amount than tyrosol for Mediterranean samples. Hydroxytyrosol and tyrosol contents of Ayvalık and Memecik were higher than Spanish and Tunisian olive oils (Dhifi et al. 2004). Gallic acid amount in all samples ranged from 1.55 to 17.3 mg/kg. While Nizip yaglık had the highest gallic acid amount, Erkence/Savandere (Sav) had the lowest amount.
Tocopherol Content
Tocopherols are main lipophilic antioxidant and have an important effect to prevent lipid oxidation. Tocopherol isomers of olive oils are presented in Table 6 and 7; α-tocopherol was the main tocopherol isomer in olive oils. α-Tocopherol content varied from 115.2 to 279.5 mg/kg oil in Mediterranean region and varied from 22.2 to 263.4 mg/kg oil in Aegean region. Gemlik cultivar had the highest α-tocopherol content (279.5 mg/kg oil); also it had the highest TPC content (1064.5 μg gallic acid/g oil). β-Tocopherol content ranged from 9.9 to 13.8 mg/kg oil. γ-Tocopherol content varied from 10.4 to 21.47 mg/kg oil, and δ- tocopherol content varied from 2.8 to 13.2 mg/kg oil in all samples. Differences in total tocopherol content may also be related to variations in climatic conditions as known high amounts of rainfall result in higher levels of tocopherol in olives (Ilyasoglu et al. 2010), as it is known that Mediterranean region received rain more than Aegean region at the same cultivation time. This result is that average α-tocopherol content of Mediterranean samples is higher than Aegean samples. α-Tocopherol content of Turkish olive oil was determined before for Memecik and Ayvalık; our results were similar to their findings (Andjelkovic et al. 2009; Ilyasoglu et al. 2010), but all of the tocopherol isomers were determined for the first time. α-Tocopherol contents of Gemlik and Memecik cultivars were similar to some Spanish olive oil, but the other cultivars were lower than Tunisian and some Greece olive oils (Benito et al. 2010; Dhifi et al. 2004; Haddada et al. 2008)
Chlorophyll and Carotenoid Content
Chlorophyll and carotenoids are the main light-harvesting pigments in vegetable oils. Pigment content of olive oil is affected by various factors including fruit variety and ripening stage (Ilyasoglu et al. 2010). Color values and β-carotene content of oils are shown in supplementary material. The chlorophyll pigment varied from 1.68 to 4.69 mg/kg, and β-carotene varied from 0.28 to 0.84 mg/kg oil in Mediterranean region. Mix 1 had the highest chlorophyll-a value, and Nizip yağlık had the highest β-carotenoid value. In Aegean region, chlorophyll-a value ranged from 1.12 to 6.92 mg/kg oil, and β-carotene content ranged from 0.09 to 0.86 mg/kg. Whereas Ayvalık and Erkence cultivars had higher chlorophyll content than Tunisian and Spanish olive oils, Tunisian olive oil had a richer carotenoid content than that in our findings.
Squalene Content
The primary squalene sources are shark and whale liver oils. The other sources are olive oil, wheat germ, and rice bran oils. Squalene has some beneficial health effects such as decreasing cholesterol levels and preventing colon cancer (Ghimire et al. 2009). The results are shown in supplementary material; squalene content varied from 2.6 to 11.7 g/kg oil in Mediterranean region and varied from 0.5 to 7.8 g/kg oil in Aegean region. While Sarıulak (Sru) cultivar had the highest squalene value, Ayvalık cultivar collected from Havran (Hav) had the lowest content. There were significant differences (p<0.05) in the squalene content of oils produced from different regions. Olive cultivar and growing region might affect squalene content of olive oils (Gokcebag et al. 2013).
