Abstract
A characterization study of Turkish monovarietal olive oils using chemical variables such as fatty acid, sn-2 fatty acid, triacylglycerol, and sterol composition is presented. A total of 101 samples of Olea europaea L. fruits from 18 cultivars were collected for two crop years from west, south, and southeast regions of Turkey. Olives were processed to oil and olive oil samples were evaluated for their triacylglycerol structures and sterol composition. Oleic acid content ranged from 60.15 to 80.46 % of total fatty acids and represented 70.90–89.02 % of sn-2 position triacylglycerols. Major triglycerides of oil samples were triolein, palmitodiolein, dioleolinolein, palmitooleolinolein, dipalmitoolein, and stearodiolein. Triolein values were between 24.72 and 48.64 % and compatible with the fatty acid composition. Total sterol content varied from 1,145.32 to 2,211.77 mg/kg and Edremit yaglık stood out because of its high sterol content. A one-way analysis of variance revealed significant differences for variables among cultivars. Principle component analysis enabled the classification of common varieties on the basis of analytical data. Sterol composition achieved more relevant discrimination than fatty acid and triglyceride composition. Classification according to geographical origin was performed by discriminant analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Virgin olive oil (VOO) is obtained from healthy olive drupes using only mechanical processing and it is therefore edible without further refining. Its consumption has increased throughout the world owing to its sensory properties and nutritional value deriving from its high monounsaturated/polyunsaturated fatty acid ratio [1] together with natural antioxidant content [2]. There are many types of olive oils available on the market with typical chemical composition and sensorial features depending on the variety and geographical district where the olives are grown. The European Union established a system known as Protected Designation of Origin (PDO) and Protected Geographical Indications (PGI) for certification of foods according to their designation of origin to ensure and improve quality of products. Olive oils with PDO certificates have higher market prices, which favors adulterations with lower grade oils or oils from different areas of origin. Hence, particular attention has been paid to accumulate knowledge regarding the chemical composition of oils obtained from various cultivars and geographical area in order to check authenticity and determine the identity of oils. Qualitative and quantitative analysis of triacylglycerols, fatty acids, sterols, tocopherols, hydrocarbons, phenols, and volatiles followed by chemometric treatments have been widely used for characterizing and confirming authenticity of virgin olive oils on the basis of their variety, growing area, and harvest year.
Triacylglycerols (TAG) are major components of oils and are derived from the esterification of glycerol with three fatty acids. Physical, nutritional, and chemical properties of oils are highly associated with the composition and location of fatty acids in TAG molecules [3]. The sn-1 and sn-3 positions of triglycerides are usually bonded to saturated fatty acids, whereas the sn-2 position includes unsaturated ones. The fatty acid composition of VOO is significantly influenced by the type of cultivar, ripeness stage of the fruit, climatic conditions, latitude, irrigation management, and zone of production [4]. There are plenty of works revealing the TAG and fatty acid profiles of olive oils for Italian [5, 6], Spanish [7, 8], Greek [9], Iranian [10], French [11, 12], Tunisian [13, 14], and Moroccan [15] cultivars. Although a moderate number of papers have been published on the fatty acid composition of Turkish olive oils [16–21], few are available on the triglyceride distribution of varieties including only prevalent ones [22–25].
Phytosterols are the main components of the unsaponifiable fraction of lipids. Sterol composition is important for assessing authenticity, detecting adulteration, and characterization purposes because it is specific for each oil. Major sterols of olive oil are β-sitosterol, Δ-5-avenasterol, and campesterol. The sterol profile of olive oil is affected by the cultivar, degree of ripening of the fruits, agronomic and climatic conditions, oil extraction technique, and storage conditions [26, 27]. Spanish [28, 29], Italian [30, 31], Portuguese [32, 33], Tunisian [13, 34], and Turkish [21, 22, 35, 36] olive varieties have been evaluated for their sterol composition.
Chemometric methods such as principal component analysis (PCA), hierarchical cluster analysis (HCA), and discriminant analysis (DA) have gained increasing importance in recent years owing to promising results from characterization studies. Analytical data obtained by chromatographic methods and quantitative information determined by spectroscopic procedures utilizing near-infrared (NIR) spectroscopy [37], Fourier transform infrared (FTIR) spectroscopy [38], and fluorescence spectroscopy [39] were evaluated by multivariate statistical techniques for classification issues in previous works. Turkish olive oils have been characterized according to mainly fatty acid composition [19, 20, 40, 41], TAG [23], fatty acid and TAG profile [25], aroma [42, 43], fatty acid and mid-infrared spectra [16], and phenolic compounds [44]. The first aim of this study was to determine fatty acid, sn-2 fatty acid, triacylglycerol, and sterol composition of olive oils obtained from 18 Turkish olive cultivars including both common and domestic ones for two successive crop years in order to establish a database for authentication and identification purposes. The second aim was to characterize and classify oils according to variety and growing region by using the acquired data. This study is the second part of a wider project and intends to complete the characterization of Turkish olive oils which began by evaluating Turkish olive cultivars and their corresponding oils in terms of their phenolic profiles [45].
