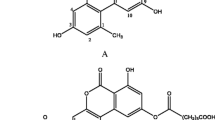
Abstract
In this paper, we report the development of a sensitive and specific monoclonal antibody-based immunodiagnostic method for the detection of iso-tenuazonic acid (ITeA), an Alternaria mycotoxin, in food samples. The ITeA was derivatized with hydrazine hydrate to produce the antigen (E)-3-(1-hydrazonoethyl)-4-hydroxy-5-isobutyl-1H-pyrrol-2(5H)-one (ITeAH) which was further reacted with glyoxalic acid to generate the hapten (E)-2-((Z)-(1-(4-hydroxy-5-isobutyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)ethylidene) (ITeAHGA) which was used as an immunogen after conjugation to bovine serum albumin (BSA). A highly specific monoclonal antibody selectively binding to ITeAH was generated via the hybridoma technique and subsequently used to construct a heterologous indirect competitive enzyme-linked immunosorbent assay (icELISA) using ITeAH as the competitive antigen for the detection of ITeA with a limit of detection (LOD) of 0.5 ng/mL. Under the optimum conditions, the developed icELISA is highly sensitive (IC50 = 7.8 ng/mL) with recovery rates ranged from 82.3 to 109.8% for spiked food samples. The comparative analysis of results revealed a good correlation between the icELISA and the standard HPLC-MS/MS method, confirming the suitability of the developed icELISA for screening and detection of mycotoxin ITeA in food samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tenuazonic acid (TeA) and its isomer, iso-tenuazonic acid (ITeA), are major metabolic toxic products of Alternaria and other fungal species such as Aspergillus flavus, Pyricularia oryzae, and Phoma sorghina (Qiang et al. 2008; Marin et al. 2013). Owing to their ability of growth at low temperature, Alternaria species are responsible for spoilage of food plants during refrigerated transport and storage, while some Alternaria mycotoxins are heat-resistant even at a relatively high temperature of 230 °C and thus cannot be detoxicated by cooking (Siegel et al. 2010). TeA-producing fungi are ubiquitous in many biological environments and capable of infecting most plant species including food crops. In fruits and vegetables, TeA has the highest contamination frequency and is present in higher concentrations compared to other Alternaria toxins (EFSA 2011). In spite of being cautious pathogens of many plant diseases, genotoxic and fetotoxic in rats, as well as being linked to the development of esophageal cancer, currently, there are no regulations on Alternaria toxins in food and feed in Europe or in other regions of the world. Furthermore, TeA is considered the most acutely toxic Alternaria mycotoxin (Shephard et al. 2012). Because of the similarities in chemical structure, it is speculated that ITeA and TeA are of similar toxicological relevance. For instance, they both exhibit remarkable toxic effects on Artemia salina with mortality rates of 68.9 and 73.6%, respectively (Qin et al. 2009). Not only the antibacterial activity of ITeA is identical to TeA (Gitterman 1965), ITeA also exhibits significant phytotoxicity, inhibiting plant growth and promoting leaf browning (Lebrun et al. 1988).
It was reported that some naturally contaminated food commodities contained only 4% ITeA in their total TeA content (Asam et al. 2013), but the high level of ITeA in sorghum-based infant food has raised increasing concerns, and more samples should be analyzed to elucidate if there is a general tendency related to sorghum (Qiang et al. 2008). Nevertheless, TeA has been found to be the predominant Alternaria mycotoxin detected in China in all tomato ketchup (10.2–1781 μg/kg) and tomato juice samples (7.4–278 μg/kg) and in 99.4% of wheat flour (1.76–520 μg/kg) (Zhao et al. 2015a, 2015b). Therefore, the total exposure of ITeA cannot be neglected due to its acute toxicity and potential harmful effects on human and animal health.
Subsequently, it is necessary to continually monitor ITeA and TeA in fruits, vegetables, cereals, and oleaginous plants intended for human consumption and feed production (Qiang et al. 2008). Although several instrumental methods exist for measuring of TeA and its analouges (Noser et al. 2011; Siegel et al. 2009; Asam et al. 2011; Prelle et al. 2013), LC-MS is the only instrumental technique available for ITeA (Asam et al. 2013). The method simultaneously detects both TeA and ITeA after their derivatization with 2,4-dinitrophenylhydrazine. While instrumental methods can offer a high level of precision and accuracy, the sophistication aspect of such analytical tools render their limited applications in routine and high throughput analysis. Immunochemical methods, on the other hand, are simple and cost effective, yet sensitive and rapid, enabling for a large array of sample screening. Immunoassay for TeA has recently been described in a couple of studies (Gross et al. 2011; Yang et al. 2012). Production of an antibody to the analyte is essential to an immunoassay. Compared with polyclonal antibody which is widely used in immunoassay, monoclonal antibody (mAb) is more specific and homogenous, also more difficult to produce. There is no report on ITeA immunoassay based on monoclonal antibody available to date to the best of our knowledge. The present study therefore aimed to develop a sensitive and specific immunochemical screening method and monitor ITeA in food products.