Antioxidant Activity Tests
ABTS, DPPH, and ORAC assays are widely used to evaluate the antioxidant activity. The results are summarized in Tables 6 and 7. ABTS-scavenging capacity ranged from 120.4 to 497.2 μg Trolox/g oil, DPPH-scavenging capacity ranged from 106.5 to 309.0 μg Trolox/g oil, and ORAC values varied from 91.2 to 800.8 μmol/100g oil, in Aegean region. ABTS-scavenging capacity ranged from 117.2 to 702.1μg Trolox/g oil, DPPH-scavenging capacity ranged from 95.6 to 407.2 μg Trolox/g oil, and ORAC values varied from 154.9 to 845.1 μmol/100g oil, in Mediterranean region. In Aegean region, Ayvalık cultivar collected from Demirciköy (Dem) cultivar had the highest ORAC-scavenging capacity (800.8 μmol/100g oil), and also Erkence cultivar collected from Mordoğan (Mor) had the highest ABTS and DPPH radical scavenging capacities. Gemlik cultivar (Nzg) had the highest antioxidant activities of three antioxidant capacity tests; also, it had the highest TPC value (1064.5 mg/kg oil) and the highest α-tocopherol (279.5 mg/kg oil) content. In the present study, phenolic content and antioxidant activity values showed a similar trend. Both phenolic and non-phenolic compounds affected antioxidant activities (Abuzaytoun and Shahidi 2006).
Statistical Analysis
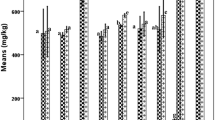
In the study, the olive oil samples from different growing regions were classified according to the seven factors (FA, TAG profiles, antioxidant activity tests, etc.) collected by HPLC and GC. The result of SIMCA test showed that the samples were distinguished according to levels of major and minor components and antioxidant activity. Cooman’s plot (Fig. 1) was generated to visualize the residual distances of all olive oil samples to each other. For SIMCA plots, boundaries marked around the sample clusters represent a 95% confidence interval for each class. When the residual variance of a sample exceeds the boundary limit for the modeled class in the data set, it was not assigned to any of the classes; it was either assigned as an outlier or belongs to a class not represented in the data set.
Soft independent modeling of class analogy (SIMCA) Cooman’s plot of non-transformed data, using seven factors collected by HPLC and GC. For SIMCA plots, boundaries marked around the sample clusters represent a 95% confidence interval for each class. When the residual variance of a sample exceeds the boundary limit for the modeled class in the data set, it was not assigned to any of the classes; it was either assigned as an outlier or belongs to a class not represented in the data set
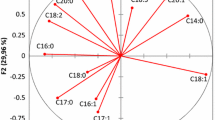
Samples that form clusters on Cooman’s plot are more similar than samples with greater distances between them. Olive oil samples from the Mediterranean region did not form better cluster in Cooman’s plot, but classification of samples from other regions was more successful, indicated by the closer clustering in the plot. This could be due to fatty acid composition, TAG composition, and antioxidant value of the Aegean region samples close to each other. SIMCA results indicated that olive oils from Aegean region were located in a smaller area than oils originating from Mediterranean region. Interclass distances between olive oils based on the SIMCA class projections of the HPLC and GC chromatograms collected for FFA, TAG, AA, sterol content, and some minor compounds were given in supplementary material. After a successful discrimination of olive oil samples, SIMCA classes were also developed (Fig. 2). The main factors of the discrimination were covered by factor 1 (26.5%), factor 2 (16.4%), and factor 3 (12.6%).
Conclusion
It has been shown that significant differences were obtained in some features of olive oils in the studied cultivars. Both genetic factors and environmental conditions are thought to be the possible reasons of the variations in FA, TAG, and sterol composition. However, for all the oils, the levels of fatty acids were in the ranges established for “extra virgin olive oil” category, indicating the high potential quality of the oils from different olive varieties of Turkey. All the described results have shown out there is a wide variability in the chemical and sensory characteristics of the virgin olive depending on the diversity of the varieties. The results indicated that the Turkish monocultivar oils from the two (Aegean and Mediterranean) regions of the Turkey would accurately be classified for olive cultivar or growing area using FA profile. We can conclude that the oil quality of Ayvalık cultivar could be the best with regard to nutritional value in the Aegean region. The results of this study provided useful information to control labeling and of building up the reference set necessary for establishing criterion of geographical origin, especially Aegean region of Turkey, and finally increasing competitiveness of these products on the market. Furthermore, the present study can be an interest in the introduction of new cultivars to the regions outside their traditional growing locations.