Materials and Methods
Reagents and Standards
Isopropyl alcohol, isooctane, lipase, silica gel, and 5α-cholestan-3β-ol were obtained from Sigma (St-Louis, USA); sodium cholate, acetonitrile, hydrochloric acid, methanol, tris(hydroxymethyl)aminomethane, hexane, formic acid, β-sitosterol, pyridine, and chloroform were purchased from Merck (Darmstadt, Germany), N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) with trimethylchlorosilane (TMSC), and a 37 fatty acid methyl ester mix were from Supelco (Bellefonte, USA). The reagents used in HPLC were of HPLC grade.
Olive Sampling and Olive Oil Extraction
The study was performed in 2006–2007 and 2007–2008 harvest years with 18 olive cultivars collected from 13 provinces placed at four different olive growing areas in Turkey (Table 1). The North Aegean region included Canakkale, Bursa, Balıkesir, Izmir, and Manisa; the South Aegean region contained Aydın and Mugla; the Mediterranean region involved Antalya, Adana, Mersin, and Hatay provinces; and the Southeast Anatolia region was composed of Gaziantep and Kilis. Memecik, Edremit yaglık (Ayvalık), and Gemlik are predominant olive varieties of Turkey and grown in more than one region; hence, more samples were available for these varieties. Halhalı was obtained from two different regions, although it has a low production potential. Kilis yaglık and Nizip yaglık are economically important varieties of Southeast Anatolia and grown in large areas. Uslu and Erkence are generally used for table olive production and are specific to the North Aegean. The remaining varieties were relatively domestic. Olive samples were mainly harvested from their respective traditional growing areas where they are cultivated as the unique and well-known variety. Olives were also tested for their physical characteristics such as fruit weight, endocarp properties, and flesh/pit ratio for clear identification of cultivars. Samples of younger trees, especially the Gemlik variety, were collected from certified orchards to ensure the correct determination of varieties.
Olea europaea L. fruits were collected from two trees in each case during the harvest period starting from the November to the end of December. After being transported to the laboratory, olives were deleafed and washed. A representative 5-kg sample of fruits was processed to oil by a laboratory scale mill (Spremoliva, Italy). The mill features a cylinder where crushing, malaxing, olive oil extraction, and waste discharge can be executed. Malaxation was performed at 27 °C for 25 min. Olive oil samples were kept at 4 °C under a nitrogen atmosphere until analyses.
Analyses
Fatty Acid Composition
Fatty acid methyl esters were prepared as described by the International Union of Pure and Applied Chemistry [46] and analyzed using a GC-2010 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a split/splitless injector (T = 290 °C) and flame ionization detector (T = 240 °C). A DB-23 fused silica capillary column (60 m × 0.25 mm i.d. and 0.25 μm film thickness; J&W Scientific) was employed to separate individual fatty acids. The oven temperature was 195 °C. The carrier gas was helium with a flow rate of 1 ml/min and the split ratio was 80:1. The results were expressed as percentage of each fatty acid.
Fatty Acids at the 2 Position of Triglycerides
Fatty acids at the 2 position of triglycerides were determined according to AOCS Official Method Ch 3-91 [47]. The gas chromatographic conditions were the same as those used for individual fatty acids.
Iodine Value and Saponification Number
The iodine value and saponification number were calculated from the fatty acid composition of the oils according to AOCS Cd 1c-85 and Cd 3a-94 methods, respectively [47].
Triglyceride Composition
Oil samples (3 %) were dissolved in acetonitrile/2-propanol/hexane (2:2:1) prior to analysis. Chromatographic separation was achieved according to the method described by Herslöf [48]. An HPLC apparatus (Shimadzu, Kyoto, Japan) equipped with a differential refractometer detector and Nucleosil 100 C18 column (25 × 0.46, 5 μm particle size; Teknochroma, Barcelona, Spain) was employed. The mobile phase was acetonitrile/2-propanol/hexane (500:118:100) with a flow rate of 1.0 ml/min. The column temperature was held at 25 °C and the injection volume was 50 μl. Triacylglycerols were separated according to equivalent carbon number (ECN) and the peaks were identified by comparing with literature data [12].
Sterol Composition
Sterol compositions were determined according to AOCS Official Method Ch 6-91 [47]. Sterol derivatives (silyl ethers) were analyzed with a gas chromatograph (GC-2010, Shimadzu, Japan) equipped with a flame ionization detector (T = 290 °C) and split/splitless injector (T = 280 °C). An HP-5 fused silica capillary column (30 m, 0.25 mm i.d., and 0.25 mm film thickness; Chrom Tech., Apple Walley, MN, USA) was employed to separate individual sterols. The oven temperature was 260 °C. The carrier gas was helium with a flow rate of 0.8 ml/min and the split ratio was 80:1. 5α-Cholestan-3β-ol was used as the internal standard for quantification.
Statistical Analysis
Statistical analysis was performed using SPSS 15 statistical software (SPSS Inc., Chicago, USA). Data were evaluated by one-way ANOVA procedure using the Duncan’s multiple range test to determine if there were any significant differences between cultivars. A p value of less than 0.05 was considered significant. Correlation analysis was performed using Pearson’s test. Data were also processed by PCA and DA using XLSTAT 2014 version (Addinsoft, New York, NY).
