Abstract
In this study, a specific monoclonal antibody (mAb) against Alternaria mycotoxin tenuazonic acid (TeA) was prepared and a sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for the detection of TeA was developed. Tenuazonic acid coupled with carboxymethoxylamine hemihydrochloride (TeA-CMO) was conjugated to keyhole limpet hemocyanin (KLH) as an immunogen for Balb/c mice injection. The hybridoma cell line 3F10 secreting the mAb specific to TeA was obtained, and then by intraperitoneal injection and the caprylic acid-ammonium sulfate precipitation, the purified mAb was prepared and identified as the immunoglobulin G1 isotypes. The affinity constant (kD) of the mAb was 3.045 E−3 M by SPR analysis. Based on this mAb, an ic-ELISA was established after optimization of assay condition, namely the concentration of coating antigen and antibody were 250 ng/mL and 125 ng/mL respectively, competition time of antigen-antibody was 40 min, and incubation time of secondary antibody was 40 min in 0.01 M PBS buffer (pH 7.4). Under the optimized condition, the IC50 value and the detection limit (LOD) were 18.50 ng/mL and 1.00 ng/mL respectively. The average recovery rate from spiked beer, apple juice, and grape juice was from 85.0 to 120.0%. A squared coefficient of correlation (R2) between ic-ELISA and HPLC method was 0.9732. The established ic-ELISA provides an acceptable technique for the detection of TeA residue in food samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tenuazonic acid (3-acetyl-5-s-butyl-4-hydroxy-3-pyrrolin-2-one, TeA) is an Alternaria toxin produced by Pyricularia, Phoma, and Alternaria alternate. Among Alternaria toxins, TeA was listed as the most toxic one by US Food and Drug Administration (Mikula et al. 2013; Ostry 2008). Improper storage or transportation was prone to induce food spoilage by TeA-producing fungi, therefore leading to contamination of TeA (Bhat et al. 2010; Fernández-Cruz et al. 2010; Yang et al. 2013). It has been reported that TeA was the predominant Alternaria toxin in food samples. For example, up to 77% cereal products were contaminated by TeA (Bruce et al. 1984). A survey about wheat flour showed 99.4% (180/181) samples contained TeA at levels ranging from 1.76 to 520 μg/kg (mean = 79.80 μg/kg) and 31.5% (67 samples) were contaminated with TeA at a concentration higher than 100 μg/kg with a maximum of 520 μg/kg (Zhao et al. 2015b). In tomato products (Stack et al. 1985), TeA level could vary from 0.4 to 70 mg/kg. Besides, TeA could be frequently detected in beers, potatoes, pepper, wines, and even animal products (Fontana et al. 2016; Li and Yoshizawa 2000; Lohrey et al. 2013; López et al. 2016; Siegel et al. 2010; Walravens et al. 2016; Zhao et al. 2015a).
However, until now, most countries have not issued a tolerance limit for TeA as well as Alternaria toxin (Janardhanan and Husain 1984; López et al. 2016; Rychlik et al. 2016; Zhou et al. 2019). Although the exposure risk of TeA existed in dietary is still unclear, its potential hazard should be further evaluated based on continuous and wider monitoring. Thus, a rapid, effective, and costly detection method for TeA would be necessary and deserves to be developed.
Compared with the analytical methods such as high-performance liquid chromatography (HPLC) (Fan et al. 2016; Myresiotis et al. 2015), liquid chromatography coupled with tandem mass spectrometry (LC-MS) (Fraeyman et al. 2015; Rasmussen et al. 2010; Xu et al. 2016), enzyme-linked immunosorbent assay (ELISA) has the advantages of ease, rapid, and accuracy as well as low cost. Previously, the competitive ELISAs based on polyclonal antibody (pAb) for the TeA derivatives, namely tenuazonic acid hemisuccinate and 5-(sec-butyl)-3-(1-hydrazonoethyl)-4-hydrooxy-2(5H)-one (TeAH), was established to detect TeA through derivatization pretreatment of the samples (Gross et al. 2011; Yang et al. 2012). However, in these assays, derivatization is complex and derivative rate for different samples might be different therefore influencing the assay accuracy. Moreover, pAbs from antiserum generally have unstable quality caused by the batch difference of immunized animals. In this study, we prepared a specific monoclonal antibody with TeA as direct target via the hybridoma technique for the first time. Furthermore, an mAb-based ic-ELISA subsequently was developed for the detection of TeA, and its accuracy was validated with spiked food samples and confirmed by HPLC analysis.