In the present study, two novel ITeA haptens, ITeAH and ITeAHGA (Fig. 1), were adopted to develop a specific anti-ITeAH antibody, using ITeAHGA as a hapten to prepare the immunogen by coupling to a carrier protein (BSA). A highly specific mAb selectively binding to ITeAH was generated via the hybridoma technique and was subsequently used to develop an indirect competitive enzyme-linked immunosorbent assay (icELISA) for the detection of ITeA. Various ELISA conditions were optimized and performance of the assay was evaluated by measuring ITeA in real food samples.
Materials and Methods
Reagents and Chemicals
Leucine, bovine serum albumin (BSA), dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), polyethylene glycol (PEG) 2000, ovalbumin (OVA), 3,3′,5,5′-tetramethylbenzidine (TMB), complete and incomplete Freund’s adjuvants, hypoxanthine-aminopterin-thymidine (HAT), hypoxanthine-thymidine (HT), culture media RPMI-1640, and pristane were obtained from Sigma (St. Louis, MO, USA). The mouse SP2/0 myeloma cell line was sourced from the Sun Yat-sen University (Guangzhou, China). Tween-20, N,N-dimethylformamide (DMF), sodium ethylate, diketene, benzene, ethyl acetate, chloroform, hydrazine hydrate, methanol, glutaric dialdehyde, and 4-hydroxybenzaldehyde were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). Horseradish peroxidase-labeled goat anti-mouse IgG (IgG-HRP) was obtained from Boster Biotech Co., Ltd. (Wuhan, China). Polystyrene microtiter plates were sourced from Jiete Biotech Co., Ltd. (Guangzhou, China). Microwell plates for cell culture were obtained from Corning Incorporated (New York, USA). All organic solvents and chemicals used were of analytical grade. Female Balb/c mice were purchased from Guangdong Medical Laboratory Animal Center. The mycotoxin standards of AOH and AME were purchased from Taileqi Technology Co., Ltd. (Beijing, China), and TeA, ITeA, and ITeAH were synthesized in the Guangdong Provincial Key Laboratory of Food Quality and Safety (Guangzhou, China).
Buffers and Solutions
Buffers were prepared and used as follows: for washing, 10 mmol/L PBST (PBS buffer containing 0.1% Tween-20); for coating, 50 mmol/L carbonate buffer (pH 9.6); for blocking, 5% of skimmed milk powder in PBS buffer; as general diluent, sodium phosphate buffers (pH 5.4); and 2 mol/L H2SO4 was used as the stop solution. Chromogenic reagent was prepared using 150 μL of the TMB solution (15 mg/mL in DMF) and 2.5 μL of 6% (w/v) H2O2 in 10 mL of 0.1 mol/L citrate.
Instruments
Centrifuge (5810R) was purchased from Eppendorf Company, USA. The LC-MS/MS analysis was carried out using a 1200 series LC system (Agilent, USA) equipped with the Agilent 6410 Triple Quad LC-MS System. The analytical column was 2.1 mm × 150 mm, 3.5 μm Zorbax SB-C18. Nuclear magnetic resonance (NMR) spectra were obtained with DRX-600 NMR spectrometers (Bruker, Germany-Switzerland). Ultraviolet-visible (UV-vis) spectra were recorded on a UV-160A Shimadzu spectrophotometer (Kyoto, Japan). Microtiter plates were washed using a Multiskan MK2 microplate washer (Thermo Labsystems, USA). The optical density (OD) of ELISA signals were measured using a Perkin Elmer 1420 Multi-label Analyzer (USA). Wrist-action shaker (Vortex Genius3) was a product of IKA Company, Germany.
Synthesis and Characterization of Haptens
ITeA Synthesis
ITeA was synthesized according to the method previously described (Yang et al. 2012) (Fig. 2). After recrystallization in chloroform, a white needle solid was obtained with a 38.6% yield. The ITeA structure was confirmed by APCI-MS and NMR analysis. Two haptens namely, ITeAH ((E)-3-(1-hydrazonoethyl)-4-hydroxy-5-isobutyl-1H-pyrrol-2(5H)-one) and ITeAHGA ((E)-2-((Z)-(1-(4-hydroxy-5-isobutyl-2-oxo-2,5-dihydro-1H-pyrrol-3-yl)ethylidene)hydrazono)acetic acid) were subsequently synthesized following the procedures shown in Fig. 2.
ITeAH Synthesis
The synthesis was carried out by the Wolff-Kishner reaction. Briefly, ITeA (1.85 g, 10 mmol) was dissolved in 20 mL chloroform and added dropwise into the flask containing 20 mL of hydrous hydrazine hydrate. After mixing for 1 h, 20 mL distilled water was added, and the mixture was then extracted twice with chloroform. The organic phase was then washed with water and dried over anhydrous magnesium sulfate. The solvent was removed to obtain a gray solid of ITeAH with a 65% yield.