Results
Fatty Acid Composition
Table 2 shows the average fatty acid composition of Turkish olive oils. The main fatty acids were palmitic (C16:0), oleic (C18:1), and linoleic (C18:2) acids. Palmitoleic (C16:1), linolenic (C18:3), arachidic (C20:0), myristic (C14:0), heptadecanoic (C17:0), heptadecenoic (C17:1), stearic (C18:0), gadoleic (C20:1), behenic (C22:0), and lignoceric (C24:0) acids were determined in low amounts. The predominant fatty acid in all samples was oleic acid ranging from 60.15 % (Domat) to 80.46 % (Girit). Linoleic acid was the second most important fatty acid varying between 5.34 % (Girit) and 18.29 % (Antalya yaglik). The oleic/linoleic acid ratio is important for oxidative stability of the oils. The Girit variety was characterized by its high oleic/linoleic acid ratio (15.06), whereas this ratio is considerably lower for Antalya yaglik (3.43), Domat (3.69), and Sarı hasebi (3.90) cultivars. Palmitic acid was the main saturated fatty acid of oil samples and present at between 9.77 (Girit) and 17.40 % (Domat). Samples having higher oleic acid content had lower palmitic or linoleic acid contents as in the Girit variety. Oils of Nizip yaglik, Kilis yaglik, Domat, Sarı Ulak, and Halhalı varieties have higher palmitic acid content, whereas oils of Antalya yaglik and Sarı hasebi varieties have higher linoleic acid content than the other cultivars.
The fatty acid compositions of the Turkish olive cultivars were within the legal limits proposed by the Turkish Food Codex [49] which is consistent with EU regulations, except for the linolenic acid content of the Domat cultivar (1.04 %), which is a local variety cultivated in a restricted area. The minimum and maximum percentages of remaining fatty acids were as follows: C14:0, 0–0.03 % (Halhalı); C16:1, 0.69 (Sarı ulak)–1.72 % (Kalamata); C17:0, 0.02 (Sarı hasebi)–0.18 % (Saurani, Sarı ulak); C17:1, 0.04 (Sarı hasebi)–0.26 % (Gulumbe); C18:0, 1.46 (Girit)–3.70 % (Saurani); C18:3, 0.56 (Halhalı)–1.04 % (Domat); C20:0, 0.21 (Kalamata)–0.57 % (Halhalı); C20:1, 0.20 (Nizip yaglık)–0.31 % (Gulumbe); C22:0, 0.04 (Kalamata)–0.16 % (Saurani); C24:0, 0.01 (Uslu, Kalamata, Sarı hasebi, Gulumbe)–0.19 % (Domat). The fatty acid profiles of the Turkish olive oil samples were also in good agreement with the values obtained for the same cultivars in some other harvest seasons [16–18, 40].
Elaidic acid (tr C18:1) was not observed in any of the samples. The total contents of trans forms of linoleic and linolenic acids (tr C18:2 + tr C18:3) were between 0 and 0.03 % (data not shown in the table), within the limits of international and Turkish regulations. A correlation study was carried out to elucidate the relationships between variables. There were high and positive correlations between C17:0 and C17:1 (r = 0.819) and C18:0 and C20:0 (r = 0.749), whereas there was a negative and strong correlation between C18:1 and C18:2 (r = −0.807).
The saponification numbers were between 192 and 193 mg KOH/g for olive oil samples. The iodine values of the samples were between 75.63 and 89.88 (data not shown), similar to those found in previous work [23, 40].
Fatty Acids at the 2 Position of Triglycerides
Table 3 shows the fatty acid composition of the sn-2 position of triglycerides. C16:0, C16:1, C17:1, C18:0, C18:1, C18:2, and C18:3 were identified in the sn-2 position of triglycerides. The sn-2 fatty acid compositions have been used for characterization of VOO and also for checking the presence of synthetic triglycerides. Unsaturated fatty acids generally occupy the 2 position of triacylglycerols. Oleic acid ranged from 70.90 % (Antalya yaglik) to 89.02 % (Girit), whereas linoleic acid ranged from 6.30 % (Girit) to 25.50 % (Antalya yaglik). Oleic acid contents were quite lower whereas linoleic acid percentages were rather higher than the ratios determined for the Cornicabra variety [7]. Linolenic acid content was between 0.60 % (Kalamata) and 1.70 % (Celebi) which is higher than the ratios reported by Aranda et al. [7] and Tekin [50]. The main saturated fatty acids, the combination of palmitic and stearic acids, were considerably higher in oil from Nizip yaglik (1.52 %), Kalamata (1.72 %), and Girit (2.37 %) varieties, which are generally cultivated in the south region of Anatolia peninsula. Positive and high correlations were determined between sn-2 C16:1 and C16:1 (r = 0.772), sn-2 C17:1 and C17:0 (r = 0.735), sn-2 C17:1 and C17:1 (r = 0.715), sn-2 C18:1 and C18:1 (r = 0.755), and sn-2 C18:2 and C18:2, whereas strong and negative ones were observed between sn-2 C18:1 and C18:2 (r = −0.911), sn-2 C18:2 and C18:1 (r = −0.741), and sn-2 C18:1 and sn-2 C18:2 (r = −0.994). To our knowledge, this is the first report evaluating the fatty acids in the sn-2 position of triglycerides of Turkish monovarietal olive oils.