Experimental Methods
Materials
The antigen tenuazonic acid (TeA), derivatized antigen tenuazonic acid coupled with carboxymethoxylamine hemihydrochloride (TeA-CMO), and the SP2/0 myeloma cell line were stored in our laboratory. Complete Freund’s adjuvants, incomplete Freund’s adjuvants, N, N-dimethylformamide (DMF), dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), polyethylene glycol (PEG) 4000, and horseradish peroxidase-labeled goat anti-mouse IgG (IgG-HRP) were purchased from Sigma company (USA). Hypoxanthine-aminopterin-thymidine (HAT) and hypoxanthine-thymidine (HT) were obtained from Gibco company (USA). Culture media RPMI-1640 was purchased from HyClone company (USA). All other organic solvents and chemicals used were of analytical grade. Female Balb/c mice were purchased from Guangdong Medical Laboratory Animal Center.
Immunization and Hybridoma selection
Hapten-protein conjugates TeA-CMO-BSA and TeA-CMO-KLH were prepared by active ester methods according to the references (Lin et al. 2019; Ni et al. 2019). Seven-week-old female Balb/c mice were firstly immunized by subcutaneous injection of 50 μg TeA-CMO-KLH emulsified with the same volume of complete Freund’s adjuvant. Booster injections were given at 2-week intervals with the immunogen and an equal volume of incomplete Freund’s adjuvant. The mice tail blood was collected at the interval of 7 days after each immunization, and detected by ic-ELISA using TeA-CMO-BSA as the coating antigen. The mouse with the highest titer and inhibition rate received a final soluble intraperitoneal injection of TeA-CMO-KLH in PBS, 3 days prior to cell fusion. Cell fusion procedures were carried out essentially as described by the methods (Xiao et al. 2018).
Preparation and Identification of mAb
The positive hybridomas were cultivated and injected into abdominal cavity of female Balb/c mice after paraffin injections 1 week in advance. The collected ascites was purified by the caprylic acid-ammonium sulfate precipitation method (Abad et al. 1999; Zhao et al. 2002). The purity of mAb was identified by 12% SDS-PAGE and the concentration was measured using a NanoDrop 2000C system (thermo scientific, USA). The isotype of mAb was identified by a mouse mAb isotyping kit (sigma, USA).
The affinity of the mAb were determined by surface plasmon resonance (SPR, BIAcore T200, USA) with a continuous flow of 10 μL/min of PBS buffer at 25 °C. The coating antigen TeA-CMO-BSA as a ligand in pH 4.0 sodium acetate was immobilized to a CM5 sensor chip by amine coupling method. Then, different concentrations of anti-TeA mAb were diluted and injected. Glycine-HCl (10 mM, pH 2.0) was used as a dissociation reagent. All data was analyzed by Biacore T200 evaluation software.
Establishment of an Indirect Competitive ELISA
The 96-well microplates were coated with 100 μL/well of TeA-CMO-BSA overnight at 37 °C, then washed two times by PBST (0.1% Tween 20 in PBS) and blocked with 120 μL/well of blocking solution (2% skimmed milk in PBS) for 3 h at 37 °C. After washing, 50 μL of different concentrations of standard TeA solution along with 50 μL of antibodies were added each well and incubated at 37 °C for 40 min. The plate was washed five times by PBST and added with 5000-fold diluted IgG-HRP for 40 min at 37 °C. The color was developed with the chromogenic reagent (100 μg/mL 3,3′,5,5′-tetramethylbenzidine and 0.02 μL/mL H2O2 in 100 mM NaOAc, pH 6.0), and the reaction was stopped with 50 μL per well of 10% H2SO4. The absorbance was read at 450 nm using a microplate reader (thermo scientific, USA).
The concentration of antibody and coating antigen were optimized via checkerboard titration (Liang et al. 2007; Xiao et al. 2018). To further improve the sensitivity and repeatability of ic-ELISA, other assay conditions were also optimized, including incubation times of antigen-antibody and secondary antibodies, as well as concentration of PBS buffer.
Under the optimized conditions, the standard curve was fitted to a four-parameter logistic equation with Origin 9.0 software. The percentage of cross-reactivity (CR) (Wang et al. 2018; Zhang et al. 2018) was calculated as follows: CR (%) = IC50 (TeA, ng/mL)/IC50 (TeA analogues, ng/mL) × 100.