ITeAHGA Synthesis
The mixture of ITeAH (1.99 g, 10 mmol) and 2-oxoacetic acid (0.89 g, 12 mmol) was dissolved in 20 mL chloroform and agitated for 2 h to produce a white solid of ITeAHGA, with a 48% yield.
Preparation and Characterization of Hapten-Protein Conjugates
The ITeAH hapten was conjugated to OVA via the glutaraldehyde method (Hamajima et al. 1995) in the following procedures and used as a coating antigen: OVA (1.66 μmol/L) and ITeAH (166 μmol/L) were first prepared in PBS (pH 7.4), and 60 μL of glutaric dialdehyde was then added dropwise. The mixture was gently stirred for 12 h at 4 °C and purified by dialyzes against PBS (10 mmol/L, pH 8.0) for 2 days. The dialyzed product was centrifuged for 10 min and the supernatant was collected and stored at 4 °C. The structures of the final conjugates were confirmed by a UV-vis (200–500 nm) spectroscopy.
The ITeAHGA hapten was conjugated to BSA and OVA via the active ester method (McAdam et al. 1992) to prepare the immunogen and coating antigen, respectively. Briefly, ITeAHGA (0.166 μmol), DCC (0.122 μmol), and NHS (0.122 μmol) were dissolved in 1.0 mL of DMF and the mixture was gently stirred at 4 °C overnight. After centrifugation for 10 min, 500 μL of the supernatant was collected and added dropwise to 10 mL of PBS (10 mmol/L, pH 8.0) containing BSA or OVA (with mole ration of carrier protein to antigen at 1:60). The mixture was agitated at 4 °C for 12 h and purified by dialyzes against PBS (10 mmol/L, pH 8.0) for 2 days. After centrifugation for 10 min, the supernatant was collected and stored at 4 °C. Formation of the conjugate was confirmed with a UV-vis spectroscopy.
Production of mAb
Six-week-old female Balb/c mice were immunized at multiple sites with 50 μg of ITeAHGA-BSA conjugate emulsified in complete Freund’s adjuvant. Booster injections were given at 2-week intervals with the same amount of conjugate emulsified in incomplete Freund’s adjuvant. Mice were tail bled, and the quality of the antiserum was assessed using an indirect ELISA. The mouse with the highest titer received a final intraperitoneal injection of 100 μg of immunogen conjugate (without adjuvant) 3 days prior to cell fusion.
Cell fusion procedures were performed as described by Moreno et al. (2001). The spleen cells (108 cells) from the selected mouse were mixed with SP2/0 myeloma cells (107 cells) at a 10:1 ratio in 50% (w/v) PEG 2000. The fused cells were distributed in 96-well plates and cultured in HAT selection medium at 37 °C in a humidified 5% CO2 incubator.
When the hybridoma cells reached around 30–40% confluence, culture supernatants were screened for their binding activities to ITeAHGA-OVA with an indirect ELISA. The hybridomas showing the desired specificity were sub-cloned for multiple rounds by the limiting dilution method until a pure and stable antibody-producing clone was obtained. The positive clones were injected into female Balb/c mice to obtain ascitic fluid for antibody production. Antibodies in the fluid were purified by the caprylic acid-ammonium sulfate precipitation method (Zhao et al. 2002) and stored at −20 °C.
Indirect Competitive ELISA
icELISA Protocol
Ninety-six well microtiter plates were coated with 100 μL/well of ITeAH-OVA overnight at room temperature. The plates were washed and incubated with 120 μL/well of blocking solution for 3 h at 37 °C. After washing, 50 μL of the standard solution or sample extracts along with 50 μL of antibodies were added. Plates were incubated for 40 min and washed. Goat anti-mouse IgG-HRP was added (100 μL/well) and incubated for 30 min at 37 °C. After washing, 100 μL of the chromogenic reagent was added and incubated for 10 min. The reaction was stopped by adding 50 μL of stop solution, and the absorbance was measured at 450 nm using a Plate Reader.
The results were expressed as the percentage of inhibition (B/B 0), where B and B 0 are the absorbance values of the wells with and without standard solution, respectively. The competitive standard curve was constructed by plotting the B/B 0 values against the logarithm of analyte concentration. Sigmoid curve was obtained using the OriginPro 8.5 software (OriginLab Corp., Northampton, USA). The limit of detection (LOD) was determined as the 10% inhibiting concentration (IC10) (Henniona and Barcelob 1998). The linear range was defined as the detection regime between the lower and upper limits of quantification, i.e., the IC20-IC80 working range.
Optimization of Assay Conditions
The most sensitive reaction condition of the icELISA assay was achieved when using ITeAHGA-BSA, ITeAH-OVA, and ITeAH as the immunogen, coating antigen, and competition analyte, respectively. Other experimental parameters were also optimized to further improve the assay sensitivity including checkerboard titrations of coating antigens and antibody dilutions, different incubation times of antigen-antibody and secondary antibodies, as well as various buffer systems.