Triglyceride Composition
The triacylglycerol composition of the Turkish olive oils is given in Table 4 and an HPLC chromatogram of an oil sample is shown in Fig. 1. The main triglycerides determined for the Turkish olive oils were triolein (OOO), palmitodiolein (OOP), dioleolinolein (OOL), palmitooleolinolein (PLO), dipalmitoolein (POP), and stearodiolein (SOO). Trilinolein (LLL), oleolinoleolinolenin (OLLn), palmitolinoleolinolenin (PLLn), oleodilinolein (OLL), dioleolinolenin (OOLn), palmitodilinolein (PLL), palmitooleolinolenin (POLn), palmitoleodiolein (OOPo), palmitoleopalmitoolein (PoOP), dipalmitolinolein (PPL), stearooleolinolein (SOL), and palmitostearoolein (POS) were also observed in all analyzed samples. The major triglyceride, triolein (OOO), was present at between 24.72 % (Domat) and 48.64 % (Gulumbe) in agreement with the fatty acid results. Aranda et al. [7] reported 51.7 % for the Cornicabra variety, Graciani [51] determined 48.4 % for Picual, Ollivier et al. [11] reported 27.32–58.76 % for French cultivars, whereas Baccouri et al. [52] described 24.6–49.2 % and 45.6–63.2 % for Tunusian and Sicillian varieties, respectively. OOP, the second most important triglyceride, had values between 21.09 % (Erkence) and 28.86 % (Nizip yaglik), higher than the results obtained for some Tunusian [13, 34] and French [12] cultivars but in accordance with the results obtained for Italian [53] and Iranian [10] varieties. OOL (accomplished with PPLn) was between 8.19 % (Girit) and 15.80 % (Domat) consistent with the values reported by Piravi-Vanak et al. [10], Ollivier et al. [12], and Chiavaro et al. [53], but higher than those described by Ben Temime et al. [34] and Baccouri et al. [52]. PLO (accomplished with SLL) was between 4.82 % (Girit) and 13.87 % (Domat) comparable to the previous work [13]. Similar triglyceride profiles were reported for Memecik, Edremit yaglık [22]; Gemlik [24]; and Ayvalık (Edremit yaglık), Uslu, Nizip yaglık, Memecik varieties [25].
Olive oil has mainly triglycerides with equivalent chain numbers (ECNs) of 44, 46, 48, and 50. ECN 42 triglycerides are indicators of more unsaturated oils. Trilinolein and ECN 42 levels of the Turkish olive oils ranged from 0.04 % (Celebi) to 0.51 % (Sarı hasebi) and 0.19 % (Gulumbe) to 1.00 % (Antalya yaglik), respectively. ECN 44, ECN 46, ECN 48, and ECN 50 values of samples were 2.35 (Gulumbe)–8.77 % (Antalya yaglık), 15.49 (Girit)–36.27 % (Antalya yaglık), 50.01 (Antalya yaglık)–75.15 % (Girit), and 3.60 (Mersin yaglık)–8.52 % (Saurani) in the same order.
Strong positive correlations were determined between OLL and C18:2 (r = 0.886), PLL and C18:2 (r = 0.735), PLO and C18:2 (r = 0.792), OOO and C18:1 (r = 0.776), OLL and sn-2 C18:2 (r = 0.910), OLL and LLL (r = 0.757), OLL and PLL (r = 0.782), PoOP and POLn (r = 0.702), PLO and OLL (r = 0.739), PLO and PLL (r = 0.730), PLL and sn-2 C18:2 (r = 0.746), PLO and sn-2 C18:2 (r = 0.794), and OOO and sn-2 C18:1 (r = 0.764). Conversely, there were high negative correlations between OLL and C18:1 (r = −0.728), PLO and C18:1 (r = −0.733), OOO and C18:2 (r = −0.747), OLL and sn-2 C18:1 (r = −0.911), PLL and sn-2 C18:1 (r = −0.756), PLO and sn-2 C18:1 (r = −0.799), and OOO and sn-2 C18:2 (r = −0.744).
Sterol Content and Composition of Turkish Olive Oils
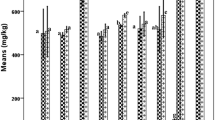
Table 5 shows the individual sterol content and triterpene dialcohol ratio of the Turkish olive oils. The main sterols found in olive oil were β-sitosterol, Δ-5-avenasterol, and campesterol. Cholesterol, brassicasterol, 24-methylene-cholesterol, campestanol, stigmasterol, Δ-7-campesterol, clerosterol, sitostanol, Δ-5,24-stigmastadienol, Δ-7-stigmastenol, Δ-7-avenasterol, and two triterpene dialcohols (erythrodiol and uvaol) were also determined in smaller amounts. The total sterol contents of all samples were above the legal threshold value (1,000 mg/kg) determined for extra virgin olive oil by EU regulations, ranging from 1,145.32 (Kalamata) to 2,211.77 mg/kg (Edremit yaglık). Edremit yaglık has also been well discriminated with its high sterol content in previous works [22, 36]. β-Sitosterol, the predominant sterol of olive oil, varied between 976.66 (Kalamata) and 1,836.26 mg/kg (Edremit yaglik). β-Sitosterol was the most densely present in the Memecik variety (89.25 %) and least concentrated in Saurani (82.04 %). The second most abundant sterol was Δ-5-avenasterol fluctuating between 38.54 (Sarı ulak) and 197.77 mg/kg (Edremit yaglık). Matthäus and Özcan [21] reported similar Δ-5-avenasterol values for Edremit and Domat, but lower values for Gemlik and Sarı ulak varieties. β-Sitosterol and Δ-5-avenasterol contents have a strong negative correlation (r = −0.982), possibly because of desaturase enzyme activity transforming β-sitosterol to Δ-5-avenasterol [54]. Regarding campesterol, Edremit yaglık had the highest (67.50 mg/kg) and Kalamata had the lowest (22.82 mg/kg) values. The amounts of the other sterols, such as 24-methylene-cholesterol, campestanol, and Δ-7-campesterol, were less than 6 ppm. Sarı ulak, Edremit yaglık, and Saurani varieties contained the highest levels of sitosterol, Δ-5,24-stigmastadienol, and Δ-7-avenasterol correspondingly.