References
Abuzaytoun R, Shahidi F (2006) Oxidative stability of algal oils as affected by their minor components. J Agric Food Chem 54(21):8253–8260. https://doi.org/10.1021/jf061047s
Andjelkovic M, Acun S, Van Hoed V, Verhe R, Van Camp J (2009) Chemical composition of Turkish olive oil--Ayvalik. J Am Oil Chem Soc 86(2):135–140. https://doi.org/10.1007/s11746-008-1330
AOAC (n.d.) Official Methods 965.33, A. O. M. Peroxide value of oils and fats. Official Methods of Analysis of AOAC International,17th ed., . Gaithersberg: AOAC International 965.33, A. O. M. Peroxide value of oils and fats
Baiano A, Terracone C, Viggiani I, Del Nobile MA (2013) Effects of cultivars and location on quality, phenolic content and antioxidant activity of extra-virgin olive oils. J Am Oil Chem Soc 90:103–111
Benito M, Oria R, Sanchez-Gimeno AC (2010) Characterization of the olive oil from three potentially interesting varieties from Aragon (Spain). Food Sci Technol Int 16(6):523–530. https://doi.org/10.1177/1082013210367542
Bondet V, BrandWilliams W, Berset C (1997) Kinetics and mechanisms of antioxidant activity using the DPPH free radical method (vol 30, pg 609, 1997). Food Sci Technol Lebensmittel-Wissenschaft & Technologie 30(7):772–772
BS EN ISO 660:2009, O. m. o. a. (2009) Animal and vegetables fats and oils-determination of acid value and acidity
Canözer O (1991) The catalogue of standard olive cultivars. Ministry of Agriculture and Rural Affairs. The General Directorate of Agricultural Production and Development: Professional publications
Criado MN, Romero MP, Casanovas M, Motilva MJ (2008) Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem 110(4):873–880. https://doi.org/10.1016/j.foodchem.2008.02.075
Dag C, Demirtas I, Ozdemir I, Bekiroglu S, Ertas E (2015) Biochemical characterization of Turkish extra virgin olive oils from six different olive varieties of identical growing conditions. J Am Oil Chem Soc 92(9):1349–1356. https://doi.org/10.1007/s11746-015-2691-7
Dhifi W, Hamrouni I, Ayachi S, Chahed T, Saidani M, Marzouk B (2004) Biochemical characterization of some Tunisian olive oils. J Food Lipids 11(4):287–296. https://doi.org/10.1111/j.1745-4522.2004.01148.x
Diraman H, Dibeklioglu H (2009) Characterization of Turkish virgin olive oils produced from early harvest olives. J Am Oil Chem Soc 86(7):663–674. https://doi.org/10.1007/s11746-009-1392-5
Diraman H, Saygi H, Hisil Y (2010) Relationship between geographical origin and fatty acid composition of Turkish virgin olive oils for two harvest years. J Am Oil Chem Soc 87(7):781–789. https://doi.org/10.1007/s11746-010-1557-2
Diraman H, Saygi H, Hisil Y (2011) Classification of three Turkish olive cultivars from Aegean region based on their fatty acid composition. Eur Food Res Technol 233(3):403–411. https://doi.org/10.1007/s00217-011-1511-z
E. U. C. R. E. Amending Regulation (EEC) (1989/2003) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis, Official Journal of the European Union L295.