Cross-Reactivity
The specificity of the generated monoclonal antibody was assessed for its cross-reaction rate (CR) with a group of structurally similar analogues based on the IC50 data calculated according to the following equation (Cui et al. 2011):
Sample Collection and Preparation
Twenty samples were obtained from the local supermarket, apple juice (n = 5), beer (n = 5), tomato ketchup (n = 4), and dried fruit (n = 6). The liquid samples (1 mL) were extracted with 2 mL of chloroform on a wrist-action shaker for 1 min. This was repeated two times followed by centrifugation (1000×g, 10 min). The dried fruit samples were extracted with 2:3:3 methanol-acetonitrile-water (v/v/v) for 25 min and 4:1 chloroform-ethanol (v/v) for 1 min successively at room temperature (Stinson et al. 1981). ITeA in the samples was first reduced to ITeAH using hydrazine hydrate prior to detection using the following procedures. The organic phase containing the ITeA was mixed with 100 μL hydrazine hydrate and vigorously agitated for 30 min at room temperature. The reaction was stopped by addition of 500 μL of H2O, and the mixture was transferred into a 25-mL round-bottom flask where the solvent was evaporated to dryness in a rotary evaporator at 60 °C under reduced pressure. The residue was then resuspended in 1 mL H2O. To eliminate sample matrix effects, the apple juice, beer solution, and the tomato ketchup were further diluted 35–45 times with the assay buffer prior to icELISA analysis. All samples were subject to analysis by both icELISA and HPLC-MS/MS.
Recovery Tests
ITeA was added to apple juice (1 mL) to give the final concentrations at 30, 150, and 300 ng/mL, respectively. For the beer sample (1 mL) and tomato ketchup (1 g), the final concentrations were 150, 300, and 720 ng/mL or ng/g, respectively. All of the spiked samples were prepared as described in the “Sample Collection and Preparation” section and measured with the developed icELISA. Calibration curve was constructed with a serial dilutions of ITeAH (0, 0.064, 0.32, 1.6, 8, 40, 200, and 1000 ng/mL) and used to measure the concentration of ITeA from different extracted samples based on the reduction rate of 65% (ITeA to ITeAH).
HPLC-MS/MS Analysis
The mobile phase was a mixture of the ammonium formate solution (5 mmol/L in water, adjusted to pH 7.8 with ammonia) (A) and acetonitrile (B), which was used in the following linear binary gradient—0–3 min, 5% B; 3–5 min, 5–15% B; 5–8 min, 15–100% B; and 8–11 min, 100% B. The injection volume and flow rate were 50 μL and 0.4 mL/min, respectively. Analytes were determined by ESI-MS/MS in the positive mode. Other parameters were as follows: gas temperature, 350 °C; gas flow, 10 L/min; nebulizer gas, 50 psi; and capillary voltage, 3.5 kV.
Results and Discussion
Hapten Synthesis and Conjugate Preparation
The design and production of functional haptens is the first and a critical step in any immunoassay development. Similar to many other small molecules, ITeA (197 Da) is not immunogenic itself and lacks an available chemical group for protein conjugation. In this work, two novel ITeA haptens are illustrated (Fig. 2). An intermediate hapten ITeAH was first prepared by condensation of hydrazine hydrate to the ketone group of ITeA. It was then reacted with glyoxalic acid to introduce the carboxyl group to obtain the tentative hapten ITeAHGA with a short aliphatic spacer arm. It has been suggested that a linear interval arm with aliphatic linkers comprised of a semi-rigid unsaturated double bond structure with three to six carbon atoms is generally good for producing the desired antibodies (Shen et al. 2007; Mercader et al. 2008). Using the same strategy, we previously reported the successful production of the anti-TeA antibody and subsequent development of an ELISA for TeA (Yang et al. 2012). The successful syntheses of ITeA, ITeAH, and ITeAHGA were confirmed by MS and NMR data:
-
ITeA: APCI-MS, m/z 196.4 ([M-H]−). 1H NMR (600 MHz, CDCl3, TMS): δ 0.96 (d, J = 6.37 Hz, 3H, CH3), 0.98 (d, J = 6.46 Hz, 3H, CH3), 1.45 (m, 1H, CH), 1.84–1.67 (m, 2H, CH2), 2.46 (s, 3H, CH3), 3.85 (ddd, J = 9.80, 3.59, 0.88 Hz, 1H, CH), and 6.03 (s, br, 1H, NH).
-
ITeAH: The APCI-MS was m/z 212.1([M + H]+). The 1H NMR (600 MHz, CDCl3, TMS): δ 0.96 (d, J = 6.43 Hz, 3H, CH3), 0.95 (d, J = 6.34 Hz, 3H, CH3), 1.33–1.41 (m, 1H), 1.72 (m, 2H), 2.67 (s, 3H, CH3), and 3.48–4.04 (m, 1H).