Sterol percentages of the Turkish olive oils were in agreement with both national and international directives with some exceptions. The Girit variety exceeded both cholesterol and campesterol upper limits with 0.54 and 4.09 % ratios. Likewise, Δ-7-stigmastenol contents of Erkence, Mersin yaglık, Saurani, Kilis yaglık, and Sarı hasebi oils were over the regulatory maximum limit of 0.5 %, specifically 0.57, 0.90, 0.80, 0.53, and 0.70 %, respectively. Although Mersin yaglık, Saurani, Sarı hasebi, and Girit are relatively domestic varieties, Kilis yaglık and Erkence have higher economic value because they comprise 2.8 % and 3.5 % of the total olive trees in Turkey [55]. All olive varieties having incompatible ratios with legal regulations in terms of individual sterols account for about 8 % of the total olive trees.
The sum of tritepene dialcohols (erythrodiol and uvaol) must be lower than 4.5 % of total sterols. The totals of those tritepene dialcohols were within the limits varying between 0.69 (Sarı ulak) and 4.42 % (Antalya yaglık).
Multivariate Analysis
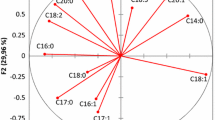
PCA is a multivariate analysis method frequently used for classification purposes. The method reduces the variables to a relatively small number of principal components but maximizes the variability. In this study, analytical data were arranged in a matrix of 101 samples × 52 variables. PLLn, OOPo, PoOP, PPL, 24-methylene-cholesterol, clerosterol, and Δ-7-stigmastanol were discarded because they did not generate significant differences among cultivars. Variables which had a Kaiser–Meyer–Olkin measure of sampling adequacy index lower than 0.5 were also removed. The remaining adequate variables were C16:0, C16:1, C17:0, C17:1, C18:0, C18:1, C18:2, C18:3, C20:0, C20:1, C22:0, C24:0, LLL, OLLn + PoLL, OLL + OLPo, OOLn, PLL, POLn, PLO + SLL, OOO, POS, cholesterol, campesterol, stigmasterol, Δ-7-campesterol, β-sitosterol, Δ-5-avenasterol, Δ-5,24-stigmastadienol, and Δ-7-avenasterol, forming a matrix of 101 samples × 29 variables. Loading plot and factor score plots are given in Figs. 2 and 3, respectively. The first two principle components explained 40.34 % of the total variance [factor 1 (F1), 24.55 %; factor 2 (F2), 15.79 %]. F1 shows high and positive correlations with C18:2, OLL + OLPo, PLL, and PLO + SLL, whereas high and negative correlations with OOO, C18:1, C18:0, and C17:0 positively affect F2. The score plot revealing the relations among varieties shows that Antalya yaglık, Mersin yaglık, Erkence, Celebi, Sarı ulak, Sarı hasebi, and Domat varieties have positive scores, whereas Gulumbe, Girit, and Kalamata varieties have negative scores on F1. Memecik oils were all negatively correlated with F2.
