Abstract
A fast, simple, and cost-effective miniaturized solid-phase extraction method using calixarenes as adsorbent was developed for the micro-extraction of cyanazine, simazine, atrazine, and propazine from complex samples. The ultrahigh-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry was employed for the structural identification of target compounds. The parameters affecting the performance of extraction, such as the type of sorbents, the amount of the sorbent, desorption solvents, breakthrough volume, and adsorption capacity, were optimized. Under optimal conditions, the linear range was 6–600 ng mL−1 with the coefficients of determination from 0.9943 to 0.9998. The limits of detection and quantification for the four triazines were 0.01–0.04 and 0.04–0.15 ng mL−1, respectively. The proposed method was successfully used to determine triazine herbicides in the honey and milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calixarenes are a class of cavitands consisting of hydroxyalkylation products of phenol and aldehyde. In particular, calixarenes have specific cavities and molecular skeleton that can hold smaller molecules or ions through multiple non-covalent interactions. In recent years, calixarenes have triggered substantial interest in various disciplines due to their fascinating cavities, unique physical and chemical properties (Zhang et al. 2016). Owing to conformational flexibility, stability, and facile functionalization of calixarenes, they were used in a wide range of applications such as synthesis and catalyst (Wang et al. 2017b), drug design (Hussain et al. 2017), sensing (Prata and Barata 2016), chromatography (Hu et al. 2016), and extraction (Li et al. 2016b). However, the use of single calixarene for complex samples has seldom been reported to date.
It is well known that preparing a sample for analysis is labor intensive and time-consuming due to complex sample matrices and insufficient sensitivity of some analytes, so an appropriate sample pretreatment is always required before the determination step. Some analytical procedures for pretreatment techniques have been developed which included cloud point extraction (CPE) (Giebultowicz et al. 2015), liquid-liquid extraction, matrix solid-phase dispersion (MSPD) (Cao et al. 2016; Albero et al. 2017), solvent extraction (Cao et al. 2014), dispersive micro-solid-phase extraction (D-μ-SPE) (Xu et al. 2015), liquid phase micro-extraction (LPME) (Nazari et al. 2017), miniaturized solid-phase extraction (mini-SPE) (Płotka-Wasylka et al. 2015; Cao et al. 2015), homogeneous liquid-liquid extraction (HLLE) (Farajzadeh et al. 2016; Pochivalov et al. 2017), magnetic dispersive solid-phase extraction (MDSPE) (Zhang et al. 2017; Farajzadeh and Mohebbi 2018; Chen et al. 2014), and so on. Among these methods, mini-SPE is one of the most widely used sample pretreatment techniques due to its simplicity, high enrichment factor, fast extraction, low consumption of organic solvents, low column pressure, and high selectivity. It is noteworthy that the choice of the sorbent is crucial because it can determine the selectivity of the extraction method and influence the enrichment efficiency and adsorption capacity of target analytes. In the last few years, several sorbents for mini-SPE have been reported, including cyclodextrin (Peng et al. 2017), nanofibers (Tavengwa et al. 2016), molecular sieve (Cao et al. 2015), Oasis HLB (Li et al. 2016a), and chitosan-modified graphitized multiwalled carbon nanotubes (Xu et al. 2016). However, no previous application of calixarenes in mini-SPE has been studied thus far.
Triazines are one of the most widely used herbicides for the control of grasses and broadleaf weeds in agriculture activities around the world because they inhibit photosynthetic reactions of many weeds. However, the presence of triazines residues had the potential to threaten human health, such as birth defects, cancers, and interruption of hormone functions, due to their toxicity and high distribution in the environment. Consequently, the monitoring of trace triazine herbicides residues in different types of matrices is crucial and essential. There are many chromatographic techniques that have been used to determine triazine herbicides in different complex real samples, such as pulse glow discharge-ion mobility spectrometry (Zou et al. 2016), high-performance liquid chromatography (HPLC) (Wang et al. 2017a; Rodríguez-González et al. 2017), and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (Zhou et al. 2016). In the technologies mentioned above, the main extraction methods of triazine pesticides were stir bar sorptive extraction (Zou et al. 2016), ionic liquid micro-extraction (Wang et al. 2017a), MSPD (Rodríguez-González et al. 2017), and solid-phase extraction (SPE) (Zhou et al. 2016). However, these extraction procedures more or less exhibit some drawbacks, such as poor extraction efficiencies, complicated preparation processes, time-consuming extraction, and low sensitivity. Therefore, it is imperative to establish a simple, effective, economical, and miniaturized sample preparation technique for the extraction of triazine residues.