-
ITeAHGA: APCI-MS, m/z 266.0 ([M-H]−). The(Qiang et al. 2008)HNMR (600 MHz, DMSO-d 6, TMS): δ 0.88 (d, J = 6.59 Hz, 6H, 2CH3), 1.29 (ddd, J = 13.90, 9.47, 4.73 Hz, 1H, HaCHb), 1.48 (ddd, J = 13.50, 9.31, 4.08 Hz,1H, HaCHb), 1.86–1.75 (m, 1H, CH), 2.61 (s, 3H, CH3), 3.73 (dd, J = 9.10, 4.00 Hz, 1H), 7.69 (s, 1H), 6.40 (s, br, 1H, NH), and 13.13 (s, br, 1H, COOH).
The production of immunogen and the homologous coating antigen was carried out by coupling the hapten ITeAHGA to the carrier protein (BSA/OVA) via the common N-hydroxysuccinimide active ester method, while ITeAH was conjugated to OVA and used as the heterologous coating antigen through the cross-linking agent glutaraldehyde. Successful conjugations were confirmed by the UV-vis data (data not shown). The antigen was added in molar excess over that of the carrier protein in order to bind sufficiently (Hamajima et al. 1995). Most reported hapten/protein ratios are between 50:1 and 100:1 which resulted in an ideal artificial antigen and subsequently produced ideal antibodies (Hamajima et al. 1995; McAdam et al. 1992; Shen et al. 2007).
mAb Production and Identification
In our experiment, the mAb raised against ITeAH was successfully produced from a selected single hybridoma (2E8) and used to establish the icELISA detection system for ITeA. The derivation strategy that is based on the special antibody to the derivative of the determinants has been successfully used by other researchers to establish immunoassays for several haptens including 1-aminohydantoin (AHD) (Jiang et al. 2012), furaltadone metabolite AMOZ (Shen et al. 2012), and TeA (Prelle et al. 2013). This strategy is particularly useful when production of antibody to toxic compound is in question, whether it is due to the extreme toxicity or the lack of toxic reagent itself. Considering the homologous coating antigens for which antibodies generally have weaker affinity towards (Xu et al. 2012; Galve et al. 2002), two coating antigens, ITeAHGA-OVA and ITeAH-OVA, were compared in the present study and the results (Fig. 3) clearly indicated that the heterologous coating antigen ITeAH-OVA (IC50 = 14.5 ng/mL) was superior to the homologous coating antigen ITeAHGA-OVA (IC50 = 27.2 ng/mL). This is in accordance with other research finding that the use of an appropriate heterologous coating antigen can significantly improve sensitivity of the assay (Qi et al. 2012). Subsequent experiments were therefore carried out based on the coating antigen of ITeAH-OVA.
ELISA Optimization
To optimize the developed ELISA, we tested various concentrations of the coating antigen and antibody, reaction time of antigen with antibody, incubation time of HRP labeled secondary antibody, different analyte/antibody ratio, and buffering system. The A max/IC50 ratio (A max is the maximum value of absorbance) from the competition curves of ITeAH was used to estimate the influence of each condition on assay performance, and the higher ratio suggests the higher sensitivity of the assay (Liang et al. 2007). Figure 4a shows an optimal combination of a coating antigen at a concentration of 15.6 ng/mL and an antibody at 1:2000 dilution, exhibiting the lowest IC50 value of 5 ng/ml. Other optimized assay conditions include 40 min reaction time (Fig. 4b) for incubation of the antigen-antibody, 30 min for the anti-IgG-HRP antibody (Fig. 4c), and H2O was found as the most suitable diluent for the analyte (Fig. 4(d)). Under these conditions, a calibration curve was constructed for ITeAH at concentrations ranged from 0.064 to 1000 ng/mL with a linear working range between 1.7 and 36.4 ng/mL (R(Marin et al. 2013 )=0.9944) (Fig. 5). The established icELISA system is highly sensitive with an IC50 value of 7.8 ng/mL and a LOD of 0.5 ng/mL for ITeAH. An enzyme immunoassay has been reported for TeA in apple and tomato products with an IC50 of 320 ± 130 ng/ml for TeA, but a much lower IC50 of 23.3 ± 7.5 ng/ml for the TeA acetate. When TeA acetate was employed as the standard in the EIA to measure the acetylated TeA, an IC30 of 5.4 ± 2.0 ng/ml was resulted (Gross et al. 2011). Ackermann et al. (2011) described the development of an EIA for rapid determination of alternariol, another Alternaria mycotoxin, with a detection limit of 1–2 μg/kg.
Cross-Reactivity Studies
The specificity of the developed monoclonal antibody was examined by testing the cross-reactivity (CR) rates of several Alternaria mycotoxin analogues (Table 1), including iso-tenuazonic acid (ITeA), tenuazonic acid (TeA), alternariol (AOH), alternariol methyl ether (AME), and hydrazine hydrate. The results were all less than 0.1%, suggesting the high specificity of the produced monoclonal antibody towards ITeAH, which is vital in the developed ELISA system.