Factor score plot of Turkish olive oils obtained from PCA of data using relevant fatty acids, triacylglycerols, and sterols on the plane identified by two principal components (Uslu: plus, Gemlik: multiplication, Memecik: open triangle, Edremit: open circle, Nizip yaglık: filled inverted triangle, Kilis yaglık:right filled triangle, Erkence: asterik, Gulumbe: dagger, Mersin yaglık: open square, Saurani: S, Antalya yaglık: filled square, Domat: D, Celebi: C, Sarı ulak: open diamond, Halhalı: H, Sarı hasebi: filled diamond, Girit: minus, Kalamata: filled circle)
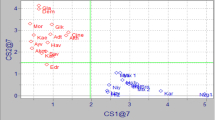
In this study, some common cultivars were represented with higher numbers of samples than some local cultivars. To avoid the scattering of values in PCA, less common cultivars (Gulumbe, Antalya yaglık, Mersin yaglık, Erkence, Celebi, Girit, Domat, Kalamata, Sarı hasebi, Sarı ulak, Halhalı, Uslu, Saurani) represented with fewer samples were eliminated to obtain clearer separations among widespread varieties (Memecik, Gemlik, Edremit, Uslu, Nizip yaglık, Kilis yaglık). Nizip and Kilis yaglık were chosen to be economically important cultivars for their growing areas. Additional PCAs were performed using fatty acid, triacylglycerol, and sterol composition of common olive oils separately. Results revealed that sterol composition was more evident than fatty acid and triacylglycerol profiles for discrimination of varieties. PCA based on sterol profile allowed one to group Memecik and Edremit yaglık cultivars as shown in Fig. 4. The first two principle components explained 63.27 % of the total variance (F1, 45.78 %; F2, 17.49 %). Memecik oils had negative scores (except one sample) and Edremit yaglık oils had positive scores on F1. The first principle component was highly correlated with Δ-5,24-stigmastadienol and β-sitosterol. A global PCA including all relevant analytical data was also employed to distinguish common cultivars resulting in a total variance of 46.08 % (F1, 27.05 %; F2, 19.03 %) (Fig. 5). Memecik and Edremit yaglık oils were grouped on negative and positive sides of F1 respectively. But a clear cluster was not observed for other varieties. F1 was highly correlated with Δ-5,24-stigmastadienol, β-sitosterol, and campesterol.
Factor score plot of Turkish olive oils of common varieties obtained from PCA of data using sterols on the plane identified by two principal components (Uslu: plus, Gemlik: multiplication, Memecik: open triangle, Edremit: open circle, Nizip yaglık: filled inverted triangle, Kilis yaglık: right filled triangle)
Factor score plot of Turkish olive oils of common varieties obtained from PCA of data using fatty acids, triacylglycerols and sterols on the plane identified by two principal components (Uslu: plus, Gemlik: multiplication, Memecik: open triangle, Edremit: open circle, Nizip yaglık: filled inverted triangle, Kilis yaglık: right filled triangle)
The classification of olive oils according to geographical origin was achieved by discriminant analysis. Oils obtained from North Aegean, South Aegean, Mediterranean, and Southeast Anatolia were grouped according to their origin as shown in Fig. 6. The first two discriminant functions explained 75.61 % of the total variance. Olive oils from various geographical areas were not separated clearly and partially overlapped. β-Sitosterol, Δ-5,24-stigmastadienol, C18:1, C18:2, OLL, OOL, PLO, OOO, and OOP had strong standardized discriminant coefficients. The percentage of correct classification of the samples was 93.14 %.
In the context of the current work, variability between two crop years and the contribution of harvest time to the classification of oils were also investigated. However an apparent discrimination according to harvest year could not be accomplished. Also, the relations between geographic area and cultivar were examined using one-way ANOVA procedure. Four of the 18 varieties were collected from more than one location; Gemlik and Edremit yaglik were harvested from three different growing areas, whereas Memecik and Halhalı were obtained from two locations. There were in total 53 variables (fatty acids, sn-2 fatty acids, triacylglycerols, sterols). Among them, four variables generated statistically significant differences for Gemlik, three variables for Memecik, 10 variables for Edremit, and 26 variables for Halhalı (Table 6). Halhalı was clearly affected by the growing region because half of the variables differed because of the geographic area and those variables were composed of major lipid components such as C18:1, sn-2 C18:1, OOO, and β-sitosterol. A limited number of variables of Gemlik and Memecik oils were affected by location and they were mainly minor ones except the palmitic acid content of Gemlik. Edremit oils’ individual sterols were influenced by the growing area.
Conclusion
This work reports the varietal and geographical differentiation of Turkish olive oils based on fatty acid, sn-2 fatty acid, triglyceride, and sterol composition. The results expose that some chemical compounds exceed the legal limits as a result of varietal characteristics which should be taken into consideration in commercial dealings and regulatory studies. In Turkey, there are four regions with PDO certificates: Edremit Gulf Olive Oils, Ayvalık Olive Oils, South Aegean Olive Oils, and Nizip Olive Oils. Olive oils certified with PDOs have higher market values owing to their quality and authenticity. The data reported herein can be used for newer PDO applications.