FAO (2018) Statistical databases
Ghimire GP, Lee HC, Sohng JK (2009) Improved squalene production via modulation of the methylerythritol 4-phosphate pathway and heterologous expression of genes from Streptomyces peucetius ATCC 27952 in Escherichia coli. Appl Environ Microbiol 75(22):7291–7293. https://doi.org/10.1128/Aem.01402-09
Gokcebag M, Diraman H, Ozdemir D (2013) Classification of Turkish monocultivar (Ayvalik and Memecik cv.) virgin olive oils from north and south zones of Aegean region based on their triacylglycerol profiles. J Am Oil Chem Soc 90(11):1661–1671. https://doi.org/10.1007/s11746-013-2308-y
Gurdeniz G, Ozen B, Tokatli F (2008) Classification of Turkish olive oils with respect to cultivar, geographic origin and harvest year, using fatty acid profile and mid-IR spectroscopy. Eur Food Res Technol 227(4):1275–1281. https://doi.org/10.1007/s00217-008-0845-7
Gutfinger T (1981) Polyphenols in olive oils. J Am Oil Chem Soc 58:966–968
Haddada FM, Krichiene D, Manai H, Oueslati I, Daoud D, Zarrouk M (2008) Analytical evaluation of six monovarietal virgin olive oils from northern Tunisia. Eur J Lipid Sci Technol 110(10):905–913. https://doi.org/10.1002/ejlt.200700251
Huang DJ, Ou BX, Hampsch-Woodill M, Flanagan JA, Deemer EK (2002) Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated beta-cyclodextrin as the solubility enhancer. J Agric Food Chem 50(7):1815–1821. https://doi.org/10.1021/jf0113732
Ilyasoglu H, Ozcelik B, Van Hoed V, Verhe R (2010) Characterization of Aegean olive oils by their minor compounds. J Am Oil Chem Soc 87(6):627–636. https://doi.org/10.1007/s11746-009-1538-5
IOC (2015) Trade standard applying to olive oils and olive pomace oils. International Olive Council, COI/T.15/NC No 3/Rev. 8
Kesen S, Kelebek H, Selli S (2014) LC-ESI-MS characterization of phenolic profiles Turkish olive oils as influenced by geographic origin and harvest year. J Am Oil Chem Soc 91(3):385–394. https://doi.org/10.1007/s11746-013-2380-3
Lanza CM, Russo C, Tomaselli F (1998) Relationship between geographical origin and fatty acid composition of extra-virgin olive oils produced in three areas of eastern Sicily. Ital J Food Sci 10(4):359–366
Lopez-Miranda J, Perez-Jimenez F, Ros E, De Caterina R, Badimon L, Covas MI et al (2010) Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr Metab Cardiovasc Dis 20(4):284–294. https://doi.org/10.1016/j.numecd.2009.12.007
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9-10):1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Uluata S, Ozdemir N (2012) Antioxidant activities and oxidative stabilities of some unconventional oilseeds. J Am Oil Chem Soc 89(4):551–559. https://doi.org/10.1007/s11746-011-1955-0
Uluata S, Altuntas U, Ozcelik B (2016) Biochemical characterization of Arbequina extra virgin olive oil produced in Turkey. J Am Oil Chem Soc 93(5):617–626. https://doi.org/10.1007/s11746-016-2811-z
Ulupınar Y, Özcan H, Demircan M, Kervankıran S (2012) 2011 Yılı Iklim Değerlendirmesi. from General directorate of meteorological service
Verleyen T, Forcades M, Verhe R, Dewettinck K, Huyghebaert A, De Greyt W (2002) Analysis of free and esterified sterols in vegetable oils. J Am Oil Chem Soc 79(2):117–122. https://doi.org/10.1007/s11746-002-0444-3
Yorulmaz A, Yavuz H, Tekin A (2014) Characterization of Turkish olive oils by triacylglycerol structures and sterol profiles. J Am Oil Chem Soc 91(12):2077–2090. https://doi.org/10.1007/s11746-014-2554-7
Acknowledgements
The authors thank Zeytindostu Agency for supplying extra virgin olive oils.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with humans.
Consent to Participate
Informed consent is not applicable. All the authors named in the manuscript are entitled to the authorship and have approved the final version of the submitted manuscript.
Conflict of Interest
Sibel Uluata declares that he has no conflict of interest. Umit Altuntaş declares that he has no conflict of interest. Beraat Özçelik declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Uluata, S., Altuntaş, U. & Özçelik, B. Characterization of Turkish Extra Virgin Olive Oils and Classification Based on Their Growth Regions Coupled with Multivariate Analysis. Food Anal. Methods 14, 1682–1694 (2021). https://doi.org/10.1007/s12161-021-01996-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-01996-4