Analysis of Spiked Samples
The spiked apple juice, beer, and tomato ketchup samples were analyzed using the developed ELISA. It is generally recognized that the reasonable sample preparation can effectively reduce the matrix effect (Sheng et al. 2012). A simple H2O dilution of samples was used in this study, i.e., 35 times dilution for the extracts of apple juice and beer, and 45-fold dilution for extract of tomato ketchup. Samples were spiked with ITeA at different concentrations to evaluate the recovery rates of the developed immunoassay. As shown in Table 2, the recoveries of ITeA ranged from 93.3 to 109.8% for apple juice, 82.3 to 93.2% for beer, and 93.2 to 107.3% for tomato ketchup, respectively, and that was within the general requirement of 70–120% for screening immunoassays (Kondo et al. 2012; Wang et al. 2011). The coefficients of variation (CV) were all found to be less than 15%, indicating a good level of precision of the developed ELISA.
Comparison of the ELISA and HPLC-MS/MS Method
The ELISA results were compared and confirmed by the HPLC-MS/MS method. The linear relationship between the two methods was at y = 0.7660x + 45.52 with a squared coefficient of correlation (R(Marin et al. 2013 )) of 0.9557 for the spiked food samples (Fig. 6), suggesting a good agreement between the screening ELISA and confirmatory HPLC-MS/MS methods. These results also demonstrated the suitability of the developed ELISA for the detection of trace levels of ITeA in food samples. Asam et al. (2013) reported the development of analytical methods for detection of TeA and its analogues in foods with an LOD of 1 μg/kg (TeA) and 3 μg/kg (ITeA) for derivatized samples and 60 μg/kg (TeA and ITeA) for samples without derivatization, while the ELISA method we developed here has a significantly lower LOD of 0.5 μg/L for ITeA. Taking consideration of the generally lower level of ITeA than TeA in fruits and vegetables, the present ITeA ELISA possesses a lower detection limit and higher sensitivity.
Detection of ITeA in Real Samples
The commercial food samples (n = 20) were analyzed using both ELISA and HPLC-MS/MS methods. As shown in Table 3, results revealed a high conformity between the two methods, i.e., all samples were found to be positive by the developed ELISA and their quantified results are in consistence with the HPLC-MS/MS data. This further confirms the reliability of the established ELISA method as a fit-for-purpose screening tool for quantitative analysis of ITeA in food samples. ITeA was detected with varying concentrations in all samples tested, i.e., 39.2–110.3 ng/mL in apple juice, 45.4–79.3 ng/mL in beer, 41.5–81.1 ng/mL in tomato ketchup, and 43.4–157.2 ng/mL in dried fruits. Using the UPLC-ESI-MS/MS, Walravensa et al. (2014) reported around 71% of rice samples and 31% of oat flake samples obtained in Belgium were contaminated with TeA at concentrations ranging from 1.90 to 113 μg/kg and 2.13 to 39 μg/kg, respectively. Such high frequency and levels of contamination of Alternaria mycotoxins and TeA in particular has highlighted the importance of continued monitoring of TeA in food and feed. Likewise, similar occurrence will also be applied to ITeA contamination scenario.
Currently, there are no details of accepted daily intake (ADI) and maximum residue limits (MRL) available for ITeA. Moreover, due to a lack of information on occurrence and toxicity, the European Food Safety Authority (EFSA) stated that a risk assessment for Alternaria mycotoxins in feed was not possible (EFSA 2011). This may partially due to the lack of rapid methods such as immunoassays that are capable of screening a large number of samples within a relatively short period of time. Consequently, the liquid chromatography coupled to (tandem) mass spectrometry is the method of choice for quantification of Alternaria toxins in foods and feeds. The sensitive and reliable ELISA described in this study would therefore contribute greatly to the effective and efficient monitoring of ITeA in food and the environment.
Conclusions
In this paper, we report the development of a sensitive ELISA using a specific monoclonal antibody for reliable detection of ITeA in food samples. The optimized ELISA has an IC50 value of 7.8 ng/mL and a detection limit of 0.5 ng/mL with good extraction efficiency for apple juice, beer, and tomato ketchup samples. The established immunoassay was subsequently implemented in a mini-survey of commercial food samples with results revealing a potentially widespread contamination of ITeA (probably alongside TeA) in processed commercial foods. The close agreement between the ELISA result and HPLC-MS/MS data has confirmed the reliability of this newly developed ELISA as a versatile screening tool for monitoring ITeA in different foods. Future studies will seek its applications in a wider range of foodstuffs including animal feeds to facilitate the collection of occurrence data and estimation of dietary exposure for this Alternaria mycotoxin.