References
Kiritsakis AK (1990) Olive Oil. AOCS Press, Champaign
Boskou D (1996) Olive oil chemistry and technology. AOCS Press, Champaign
Martínez-Force E, Ruiz-López N, Garcés R (2004) The determination of the asymmetrical stereochemical distribution of fatty acids in triacylglycerols. Anal Biochem 334:175–182
Boskou D (2006) Olive oil chemistry and technology. AOCS Press, Champaign
Brescia MA, Alviti G, Liuzzi V, Sacco A (2003) Chemometric classification of olive cultivars based on compositional data of oils. J Am Oil Chem Soc 80:945–950
Vichi S, Pizzale L, Conte LS (2007) Stereospecific distribution of fatty acids in triacylglycerols of olive oils. Eur J Lipid Sci Technol 109:72–78
Aranda F, Gómez-Alonso S, Rivera del Álamo RM, Salvador MD, Fregapane G (2004) Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: comparison with other Spanish cultivars. Food Chem 86:485–492
Diaz TG, Meras TD, Casas JS, Franco AM (2005) Characterization of virgin olive oils according to its triglycerides and sterol composition by chemometric methods. Food Control 16:339–347
Stefanoudaki E, Kotsifaki F, Koutsaftakis A (1999) Classification of virgin olive oils of the two major cretan cultivars based on their fatty acid composition. J Am Oil Chem Soc 76:623–626
Piravi-Vanak Z, Ghavami M, Ezzatpanah H, Arab J, Safafar H, Jahan BG (2009) Evaluation of authenticity of Iranian olive oil by fatty acid and triacylglycerol profiles. J Am Oil Chem Soc 86:827–833
Ollivier D, Artaud J, Pinatel C, Durbec JP, Guérère M (2003) Triacylglycerol and fatty acid compositions of French virgin olive oils. Characterization by chemometrics. J Agric Food Chem 51:5723–5731
Ollivier D, Artaud J, Pinatel C, Durbec JP, Guérère M (2006) Differentiation of French virgin olive oil RDOs by sensory characteristics, fatty acid and triacylglycerol compositions and chemometrics. Food Chem 97:382–393
Haddada MF, Mania H, Oueslati I, Daoud D, Sánchez J, Osorio E, Zarrouk M (2007) Fatty acid, triacylglycerol, and phytosterol composition in six Tunisian olive varieties. J Agric Food Chem 55:10941–10946
Baccouri O, Guerfel M, Baccouri B, Cerretani L, Bendini A, Lercker G, Zarrouk M, Ben Miled D (2008) Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem 109:743–754
Boukachabine N, Ajana H, El Antari A (2011) A study of fatty acids and triglycerides oil composition and quality parameters of five autochthon olive varieties in Morocco. Leban Sci J 12:45–65
Gurdeniz G, Ozen B, Tokatli F (2008) Classification of Turkish olive oils with respect to cultivar, geographic origin and harvest year, using fatty acid profile and mid-IR spectroscopy. Eur Food Res Technol 227:1275–1281
Andjelkovic M, Acun S, Van Hoed V, Verhé R, Van Camp J (2009) Chemical composition of Turkish olive oil-Ayvalik. J Am Oil Chem Soc 86:135–140
Kıralan M, Bayrak A, Ozkaya MT (2009) Oxidation stability of virgin olive oils from some important cultivars in East Mediterranean area in Turkey. J Am Oil Chem Soc 86:247–252
Dıraman H, Saygi H, Hisil Y (2010) Relationship between geographical origin and fatty acid composition of Turkish virgin olive oils for two harvest years. J Am Oil Chem Soc 87:781–789
Dıraman H, Saygı H, Hışıl Y (2011) Classification of three Turkish olive cultivars from Aegean region based on their fatty acid composition. Eur Food Res Technol 233:403–411
Matthäus B, Özcan MM (2011) Determination of fatty acid, tocopherol, sterol contents and 1,2- and 1,3-diacylglycerols in four different virgin olive oil. J Food Process Technol 2:1–4
Yorulmaz A, Erinc H, Tekin A (2013) Changes in olive and olive oil characteristics during maturation. J Am Oil Chem Soc 90:647–658
Gökçebağ M, Dıraman H, Özdemir D (2013) Classification of Turkish monocultivar (Ayvalık and Memecik cv.) virgin olive oils from north and south zones of Aegean region based on their triacyglycerol profiles. J Am Oil Chem Soc 90:1661–1671
Sevim D, Tuncay O (2013) Effect of olive leaves addition before extraction of Turkish olive cultivars on olive oil minor components and antioxidant activity. Open Access Sci Rep 2:2–8
Dıraman H, Dibeklioğlu H (2014) Using lipid profiles for the characterization of Turkish monocultivar olive oils produced by different systems. Int J Food Prop 17:1013–1033
Cañabate-Diaz B, Segura Carretero A, Fernandez-Gutierrez A, Belmonte Vega A, Garrido Frenich A, Martinez Vidal JL, Martos JD (2007) Separation and determination of sterols in olive oil by HPLC–MS. Food Chem 102:593–598
Lerma-García MJ, Concha-Herrera V, Herrero-Martínez JM, Simó-Alfonso EF (2009) Classification of extra virgin olive oils produced at La Comunitat Valenciana according to their genetic variety using sterol profiles established by high performance liquid chromatography with mass spectrometry detection. J Agric Food Chem 57:10512–10517
Casas JS, Bueno EO, García AMM, Cano MM (2004) Sterol and erythrodiol + uvaol content of virgin olive oils from cultivars of extremadura (Spain). Food Chem 87:225–230
Álamo RM, Fregapane G, Aranda F, Gómez-Alanso G, Salvador MD (2003) Sterol and alcohol composition of Cornicabra virgin olive oil: the campesterol content exceeds the upper limit of 4% established by EU regulations. Food Chem 84:533–537