The aim of this study was to develop a simple, fast, specific, and sensitive analytical method for the simultaneous quantitation of cyanazine, simazine, atrazine, and propazine from complex samples. The calixarenes were successfully applied as mini-SPE adsorbent to extract and clean up the chemicals residues, followed by the ultrahigh-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UHPLC-Q-TOF/MS) analysis. As far as we know, studies using mini-SPE-UHPLC-Q-TOF/MS have not been done to determine the target herbicides. Some key factors affecting the extraction efficiency such as the type of sorbents, the amount of the sorbent, desorption solvents, breakthrough volume, and adsorption capacity were studied in detail. Finally, the developed method was successfully applied to the microextraction of triazine herbicides in honey and milk samples.
Materials and Methods
Reagents and Materials
4-tert-Butylcalix[4]arene (tBC), calix[6]arene, calix[8]arene, and O(1),O(3)-Bis(ethoxycarbonylmethyl)-p-tert-butylcalix[4]arene were purchased from Alfa Aesar Chemical Co., Ltd. (Shanghai, China). Cyanazine, simazine, atrazine, and propazine were supplied by Sigma-Aldrich (Shanghai) Trading Co., Ltd. (Shanghai, China). Methanol, formic acid, ethanol, and acetonitrile (ACN) of chromatographic grade were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ethyl acetate, acetone, and chloroform were purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). All reagents were used as received without further purification in the study. Pure water with a resistivity of 18.2 MΩ cm at 25 °C was prepared by using a Milli-Q® integral water purification system (Millipore Co., USA).
The honey and milk samples were purchased from a local supermarket (Hangzhou, China).
UHPLC-Q-TOF/MS System
A 1290 UHPLC Agilent Infinity system and a quadrupole combined with a time-of-flight mass spectrometer (Santa Clara, CA, USA) were coupled through an electrospray (ESI) interface. The UHPLC instrument consisted of an online vacuum degasser, an autosampler, a binary solvent pump, and a thermostated column compartment. An Agilent Eclipse Plus C18 column (2.1 mm × 50 mm, 1.8 μm Agilent Technologies Inc., USA) was employed for UHPLC analysis, and this was protected by an in-line Agilent filter with 4.6-mm-diameter frits and 0.2-μm pore size.
The mobile phase consisted of water with 0.1% formic acid (A) and methanol (B) using a gradient program of 20–40% B for 0–1 min, 40–60% B for 1–5 min, 60–70% B for 5–7 min, and 70–100% B for 7–8 min. The flow rate of mobile phase, injection volume, and column temperature were set at 0.4 mL min−1, 2 μL, and 30 °C, respectively. The ESI-Q-TOF/MS detection conditions in positive ionization mode were as follows: capillary voltage, 3500 V; drying gas (N2) flow, 12 L min−1; drying gas (N2) temperature, 350 °C; nebulizer pressure, 45 psig; fragmentor, 165 V; and skimmer, 65 V, which gave the highest response for the analytes. Data acquisition and processing were performed in the extended dynamic range 2 GHz over the m/z 100–1000 range using MassHunter Workstation Quantitative and Qualitative Analysis software (version B 05.00, Agilent, Waldbronn, Germany).
Sample Treatment
One gram of the honey or milk sample was diluted with 10 mL of ultrapure water. The resulting solutions were centrifuged at 15,000 rpm for 4 min and loaded onto a cartridge to perform the mini-SPE extraction and clean-up.