Analysis of Spiked Samples
Wheat beer, apple juice, and grape juice purchased from a local supermarket were verified as TeA-free by HPLC analysis. The negative samples were spiked with different concentrations of TeA and filtered by a 0.22-μm membrane respectively for analysis.
The accuracy of the established ic-ELISA was validated with HPLC analysis. The ODS-C18 was used as reverse phase column under 40 °C temperature. Methanol-water (v/v, 85%: 15%) containing zinc sulfate (300 mg/L) was as mobile phase at a continuous flow rate of 0.4 mL/min and the injection volume was 10 μL. Analytes were determined by UV at 280 nm wavelength.
Results and Discussion
Preparation and Identification of mAb
The mouse with high serum titer was chosen for cell fusing. After four rounds of limiting dilution, a single-cell clone 3F10-producing specific antibody was selected. Furthermore, the purified antibodies with the concentration of 6.3 mg/mL were prepared from the ascites fluid and identified by SDS-PAGE (Fig. 1a). The isotype of purified mAb was identified to be immunoglobulin G1 type (Fig. 1b), consistent with the monoclonal antibody isotype for the most of small molecule antigen reported (Liu et al. 2016). In addition, to assess the antibody quality, the affinity of antibody-analyte was determined by SPR. As shown in Fig. 1c, with the antibody concentration increasing, response unit (RU) values became higher and appeared an obvious dose-dependent relationship. The affinity constant of the mAb was 3.045E−3 M (Fig. 1d), which was among the range about binding of proteins to small molecules.
Characterization of purified anti-TeA mAb. a SDS-PAGE analysis, nonreducing gel: 1, mAb after purification, 2, mAb before purification; reducing gel: 3, mAb before purification, 4, mAb after purification; b determination of monoclonal antibody isotypes; c kinetics tests for analysis of anti-TeA mAb and analyte; and d affinity test
Establishment of ic-ELISA Based on the mAb
To establish an ic-ELISA based on mAb for the detection of TeA, different parameters were optimized firstly. The ratio of Amax/IC50 was employed to evaluate the influence of each condition, and a higher ratio indicated a higher sensitivity of the assay (Abad and Montoya 1997; Chen et al. 2014). As shown in Fig. 2, the optimized reaction conditions were 250 ng/mL of coating antigen, a 1:32000 dilutions fold antibody (125 ng/mL) in PBS buffer (pH = 7.4, 0.01 M), a 40-min competition time of antibody-antigen and 40 min IgG-HRP incubation time, respectively. Under these conditions, an ic-ELISA standard curve was finally established with the IC50 value of 18.50 ng/mL, the limit of detection (LOD) of 1.00 ng/mL, and the liner concentration range of 3.56~96.24 ng/mL (Fig. 3). The IC50 value was nearly 20 times lower than the reported pAb-based ELISA (Gross et al. 2011). Meanwhile, unlike derivative-ELISA established by previous study, in which at least 2 h is required for derivatization pretreatment of the samples (Yang et al. 2012), the whole assay in this project can be finished within 1.5 h.
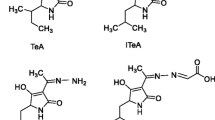
The CR of the assay was evaluated by IC50 values of seven kinds of analogues. The results indicated that mAb was specific and sensitive to TeA (Table 1) and showed less than 2% CR to all analogues including the iso-tenuazonic acid (ITeA), an isomer of TeA, which only appears methyl isomerization in spatial structure. Likewise, in our previous work (Xiao et al. 2018), an anti-ITeA mAb was prepared and showed low CR for TeA. This further confirmed the potential advantages of immunoassay based on the specific antibody to different target analysis.
Detection of TeA in Spiked Samples by ic-ELISA and HPLC
Wheat beer, apple juice, and grape juice spiked with TeA at three concentrations (0, 0.8, 2.0, and 3.2 mg/L) were detected by the developed ic-ELISA. Generally, dilution is a convenient and simple way to eliminate the matrix effect (Sheng et al. 2012), especially for liquid samples. In this study, when wheat beer, apple juice, and grape juice samples were diluted by PBS buffer to 40 times respectively, the matrix interference was effectively eliminated (Fig. 4). In addition, the ic-ELISA gives an acceptable average recovery ranged from 85.0 to 120.0% (Table 2), and the coefficients of variation (CV) were all below 15%. Furthermore, the samples spiked with TeA were also detected by HPLC to evaluate the accuracy of ic-ELISA. As shown in Fig. 5, the squared correlation coefficient (R2) of ic-ELISA and HPLC was 0.9732. This means that the established ic-ELISA could be used to monitor TeA residues in food samples with acceptable accuracy and reproducibility.