References
Ackermann Y, Curtui V, Dietrich R, Gross M, Latif H, Martlbauer E, Usleber E (2011) Widespread occurrence of low levels of alternariol in apple and tomato products, as determined by comparative immunochemical assessment using monoclonal and polyclonal antibodies. J Agric Food Chem 59:6360–6368
Asam S, Liu Y, Konitzer K, Rychlik M (2011) Development of a stable isotope dilution assay for tenuazonic acid. J. Agri. Food Chem. 59:2980–2987
Asam S, Lichtenegger M, Muzik K, Liu Y, Frank O, Hofmann T, Rychlik M (2013) Development of analytical methods for the determination of tenuazonic acid analogues in food commodities. J Chromatogr A 1289:27–36
Cui JL, Zhang K, Huang QX, Yu YY, Peng XZ (2011) An indirect competitive enzyme-linked immunosorbent assay for determination of norfloxacin in waters using a specific polyclonal antibody. Anal Chim Acta 688:84–89
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2011) Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J 9(10):2407
Galve R, Sanchez-Baeza F, Camps F, Marco MP (2002) Indirect competitive immunoassay for trichlorophenol determination. Rational evaluation of the competitor heterology effect Anal Chim Acta 452:191–206
Gitterman CO (1965) Antitumor, cytotoxic, and antibacterial activities of tenuazonic acid and congeneric tetramic acids. J Med Chem 8(4):483–486
Gross M, Curtui V, Ackermann Y, Latif H, Usleber E (2011) Enzyme immunoassay for tenuazonic acid in apple and tomato products. J Agric Food Chem 59:12317–12322
Hamajima K, Bukawa H, Fukushima J, Kawamoto S, Kaneko T, Sekigawa KI, Tanaka SI, Tsukuda M, Okuda K (1995) A macromolecular multicomponent peptide vaccine prepared using the glutaraldehyde conjugation method with strong immunogenicity for HIV-1. Clin Immunol Immunopathol 77:374–379
Henniona MC, Barcelob D (1998) Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: a review. Anal Chim Acta 362:3–34
Jiang WX, Luo PJ, Wang X, Chen X, Zhao YF, Shi W, Wu XP, Wu YN, Shen JZ (2012) Development of an enzyme-linked immunosorbent assay for the detection of nitrofurantoin metabolite, 1-amino-hydantoin, in animal tissues. Food Control 23:20–25
Kondo M, Tsuzuki K, Hamada H, Yamaguchi Y, Uchigashima M, Saka M, Watanabe E, Iwasa S, Narita H, Miyake S (2012) Development of an enzyme-linked immunosorbent assay (ELISA) for residue analysis of the fungicide azoxystrobin in agricultural products. J Agric Food Chem 60:904–911
Lebrun MH, Nicolas L, Boutar M, Gaudemer F, Ranomenjanahary S, Gaudemer A (1988) Relationship between the structure and the phytotoxicity of the fungal toxin tenuazonic acid. Phytochemistry 27(1):77–84
Liang CZ, Jin RY, Gui WJ, Zhu GN (2007) Enzyme-linked immunosorbent assay based on a monoclonal antibody for the detection of the insecticide triazophos: assay optimization and application to environmental samples. Environ Sci Technol 41:6783–6788
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237
McAdam DP, Hill AS, Beasley HL, Skerritt JH (1992) Mono- and polyclonal antibodies to the organophosphate fenitrothion.1.Approaches to hapten-protein conjugation. J. Agric. Food Chem. 40:1466–1470
Mercader JV, Suárez-Pantaleón C, Agulló C, Abad-Somovilla A, Abad-Fuentes A (2008) Production and characterization of monoclonal antibodies specific to the strobilurin pesticide pyraclostrobin. J. Agri. Food Chem. 56:7682–7690
Moreno MJ, Abad A, Montoya A (2001) Production of monoclonal antibodies to the N-methylcarbamate pesticide propoxur. J Agric Food Chem 49:72–78
Noser J, Schneider P, Rother M, Schmutz H (2011) Determination of six Alternaria toxins with UPLC-MS/MS and their occurrence intomatoes and tomato products from the Swiss market. Mycotoxin Res 27:265–271
Prelle A, Spadaro D, Garibaldi A, Gullino ML (2013) A new method for detection of five alternaria toxins in food matrices based on LC-APCI-MS. Food Chem 140:161–167
Qi YH, Shan WC, Liu YZ, Zhang YJ, Wang JP (2012) Production of the polyclonal antibody against Sudan 3 and immunoassay of Sudan dyes in food samples. J Agri Food Chem 60:2116–2122
Qiang S, Dong YF, An CF, Zhou B, Zhu YZ, Chen SG, Dai XB, Dai BJ, Cai JG (2008) Biological control of weeds using the metabolites of Alternaria alternata. United States Patent Application Publication. U.S.Patent 2008/0280761 A1, 13 Nov 2008
Qin JC, Zhang YM, Hu L, Ma YT, Gao JM (2009) Cytotoxic metabolites produced by Alternaria No.28, an endophytic fungus isolated from Ginkgo biloba. Nat. Prod. Commun. 4(11):1473–1476
Shen YD, Wang Y, Zhang SW, Xiao ZL, Sun YM, Bu XZ, Gu LQ (2007) Design and efficient synthesis of novel haptens and complete antigens for the AOZ, a toxic metabolite of furazolidone. Chinese Chem Lett 18:1490–1492