Column Preparation and Mini-SPE Procedure
The mini-SPE method was carried out as follows. Firstly, the sorbents (tBC, calix[6]arene, calix[8]arene, and O(1),O(3)-Bis(ethoxycarbonylmethyl)-p-tert-butylcalix[4]arene) were accurately weighed (15, 20, 25, 30, 35 mg) and placed into an empty 1-mL cartridge containing lower and upper sieve plates. Then, the sample solution (10 mL) was passed through the mini-SPE column, which had been preconditioned with 1 mL of methanol and 1 mL of water. During this process, the flow rate was controlled by an aspirator pump and maintained at the rate of 1 mL min−1. Subsequently, matrix interferences were removed by washing with 1 mL water, and the target analytes were eluted with different volumes (100, 200, 300, 400, and 500 μL) of desorption solvent (methanol, ethanol, acetonitrile, ethyl acetate, acetone, and chloroform). Finally, the eluents were collected in a 1.5-mL Eppendorf tube and centrifuged at 15000 rpm for 4 min before UHPLC-Q-TOF/MS analysis (Fig. 1).
Calculation of Enrichment Factor and Extraction Recovery
Two important parameters including enrichment factor (EF) and extraction recovery (ER) have been employed for evaluating the proposed method. The EF and ER were calculated for analytes based on the following equations:
where Cop is the analyte concentration in the desorption solvent, C0 is the initial concentration of analyte in the aqueous solution, and Vop and V0 represent the volume of organic phase and the aqueous solution, respectively.
Results and Discussion
Structural Characterization and Identification of Triazine Herbicides
In order to achieve the structural information and confirm the presence of triazine herbicides in the sample, fragment ions mass spectra were obtained by UHPLC/Q-TOF-MS, which was performed in positive mode. Data acquisition recorded the fragmentation patterns between the precursor ion and the most fragment ions for target analytes. As presented in Fig. 2a, the abundant ions of cyanazine generated were m/z 214.0843, 174.0530, and 104.0008. In this run, cyanazine fractured with a neutral loss of 27, 40, and 70 Da. These may correspond to a fracture of a HCN, C3H4, and C3H6N2 group, respectively. The precursor ion of simazine was m/z 202.0849 in full scan spectrum to collision energy where 30 eV was applied (Fig. 2b). It can be found that the minor product has mass of 174.0534 Da (loss of C2H4), and the two abundant products formed were m/z 132.0319 (loss of C2H4, C2H5N) and m/z 104.0011 (loss of C2H4, C2H5N, CH2N2). The mass spectrometry of atrazine (Fig. 2c) corresponded to the following ion: m/z 104.0015 and m/z 174.0525, which were the same compound structure as above. Furthermore, the fragmentation appeared to involve cleavage of C2H4 unit leading to the mass 146.0237 Da. By further collisional dissociation of propazine (Fig. 2d), the ion at m/z 188.0687 was observed. Moreover, propazine was undergoing same transformation fragmentation as atrazine, m/z 146.0221 and m/z 104.0012.
Effect of the Type of Sorbents
The type of adsorbent is an important factor that affected the extraction efficiency of micro-SPE. As is known to all, calixarenes have a favorable adjustable cavity for inclusion of target analytes. The cavity size of calixarenes is one of the primary parameters for the adsorption of triazine herbicides. To enhance the extraction efficiency, the micropore diameter of ideal adsorbent should be slightly larger than the kinetic diameter of the triazine herbicides. In our work, four kinds of calixarenes, including tBC, calix[6]arene, calix[8]arene, and O(1),O(3)-Bis(ethoxycarbonylmethyl)-p-tert-butylcalix[4]arene, were tested. As the results showed in Fig. 3a, tBC performs the highest extraction ability for the triazine herbicides compared to the other adsorbents. tBC exhibited the highest peak abundances in the series of calixarenes because the cavity diameter of the tBC was just suitable for the triazine herbicides. Adsorption abilities of calix[6]arene and calix[8]arene were poor among these adsorbents tested because they were difficult to fit the triazine herbicides in the bigger cavity. Low peak abundances were exhibited for O(1),O(3)-Bis(ethoxycarbonylmethyl)-p-tert-butylcalix[4]arene which was applied as adsorbents. This may be due to dissolution and interaction of the adsorbent and elution solvent that led to poor extraction efficiency. Therefore, tBC was chosen and employed for the further experiments.