Conclusions
In this study, a sensitive and specific ic-ELISA based on monoclonal antibody was developed for the determination of TeA in food samples. A novel monoclonal antibody against TeA was prepared for the first time in this study, and the SPR results showed its high affinity. After optimization of four parameters, a mAb-based ic-ELISA was established with high sensitivity and specificity. Furthermore, ideal recoveries in spiked food samples were obtained and its accuracy was confirmed via HPLC method. Overall, this work should broaden the application of immunological methods involved in hazardous mycotoxins. Future studies will be focused on the optimization of screening hybridoma process for anti-TeA mAb with higher affinity and the development of a rapid immunoassay kit.
References
Abad A, Montoya A (1997) Development of an enzyme-linked immunosorbent assay to carbaryl. 2. assay optimization and application to the analysis of water samples. J Agric Food Chem 45:1495–1501. https://doi.org/10.1021/jf950691w
Abad A, Moreno MJ, Montoya A (1999) Development of monoclonal antibody-based immunoassays to the n-methylcarbamate pesticide carbofuran. J Agric Food Chem 47:2475–2485. https://doi.org/10.1021/jf981184s
Bhat R, Sridhar KR, Karim AA (2010) Microbial quality evaluation and effective decontamination of nutraceutically valued lotus seeds by electron beams and gamma irradiation. Radiat Phys Chem 79:976–981. https://doi.org/10.1016/j.radphyschem.2010.04.002
Bruce VR, Stack ME, Mislivec PB (1984) Incidence of toxic Alternaria species in small grains from the USA. J Food Sci 49:1626–1627. https://doi.org/10.1111/j.1365-2621.1984.tb12864.x
Chen LY, Wang MC, Xiang HF, Lin XJ, Cao DH, Ye LY (2014) Prediction of effect-site concentration of sufentanil by dose-response target controlled infusion of sufentanil and propofol for analgesic and sedation maintenance in burn dressing changes. Burns 40:455–459. https://doi.org/10.1016/j.burns.2013.08.002
Fan C, Cao XL, Liu M, Wang W (2016) Determination of Alternaria mycotoxins in wine and juice using ionic liquid modified countercurrent chromatography as a pretreatment method followed by high-performance liquid chromatography. J Chromatogr A 1436:133–140. https://doi.org/10.1016/j.chroma.2016.01.069
Fernández-Cruz ML, Mansilla ML, Tadeo JL (2010) Mycotoxins in fruits and their processed products: analysis, occurrence and health implications. J Adv Res 1:113–122. https://doi.org/10.1016/j.jare.2010.03.002
Fontana AR, Prendes LP, Morata VI, Bottini R (2016) High-throughput modified QuEChERS method for the determination of the mycotoxin tenuazonic acid in wine grapes. RSC Adv 6:95670–95679. https://doi.org/10.1039/C6RA22990E
Fraeyman S, Devreese M, Broekaert N, Mil TD, Antonissen G, Baere SD, Backer PD, Rychlik M, Croubels S (2015) Quantitative determination of tenuazonic acid in pig and broiler chicken plasma by LC-MS/MS and its comparative toxicokinetics. J Agric Food Chem 63:8560–8567. https://doi.org/10.1021/acs.jafc.5b02828
Gross M, Curtui V, Ackermann Y, Latif H, Usleber E (2011) Enzyme immunoassay for tenuazonic acid in apple and tomato products. J Agric Food Chem 59:12317–12322. https://doi.org/10.1021/jf203540y
Janardhanan KK, Husain A (1984) Phytotoxic activity of tenuazonic acid isolated from Alternaria alternata (Fr.) Keissler causing leaf blight of Datura innoxia Mill. and its effect on host metabolism. J Phytopathol 111:305–311. https://doi.org/10.1111/j.1439-0434.1984.tb00774.x
Li FQ, Yoshizawa T (2000) Alternaria mycotoxins in weathered wheat from China. J Agric Food Chem 48:2920–2924. https://doi.org/10.1021/jf0000171