Marini F, Balestrieri F, Bucci R, Magrì AD, Magrì AL, Marini D (2004) Supervised pattern recognition to authenticate Italian extra virgin olive oil varieties. Chemom Intell Lab Syst 73:85–93
Cercaci L, Passalacqua G, Poerio A, Rodriguez-Estrada MT, Lercker G (2007) Composition of total sterols (4-desmethyl-sterols) in extra virgin olive oils obtained with different extraction technologies and their influence on the oil oxidative stability. Food Chem 102:66–76
Cunha SS, Fernandes JO, Oliveira MBPP (2006) Quantification of free and esterified sterols in Portuguese olive oils by solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A 1128:220–227
Matos LC, Cunha SC, Amaral JS, Pereira JA, Andrade PB, Seabra RM, Oliveira BPP (2007) Chemometric characterization of three varietal olive oils (Cvs. Cobrançosa, Madural and Verdeal Transmontana) extracted from olives with different maturation indices. Food Chem 102:406–414
Ben Temime S, Manai H, Methenni K, Baccouri B, Abaza L, Daoud D, Casas JS, Bueno EO, Zarrouk M (2008) Sterolic composition of Cheutoi virgin olive oil: influence of geographical origin. Food Chem 110:368–374
Gül MK, Seker M (2006) Comparative analysis of phytosterol components from rapeseed (Brassica napus L.) and olive (Olea europaea L.) varieties. Eur J Lipid Sci Technol 108:759–765
Ilyasoglu H, Ozcelik B, Hoed VV, Verhe R (2010) Characterization of Aegean olive oils by their minor compounds. J Am Oil Chem Soc 87:627–636
Bertran E, Blanco M, Coello J, Iturriaga H, Maspoch S, Montolin I (2000) Near-infra-red spectrometry and pattern recognition as screening methods for the authentification of virgin olive oils of very close geographical origin. J Near Infrared Spectrosc 8:45–52
Tapp HS, Defernez M, Kemsley EK (2003) FTIR spectroscopy and multivariate analysis can distinguish the geographic origin of extra virgin olive oils. J Agric Food Chem 51:6110–6115
Dupuy N, Le Dreäau Y, Ollivier D, Artaud J, Pinatel C, Kister J (2005) Origin of French virgin olive oil registered designation of origins predicted by chemometric analysis of synchronous excitation—emission fluorescence spectra. J Agric Food Chem 53:9361–9368
Dıraman H, Dibeklioğlu H (2009) Characterization of Turkish virgin olive oils produced from early harvest olives. J Am Oil Chem Soc 86:663–674
Dıraman H, Saygı H, Hışıl Y (2011) Geographical classification of Turkish virgin olive oils from the Aegean region for two harvest years based on their fatty acid profiles. J Am Oil Chem 88:1905–1915
Kadiroglu P, Korel F, Tokatlı F (2011) Classification of Turkish extra virgin olive oils by a SAW detector electronic nose. J Am Oil Chem Soc 88:639–645
Ilyasoglu H, Ozcelik B, Hoed VV, Verhe R (2011) Cultivar characterization of Aegean olive oils with respect to their volatile compounds. Sci Hortic 129:279–282
Ocakoglu D, Tokatli F, Ozen B, Korel F (2009) Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chem 113:401–410
Yorulmaz A, Poyrazoglu ES, Ozcan MM, Tekin A (2012) Phenolic profiles of Turkish olives and olive oils. Eur J Lipid Sci Technol 114:1083–1093
IUPAC (1987) Standard methods for analysis of oils, fats and derivates, 7th edn. IUPAC method 2.301. Blackwell, Oxford
AOCS (1989) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS Press, Champaign
Herslöf BG (1981) HPLC of triglycerides using UV detection. J High Resolut Chromatogr 4:471–473
(2010) Turkish food codex, Communique on olive oil and pomace oil The Official Gazette of Republic of Turkey, Number 27665, Ankara
Tekin A (1997) Bazı bitkisel yağların gliserit yapılarının belirlenmesi. Gıda 22:407–411
Graciani E (1988) Caracterización del aceite de oliva virgen español.III. Posibilidad de caracterización por variedades de aceituna o porzonas de producción de acuerdo con su contenido en triacilgliceroles. Grasas Aceites 39:105–110
Baccouri O, Cerretani L, Bendini A, Caboni MF, Zarrouk M, Pirrone L, Miled DDB (2007) Preliminary chemical characterization of Tunisian monovarietal virgin olive oils and comparison with Sicilian ones. Eur J Lipid Sci Technol 109:1208–1217
Chiavaro E, Vittadini E, Rodriguez-Estrada MT, Cerretani L, Bonoli M, Bendini A, Lercker, G (2007) Monovarietal extra virgin olive oils: correlation between thermal properties and chemical composition. J Agric Food Chem 55:10779–10786
Gutiérrez F, Jimenez B, Ruiz A, Albi MA (1999) Effect of olive ripeness on the oxidative stability of virgin olive oil extracted from the varieties Picual and Hojiblanca and on the different components involved. J Agric Food Chem 47:121–127
Canözer Ö (1991) The catalogue of standard olive cultivars. Professional publications no 334, p 104. Ministry of Agriculture and Rural Affairs, The General Directorate of Agricultural Production and Development, Ankara
Acknowledgments
Authors thank to Scientific and Technical Research Council of Turkey (TUBITAK), Project No 105O395, for financial support.
Conflict of interest
The authors certify that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yorulmaz, A., Yavuz, H. & Tekin, A. Characterization of Turkish Olive Oils by Triacylglycerol Structures and Sterol Profiles. J Am Oil Chem Soc 91, 2077–2090 (2014). https://doi.org/10.1007/s11746-014-2554-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2554-7