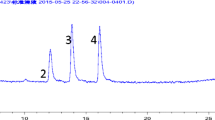
The optimization of mini-SPE process. Analytes: (1) cyanazine, (2) simazine, (3) atrazine, (4) propazine. a Selection of type of sorbent. b Effect of the amount of calixarenes on the extraction efficiency of triazine herbicides. c Effect of the type of elution solvent on the extraction efficiency of triazine herbicides
Effect of the Amount of the Sorbent
The amount of the adsorbent is one of the key factors in any extraction process. To achieve an optimal amount of tBC for enrichment of triazine herbicides, the effect of adsorbent amount on the extract ability was assessed at five different levels (15, 20, 25, 30, and 35 mg). The result, which is showed in Fig. 3b, presents the peak abundances for the target analytes increased drastically when the amount of tBC was increased from 15 to 25 mg. This may be due to the number of active sites and high surface areas of tBC that increased the extraction efficiency rapidly. However, when further increasing the amount of tBC (25 mg) was applied to the extraction procedure, a slight diminution was observed to peak areas for all target analytes. The reason may be that strong adsorption leads to the difficulty which incurred during the desorption process. With higher the amount of tBC, the peak abundances did not present a marked enhancement and even decreased. Therefore, for the investigated amount ranges, 25 mg of tBC was selected as the optimum value to achieved effective extraction.
Effect of the Desorption Solvents
The organic solvents applied to elute the target analytes observably affected the degree of desorption of compounds from the adsorbent. According to the principle of like dissolves like, polar solvents are useful for dissolution of polar analytes. Based on this principle, six kinds of desorption solvents with different polarity were evaluated, including methanol, ethanol, acetonitrile, ethyl acetate, acetone, and chloroform. Since the studied triazine herbicides are relatively polar compounds, polar solvents should give better elution ability than nonpolar solvents. It was evident that methanol has higher polar characteristic than other organic solvents (Fig. 3c). On the other side, being relatively nonpolar, ethyl acetate, acetone, and chloroform revealed less desorption efficiency of target analytes. For all the compounds, desorption power of the elution solvents did not only lay on their polarity. Other parameters, such as the dissolution of the adsorbent by the elution solvent, may have an important influence on the elution procedure. For ethanol, ethyl acetate, acetone, and chloroform, dissolution and interaction of the adsorbent and elution solvent decreased extraction efficiency of target analytes. As a result, methanol was chosen as elution solvent for following experiments to make sure the entire elution of target analytes.
Effect of the Volume of Desorption Solvents
The volume of desorption solvent was another significant parameter that closely correlated with the extraction efficiency. The desorption solvent volume affected the sensitivity of the method, as it decided the maximum EF that could be achieved for the target analytes. To obtain quantitative and efficient elution of target analytes, the volumes of desorption solvent were evaluated ranging from 100 to 500 μL. Lower volumes were not considered, as they were difficult to process. According to the experiments (presented in Fig. 3d), it is found that 200 μL is sufficient to obtain satisfactory extraction efficiency and acceptable EF. As it was expected, the extraction efficiency increased with the volume of desorption solvent up to 200 μL, while a slight diminution was observed when 300 μL was used. This reason could be ascribed to the fact that the force between target analytes and sorbent was strong and analytes needed more desorption solvent for complete elution. However, when the volume of desorption solvent more than 200 μL, EF decreased due to the dilution effect. Hence, 200 μL of methanol was chosen as optimal in the following experiments.
Breakthrough Volume
The breakthrough volume (VB) is one of the factors characterizing the micro-SPE adsorbent, which can be calculated by the breakthrough curve. Generally, the breakthrough curve generated by the relationship between the concentration of the compound in the effluent and the corresponding aliquot volume of the sample passed through the adsorbent. Under optimal conditions, the breakthrough curve has a logarithmic shape and the relative equation is expressed as follows:
where VR stands for the retention volume, which can be defined vividly from the figure; it is in accord with half of the max concentration of the analyte in the effluent. σv is the standard deviations of the logarithmic curve definite diagrammatically from the breakthrough curve. Because of its asymmetry, these are given properly as value is in correspondence to 0.159 of the average value of maximum analyte concentration in the effluent. The breakthrough volume can be determined as:
where VB expressed the capacity of adsorbent that corresponds (on the breakthrough curve) to 1% of maximum analyte concentration in the effluent.