Liang CZ, Jin RY, Gui WJ, Zhu GN (2007) Enzyme-linked immunosorbent assay based on a monoclonal antibody for the detection of the insecticide triazophos: assay optimization and application to environmental samples. Environ Sci Technol 41:6783–6788. https://doi.org/10.1021/es070828m
Lin L, Wu XL, Luo PJ, Song SS, Zheng QK, Kuang H (2019) Ic-ELISA and immunochromatographic strip assay based monoclonal antibody for the rapid detection of bisphenol S. Food Agric Immunol 30:633–646. https://doi.org/10.1080/09540105.2019.1612330
Liu JW, Lu CC, Liu BH, Yu FY (2016) Development of novel monoclonal antibodies-based ultrasensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for aflatoxin B1 detection. Food Control 59:700–707. https://doi.org/10.1016/j.foodcont.2015.06.038
Lohrey L, Marschik S, Cramer B, Humpf HU (2013) Large-scale synthesis of isotopically labeled 13C2-tenuazonic acid and development of a rapid HPLC-MS/MS method for the analysis of tenuazonic acid in tomato and pepper products. J Agric Food Chem 61:114–120. https://doi.org/10.1021/jf305138k
López P, Venema D, Td R, Ad K, Scholten JM, Mol HGJ, Md N (2016) Occurrence of Alternaria toxins in food products in the Netherlands. Food Control 60:196–204. https://doi.org/10.1016/j.foodcont.2015.07.032
Mikula H, Horkel E, Hans P, Hametner C, Fröhlich J (2013) Structure and tautomerism of tenuazonic acid – a synergetic computational and spectroscopic approach. J Hazard Mater 250-251:308–317. https://doi.org/10.1016/j.jhazmat.2013.02.006
Myresiotis CK, Testempasis S, Vryzas Z, Karaoglanidis GS, Papadopoulou-Mourkidou E (2015) Determination of mycotoxins in pomegranate fruits and juices using a QuEChERS-based method. Food Chem 182:81–88. https://doi.org/10.1016/j.foodchem.2015.02.141
Ni TT, Peng DP, Wang YX, Pan YH, Xie SY, Chen DM, Wang YL, Ta YF, Yuan ZH (2019) Development of a broad-spectrum monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for the multi-residue detection of avermectins in edible animal tissues and milk. Food Chem 286:234–240. https://doi.org/10.1016/j.foodchem.2019.02.011
Ostry V (2008) Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J 1:175–188. https://doi.org/10.3920/WMJ2008.x013
Rasmussen RR, Storm IMLD, Rasmussen PH, Smedsgaard J, Nielsen KF (2010) Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal Bioanal Chem 397:765–776. https://doi.org/10.1007/s00216-010-3545-7
Rychlik M, Lepper H, Weidner C, Asam S (2016) Risk evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 68:181–185. https://doi.org/10.1016/j.foodcont.2016.03.035
Sheng YJ, Jiang WX, Saeger SD, Shen JZ, Zhang SX, Wang ZH (2012) Development of a sensitive enzyme-linked immunosorbent assay for the detection of fumonisin B1 in maize. Toxicon 60:1245–1250. https://doi.org/10.1016/j.toxicon.2012.08.011
Siegel D, Merkel S, Koch M, Nehls I (2010) Quantification of the Alternaria mycotoxin tenuazonic acid in beer. Food Chem 120:902–906. https://doi.org/10.1016/j.foodchem.2009.10.070
Stack ME, Mislivec PB, Roach JAG, Pohland AE (1985) Liquid chromatographic determination of tenuazonic acid and alternariol methyl ether in tomatoes and tomato products. J Assoc Off Anal Chem 68:640–642. https://doi.org/10.1007/BF02166275
Walravens J, Mikula H, Rychlik M, Asam S, Devos T, Ediage EN, Mavungu JDD, Jacxsens L, Landschoot AV, Vanhaecke L, Saeger SD (2016) Validated UPLC-MS/MS methods to quantitate free and conjugated Alternaria toxins in commercially available tomato products, fruit and vegetable juices in Belgium. J Agric Food Chem 64:5101–5109. https://doi.org/10.1021/acs.jafc.6b01029
Wang F, Cai J, Eremin SA, Xiao ZL, Shen YD, Tian YX, Xu ZL, Yang JY, Lei HT, Sun YM, Wang H (2018) Fluorescence polarization immunoassay for Alternaria mycotoxin tenuazonic acid detection and molecular modeling studies of antibody recognition. Food Anal Methods 11:2455–2462. https://doi.org/10.1007/s12161-018-1236-8