Shen YD, Xu ZL, Zhang SW, Wang H, Yang JY, Lei HT, Xiao ZL, Sun YM (2012) Development of a monoclonal antibody-based competitive indirect enzyme-linked immunosorbent assay for furaltadone metabolite AMOZ in fish and shrimp samples. J Agric Food Chem 60:10991–10997
Sheng YJ, Jiang WX, Saeger SD, Shen JZ, Zhang SX, Wang ZH (2012) Development of a sensitive enzyme-linked immunosorbent assay for the detection of fumonisin B1 in maize. Toxicon 60:1245–1250
Shephard GS, Berthiller F, Burdaspal PA, Crews C, Jonker MA, Krska R, MacDonald S, Malone RJ, Maragos C, Sabino M, Solfrizzo M, Van Egmond HP, Whitaker ATB (2012) Developments in mycotoxin analysis: an update for 2011-2012. World Mycotoxin J 5(1):3–30
Siegel D, Rasenko T, Koch M, Nehls I (2009) Determination of the Alternaria mycotoxin tenuazonic acid in cereals by high-performance liquid chromatography-electrospray ionization ion-trap multistage mass spectrometry after derivatization with 2,4-dinitrophenylhydrazine. J Chromatogr A 1216:4582–4588
Siegel D, Feist M, Proske M, Koch M, Nehls I (2010) Degradation of the Alternaria mycotoxins alternariol, alternariol monomethyl ether, and altenuene upon bread baking. J Agric Food Chem 58:9622–9630
Stinson EE, Osman SF, Heisler EG, Siciliano J, Bills DD (1981) Mycotoxin production in whole tomatoes, apples, oranges, and lemons. J Agric Food Chem 29:790–792
Walravensa J, Mikulab H, Rychlikc M, Asamd S, Ediagea EN, Mavungua JDD, Landschoote AV, Vanhaeckef L, Saeger SD (2014) Development and validation of an ultra-high-performance liquid chromatography tandem mass spectrometric method for the simultaneous determination of free and conjugated Alternaria toxins in cereal-based foodstuffs. J Chromatogr A 1372:91–101
Wang JP, Yu GC, Sheng W, Shi M, Guo BX, Wang S (2011) Development of an enzyme-linked immunosorbent assay based a monoclonal antibody for the detection of pyrethroids with phenoxybenzene multiresidue in river water. J Agric Food Chem 59:2997–3003
Xu ZL, Shen YD, Sun YM, Campbell K, Tian YX, Zhang SW, Lei HT, Jiang YM (2012) Novel hapten synthesis for antibody production and development of an enzyme-linked immunosorbent assay for determination of furaltadone metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). Talanta 103:306–313
Yang XX, Liu XX, Wang H, Xu ZL, Shen YD, Sun YM (2012) Development of an enzyme-linked immunosorbent assay method for detection of tenuazonic acid. Chinese J Anal Chem 40(9):1347–1352
Zhao MP, Li YZ, Guo ZQ, Zhang XX, Chang WB (2002) A new competitive enzyme-linked immunosorbent assay (ELISA) for determination of estrogenic bisphenols. Talanta 57:1205–1210
Zhao K, Shao B, Yang DJ, Li FQ (2015a) Natural occurrence of four alternaria mycotoxins in tomato- and citrus- based foods in China. J Agric Food Chem 63:343–348
Zhao K, Shao B, Yang DJ, Li FQ, Zhu JH (2015b) Natural occurrence of alternaria Toxins in wheat-based products and their dietary exposure in China. PLoS One 10(6):e0132019
Funding
This study was funded by the National Natural Science Foundation of China (31271866), the National Key Research and Development Program of China (2016YFE0106000), the Science and Technology Project of Guangzhou City (2014J4100185), and the Science and Technology Project of Guangdong Province (2014A050503059, 2014A030311043).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Zhi-Li Xiao declares that she has no conflict of interest.
Ya-Li Wang declares that she has no conflict of interest.
Yu-Dong Shen declares that he has no conflict of interest.
Zhen-Lin Xu declares that he has no conflict of interest.
Jie-Xian Dong declares that she has no conflict of interest.
Hong Wang declares that she has no conflict of interest.
Chen Situ declares that she has no conflict of interest.
Feng Wang declares that he has no conflict of interest.
Jin-Yi Yang declares that he has no conflict of interest.
Hong-Tao Lei declares that he has no conflict of interest.
Yuan-Ming Sun declares that he has no conflict of interest.
Ethical Approval
All procedures involving animals were approved and performed in accordance with the relevant protective and administrative guidelines for laboratory animals of China.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Zhi-Li Xiao and Ya-Li Wang are equal contributors.
Rights and permissions
About this article
Cite this article
Xiao, ZL., Wang, YL., Shen, YD. et al. Specific Monoclonal Antibody-Based Enzyme Immunoassay for Sensitive and Reliable Detection of Alternaria Mycotoxin Iso-Tenuazonic Acid in Food Products. Food Anal. Methods 11, 635–645 (2018). https://doi.org/10.1007/s12161-017-1033-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1033-9