In our work, the breakthrough volume of micro-SPE column was presented by percolating multifarious volumes of samples (1, 2.5, 5, 10, 15, 20, 25, and 30 mL). The results indicated (Fig. 4) that the breakthrough volumes obtained were 1.69, 1.73, 2.22, and 0.52 mL for cyanazine, simazine, atrazine, and propazine, respectively.
Adsorption Capacity
In order to determine and evaluate the maximum adsorption capacity of tBC for triazine herbicides, the experiments were performed in a range of sample solution with different initial concentration (0.5–9 mg/L). The equilibrium adsorption capacity (qe, mg/g) of tBC was calculated by the change of concentrations before and after adsorption, and its value can be obtained according to the following equation:
where qe (mg/g) is the amount of herbicides adsorbed per unit weight of adsorbent at equilibrium, C0 (mg/L) and Ce (mg/L) are the initial and equilibrium concentrations of triazine herbicides in solution, V is the volume of experimental solution (L), and m (g) is the mass of tBC.
To estimate the apparent maximum binding amount of the tBC, the data was further manipulated with the Langmuir isotherm model:
In above equations, qm is the maximum sorption capacity and K is constant coefficient. The coordinates used in the transformations (Ce/qe versus Ce) provide graphically demonstrating the binding characteristics of the equilibrium sorption data in an intelligible manner. The values of qm can be acquired easily from slope of the depicted plots of Ce/qe versus Ce. In our study, the maximum sorption capacity values obtained for cyanazine, simazine, atrazine, and propazine were 0.21, 0.27, 0.32, and 0.55 mg/g, respectively.
Validation of Mini-SPE Method
The mini-SPE method was evaluated with respect to linearity, precision, limits of detection (LODs), limits of quantification (LOQs), repeatability, EF, and ER under the optimized conditions. The linearity of the method was carried out using a series of standard solutions at concentrations from 6 to 240 ng mL−1 for cyanazine, from 30 to 600 ng mL−1 for simazine, from 10 to 400 ng mL−1 for atrazine, and from 8 to 400 ng mL−1 for propazine. Calibration curves were constructed by least-squares linear regression analysis of the peak areas versus the concentration of the analytes, and good linearities of mini-SPE method were observed with correlation coefficient (r) ≥ 0.9943 as shown in Table 1. Precision of the mini-SPE method was determined by study of the intra-day and inter-day repeatability from the analysis of model sample at a spiked concentration of 50 ng mL−1. Intra-day repeatability was measured on a single day using six replicates of the spiked matrices, whereas inter-day repeatability was calculated during three consequent days using two replicates of each matrices at the same day, evaluated as relative standard deviation (RSD). The retention times and analyte situation were changed slightly in the evaluation of inter-day and intra-day precisions; it may be due to a change in the target analytes’ micro-environment. This could be HPLC column temperature fluctuations, evaporation of sample solution, column pollution, and so on, which resulted in the system was not well equilibrated. However, the intra-day repeatability of the four targeted compounds ranged from 0.90 to 2.94% and for the inter-day repeatability was lower than 5.0% for all analytes (Table 1), which demonstrated that the proposed method is capable of achieving high reproducibility with good precisions. The LODs and LOQs were determined based on the signal-to-noise ratios of 3 and 10. As shown in Table 1, the LOD and LOQ values for the four targeted analytes were in range of 0.01–0.04 and 0.04–0.15 ng mL−1, respectively. Under optimal conditions, EF ranged from 18.53 to 48.88 and ER varied between 37.06 and 97.76%, respectively.