Xiao ZL, Wang YL, Shen YD, Xu ZL, Dong JX, Wang H, Situ C, Wang F, Yang JY, Lei HT, Sun YM (2018) Specific monoclonal antibody-based enzyme immunoassay for sensitive and reliable detection of Alternaria mycotoxin iso-tenuazonic acid in food products. Food Anal Methods 11:635–645. https://doi.org/10.1007/s12161-017-1033-9
Xu WJ, Han XM, Li FQ, Zhang LS (2016) Natural occurrence of Alternaria toxins in the 2015 wheat from Anhui province, China. Toxins (Basel) 8:308. https://doi.org/10.3390/toxins8110308
Yang XX, Liu XX, Wang H, Xu ZL, Shen YD, Sun YM (2012) Development of an enzyme-linked immunosorbent assay method for detection of tenuazonic acid. Chin J Anal Chem 40:1347–1352. https://doi.org/10.3724/SP.J.1096.2012.11253
Yang JY, Li J, Jiang YM, Duan XW, Qu HX, Yang B, Chen F, Sivakumar D (2013) Natural occurrence, analysis, and prevention of mycotoxins in fruits and their processed products. Crit Rev Food Sci Nutr 54:64–83. https://doi.org/10.1080/10408398.2011.569860
Zhang YQ, Xu ZL, Wang F, Cai J, Dong JX, Zhang JR, Si R, Wang CL, Wang Y, Shen YD, Sun YM, Wang H (2018) Isolation of bactrian camel single domain antibody for parathion and development of one-step dc-FEIA method using VHH-alkaline phosphatase fusion protein. Anal Chem 90:12886–12892. https://doi.org/10.1021/acs.analchem.8b03509
Zhao MP, Li YZ, Guo ZQ, Zhang XX, Chang WB (2002) A new competitive enzyme-linked immunosorbent assay (ELISA) for determination of estrogenic bisphenols. Talanta 57: 1205-1210. https://doi.org/10.1016/s0039-9140(02)00207-2
Zhao K, Shao B, Yang DJ, Li FQ (2015a) Natural occurrence of four Alternaria mycotoxins in tomato- and citrus-based foods in China. J Agric Food Chem 63:343–348. https://doi.org/10.1021/jf5052738
Zhao K, Shao B, Yang D, Li FQ, Zhu JH (2015b) Natural occurrence of Alternaria toxins in wheat-based products and their dietary exposure in China. PLoS One 10:e0132019. https://doi.org/10.1371/journal.pone.0132019
Zhou B, Wang H, Meng B, Wei R, Wang L, An CF, Chen SG, LongYang C, Qiang S (2019) An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Managemant Science 75:2482–2489. https://doi.org/10.1002/ps.5402
Funding
This research was funded by the Key Area R&D Program of Guangdong Province (No. 2019B020211002), the National Natural Science Foundation of China (No. 31972157), the Science and Technology Planning Project of Guangzhou City (201804020077), and the International Cooperation Program of SCAU (2019SCAUGH03) and Project Supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yi-Fan Liang declares that he has no conflict of interest. Xiao-Wen Zhou declares that he has no conflict of interest. Feng Wang declares that he has no conflict of interest. Yu-Dong Shen declares that he has no conflict of interest. Zhi-Li Xiao declares that she has no conflict of interest. Shi-Wei Zhang declares that he has no conflict of interest. Yong-Jun Li declares that he has no conflict of interest. Hong Wang declares that she has no conflict of interest.
Ethical Approval
Female Balb/c mice were housed and maintained at the Guangdong Medical Laboratory Animal Center. All animal experiments were performed in compliance with the protective and administrative laws for laboratory animals of China and conducted with the approval of the Institutional Authority for Laboratory Animal Care, South China Agricultural University, Guangzhou, China.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, YF., Zhou, XW., Wang, F. et al. Development of a Monoclonal Antibody-Based ELISA for the Detection of Alternaria Mycotoxin Tenuazonic Acid in Food Samples. Food Anal. Methods 13, 1594–1602 (2020). https://doi.org/10.1007/s12161-020-01780-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01780-w