Analysis of Real Samples
To evaluate the reproducibility of the method, three calixarene-based mini-SPE columns were prepared under the instrumental conditions reported in Section 2. As shown in Table 2, the relative standard deviation values for the retention times and peak areas were less than 1.32% (n = 3) and 5.32% (n = 3), respectively. The accuracy (in terms of recovery) was assessed by analyzing three replicates of spiked samples at two spiked levels and then extracted and analyzed by the proposed method. The results demonstrated that the recovery of tested analytes was 86.7–95.2% in honey with RSD of ≤ 4.5% and 85.7–95.1% in milk with RSD of ≤ 5.5%, as shown in Table 2. The feasibility of the mini-SPE–UHPLC-Q-TOF/MS method was evaluated by extracting triazine herbicides from river water and soil samples. It was found that no triazine residues were detected in any of the analyzed samples. Figure 5 shows the extracted ion chromatograms (EICs) of spiked honey and milk samples after treated by the mini-SPE procedure. All the above results indicated that the proposed method was reliable to screen triazine herbicides in complex matrix.
Comparison with Other Methods
The analytical performance of mini-SPE combined with UHPLC-Q-TOF/MS was compared with other reported methods for extraction and determination of triazine herbicides. The extraction method, amount of sorbent, extraction time, extraction solvent, instrumental technique, and LODs are listed in Table 3. As can be observed, the analytical characteristics of the developed method were comparable or in some cases has distinct advantages over other reported studies. First of all, the amount of sorbent in this method was much lower than others, except for dispersive solid-phase extraction (DSPE) (Wu et al. 2015). Twenty-five milligrams of tBC can effectively extract target analytes. However, other sorbents used in other extraction procedures were more than 100 mg. Secondly, the extraction time needed for the mini-SPE process was only 11 min, which was lower than molecularly imprinted solid-phase extraction (MISPE, 38 min) reported by Mao et al. (2013). Thirdly, as for solvent consuming, only 200 μL of methanol was employed for desorption in this work. In addition, mini-SPE gave comparable LODs and allows the analysis of triazine herbicides at trace levels in comparison with DSPE, MISPE, and Quick, Easy, Cheap, Effective, Rugged, and Safe extraction (QuEChERS) (De Oliveira Silva et al. 2014). Thus, considering the low consumption of sorbent and extraction solvent, speed, and low LODs, mini-SPE can be a competitive analytical tool for extraction and determination of trace triazine herbicides from the honey and milk samples.
Conclusions
In this study, calixarenes were used to develop a simple and efficient mini-SPE procedure for simultaneous purification and enrichment of triazine herbicides in honey and milk. Simple operation, high extraction efficiency, and high sensitivity made the mini-SPE–UHPLC-Q-TOF/MS technique particularly attractive for trace detection of herbicides. Besides, this method could be considered as an environmental friendly method since it required just a low cost of organic reagents. The results indicated that the proposed mini-SPE provided a promising alternative green mini-SPE method for trace analysis of food samples.
References
Albero B, Sanchez-Brunete C, Miguel E, Tadeo JL (2017) Application of matrix solid-phase dispersion followed by GC-MS/MS to the analysis of emerging contaminants in vegetables. Food Chem 217:660–667
Cao W, Hu SS, Li XY, Pang XQ, Cao J, Ye LH, Dai HB, Liu XJ, Da JH, Chu C (2014) Highly sensitive analysis of flavonoids by zwitterionic microemulsion electrokinetic chromatography coupled with light-emitting diode-induced fluorescence detection. J Chromatogr A 1358:277–284
Cao W, Ye LH, Cao J, Xu JJ, Peng LQ, Zhu QY, Zhang QY, Hu SS (2015) Quantitative analysis of flavanones from citrus fruits by using mesoporous molecular sieve-based miniaturized solid phase extraction coupled to ultrahigh-performance liquid chromatography and quadrupole time-of-flight mass spectrometry. J Chromatogr A 1406:68–77
Cao J, Peng LQ, Xu JJ, Du LJ, Zhang QD (2016) Simultaneous microextraction of inorganic iodine and iodinated amino acids by miniaturized matrix solid-phase dispersion with molecular sieves and ionic liquids. J Chromatogr A 1477:1–10
Chen XH, Zhao YG, Shen HY, Zhou LX, Pan SD, Jin MC (2014) Fast determination of seven synthetic pigments from wine and soft drinks using magnetic dispersive solid-phase extraction followed by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1346:123–128
De Oliveira Silva R, De Castro RC, Milhome MAL, Do Nascimento RF (2014) Liquid chromatography–electrospray ionization–tandem mass spectrometry method for determination of twenty multi-class pesticide residues in cashew. LWT-Food Sci Technol 59:21–25
Farajzadeh MA, Mohebbi A (2018) Development of magnetic dispersive solid phase extraction using toner powder as an efficient and economic sorbent in combination with dispersive liquid–liquid microextraction for extraction of some widely used pesticides in fruit juices. J Chromatogr A 1532:10–19
Farajzadeh MA, Mohebbi A, Feriduni B (2016) Development of a simple and efficient pretreatment technique named pH-dependent continuous homogenous liquid–liquid extraction. Anal Methods 8:5676–5683
Giebultowicz J, Kojro G, Bus-Kwasnik K, Rudzki PJ, Marszalek R, Les A, Wroczynski P (2015) Cloud-point extraction is compatible with liquid chromatography coupled to electrospray ionization mass spectrometry for the determination of bisoprolol in human plasma. J Chromatogr A 1423:39–46
Hu K, Zhang W, Yang H, Cui Y, Zhang J, Zhao W, Yu A, Zhang S (2016) Calixarene ionic liquid modified silica gel: a novel stationary phase for mixed-mode chromatography. Talanta 152:392–400
Hussain MA, Ashraf MU, Muhammad G, Tahir MN, Bukhari SNA (2017) Calixarene: a versatile material for drug design and applications. Curr Pharm Des 23:2377–2388
Li X, Li S, Kellermann G (2016a) Pre-analytical and analytical validations and clinical applications of a miniaturized, simple and cost-effective solid phase extraction combined with LC-MS/MS for the simultaneous determination of catecholamines and metanephrines in spot urine samples. Talanta 159:238–247
Li JW, Wang YL, Yan S, Li XJ, Pan SY (2016b) Molecularly imprinted calixarene fiber for solid-phase microextraction of four organophosphorous pesticides in fruits. Food Chem 192:260–267
Mao BP, Jia ZW, Mao XH, Wang K, Ji S (2013) Determination of triazine residues in herbal plants using molecularly imprinted polymer extraction followed by UPLC-MS/MS. Anal Methods 5:3582–3590
Mou RX, Chen MX, Cao ZY, Zhu ZW (2011) Simultaneous determination of triazine herbicides in rice by high-performance liquid chromatography coupled with high resolution and high mass accuracy hybrid linear ion trap-orbitrap mass spectrometry. Anal Chim Acta 706:149–156
Nazari S, Mehri A, Hassannia AS (2017) Fe3O4-modified graphene oxide as a sorbent for sequential magnetic solid phase extraction and dispersive liquid phase microextraction of thallium. Microchim Acta 184:3239–3246
Peng LQ, Ye LH, Cao J, Chang YX, Li Q, An M, Tan Z, Xu JJ (2017) Cyclodextrin-based miniaturized solid phase extraction for biopesticides analysis in water and vegetable juices samples analyzed by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Food Chem 226:141–148
Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J (2015) Miniaturized solid-phase extraction techniques. Trends Anal Chem 73:19–38
Pochivalov A, Vakh C, Andruch V, Moskvin L, Bulatov A (2017) Automated alkaline-induced salting-out homogeneous liquid-liquid extraction coupled with in-line organic-phase detection by an optical probe for the determination of diclofenac. Talanta 169:156–162
Prata JV, Barata PD (2016) Fostering protein–calixarene interactions: from molecular recognition to sensing. RSC Adv 6:1659–1669
Rodríguez-González N, González-Castro MJ, Beceiro-González E, Muniategui-Lorenzo S (2017) Development of a matrix solid phase dispersion methodology for the determination of triazine herbicides in marine sediments. Microchem J 133:137–143
Sobhanzadeh E, Bakar NKA, Abas MRB, Nemati K (2011) Low temperature followed by matrix solid-phase dispersion-sonication procedure for the determination of multiclass pesticides in palm oil using LC-TOF-MS. J Hazard Mater 186:1308–1313
Tavengwa NT, Nyamukamba P, Cukrowska E, Chimuka L (2016) Miniaturized pipette-tip-based electrospun polyacrylonitrile nanofibers for the micro-solid-phase extraction of nitro-based explosive compounds. J Sep Sci 39:4819–4827
Wang K, Jiang J, Kang M, Li D, Zang S, Tian S, Zhang H, Yu A, Zhang Z (2017a) Magnetical hollow fiber bar collection of extract in homogenous ionic liquid microextraction of triazine herbicides in water samples. Anal Bioanal Chem 409:2569–2579
Wang S, Bi Y, Hang X, Zhu X, Liao W (2017b) A calixarene-based {Co9} coordination triangle as an efficient heterogeneous catalyst. Z Anorg Allg Chem 643:160–165
Wu XL, Meng LX, Wu YH, Luk YY, Ma YQ, Du YL (2015) Evaluation of graphene for dispersive solid-phase extraction of triazine and neonicotine pesticides from environmental water. J Braz Chem Soc 26:131–139
Xu JJ, Ye LH, Cao J, Cao W, Zhang QY (2015) Ultramicro chitosan-assisted in-syringe dispersive micro-solid-phase extraction for flavonols from healthcare tea by ultra-high performance liquid chromatography. J Chromatogr A 1409:11–18
Xu JJ, An M, Yang R, Tan Z, Hao J, Cao J, Peng LQ, Cao W (2016) Determination of tetracycline antibiotic residues in honey and milk by miniaturized solid phase extraction using chitosan-modified graphitized multiwalled carbon nanotubes. J Agric Food Chem 64:2647–2654
Zhang F, Sun Y, Tian D, Shin WS, Kim JS, Li H (2016) Selective molecular recognition on calixarene-functionalized 3D surfaces. Chem Commun 52:12685–12693
Zhang Y, Zhou H, Zhang ZH, Wu XL, Chen WG, Zhu Y, Fang CF, Zhao YG (2017) Three-dimensional ionic liquid functionalized magnetic graphene oxide nanocomposite for the magnetic dispersive solid phase extraction of 16 polycyclic aromatic hydrocarbons in vegetable oils. J Chromatogr A 1489:29–38
Zhou T, Hou J, Yuan D, Li H, Zhang P, Li Y, Ding HG, Chen Y, Ding L (2016) Determination of triazine herbicides from honey samples based on hydrophilic molecularly imprinted resins followed by high performance liquid chromatography-tandem mass spectrometry. RSC Adv 6:98663–98673
Zou N, Yuan C, Liu S, Han Y, Li Y, Zhang J, Xu X, Li X, Pan C (2016) Coupling of multi-walled carbon nanotubes/polydimethylsiloxane coated stir bar sorptive extraction with pulse glow discharge-ion mobility spectrometry for analysis of triazine herbicides in water and soil samples. J Chromatogr A 1457:14–21
Funding
This study was supported by the General Program of National Natural Science Foundation of China (81573552), public welfare research project of Zhejiang Province (LGF18H280006), Projects for Study Abroad Returnees of Hangzhou (China, 2017), Hangzhou 131 middle-aged and young talent training plan (China, 2017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Li-Jing Du declares that she has no conflict of interest. Yan-Hua Jiao declares that she has no conflict of interest. Li-Hong Ye declares that she has no conflict of interest. Ting-Hong Fei declares that she has no conflict of interest. Qiu-Yan Wang declares that she has no conflict of interest. Yu-Han Hu declares that he has no conflict of interest. Jun Cao declares that he has no conflict of interest. Qi-Dong Zhang declares that he has no conflict of interest. Li-Qing Peng declares that she has no conflict of interest. Yu-Bo Chen declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Du, LJ., Jiao, YH., Ye, LH. et al. Calixarene-Based Miniaturized Solid-Phase Extraction of Trace Triazine Herbicides from the Honey and Milk Samples. Food Anal. Methods 11, 3283–3292 (2018). https://doi.org/10.1007/s12161-018-1270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1270-6