Abstract
In this work, we present the development and validation of an extraction analytical method for determination and quantification of 18 pesticides belonging to the chemical classes of benzimidazoles, organophosphates, anilides, triazoles, avermectins, benzoylureas, triazines, pyrethoids, neonicotinoids, and strobilurins in industrial samples of larger beer, employing solid-phase extraction (SPE), ultra-performance liquid chromatography-mass spectrometry. The optimized SPE procedure employed 100 mL of sample, 150 mg of biochar adsorbent, and elution with 3 mL of methanol:dichloromethane (60:40, v/v), resulting in a fast, practical, and economical technique. The methodology showed good linearity (R2 > 0.99). The average recoveries (n = 5) for the lowest concentration level ranged from 61 to 102%, with relative standard deviations between 2 and 19%. Detection and quantification limits ranged from 0.05 to 0.08 µg/L and from 0.01 to 0.25 µg/L, respectively. In addition, the method was applied to 13 commercial Pilsen-type larger beer labels, in which pesticide residues were not found.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Beer is one of the most appreciated and consumed drinks in the world (Statista 2022). Beer malt is made up of cereals such as barley, corn, and rice that are capable of providing a characteristic flavor to the drink (Mega et al. 2011). These cereals are widely used in Brazilian Pilsen beer because they provide a lighter and refreshing drink (Statista 2022).
Some chemicals such as pesticides are used in various crops to protect against pests and ensure increased production. These productions can remain after the food processing stage, affecting the health of consumers. In the case of alcoholic beverages such as beer, they may contain pesticides that are used during the cultivation process of the cereals used for their production (Jha et al. 2018). Exposure to beverage contaminated with these compounds can cause diseases such as cancer, different disorders, asthma and others that can compromise the health of the consumer (Hakme et al. 2024; Jha et al. 2018; Alavanja et al. 2013; Mostafalou and Abdollahi 2013).
One of the ways to evaluate the presence of these contaminants in the drink is through sample preparation methods that are adequate and capable of providing reliable results. In addition to using an efficient instrumental analytical technique to determine each of these compounds, among the sample preparation methods, there is solid-phase extraction (SPE), which is used for liquid samples, including beer (Dusek and Jandovská 2023; Pérez-Lucas and Navarro 2023; Kaczynski et al. 2024; Hack et al. 1997; Jones et al. 1988).
SPE is one of the most used methods because it provides speed, simplicity, good repeatability, and low cost, in addition to the possibility of using different adosrbents with different physicochemical properties. There are cartridges containing commercial adsorbents such as florisil, Oasis HLB, octadecylsilane (C18), ion exchange, and other solid phases that are efficient extraction; it is necessary to use an adsorbent that presents selectivity attributes in relation to the analytes and desorption capacity of these analytes from the adsorbent surface (Demir et al. 2020; Shahryari et al. 2022). Alternative materials that have been applied to SPE, such as magnetic, polymeric, molecular printing, nanomaterials, and carbon nanotubes, are prepared and reported in the literature with great efficiency for the extraction of numerous analytes (Shahryari et al. 2022; Augusto et al. 2013; Liu et al. 2012; Valverde et al. 2014; Wen et al. 2014; Wierucka and Bizuik 2014; Birniwa et al. 2020; Haq et al. 2023).
Studies have also investigated the efficiency of alternative and low-cost adsorbents such as biochar in different contaminant extraction methods, due to it being a material obtained from different biomasses, in addition to being rich in carbon, presence of varied functional groups, high porosity, and capacity for adsorption (Ahmad et al. 2014; Tang et al. 2013). Biochar comes from residues of crops and plants produced annually with little or no added value, which can generate a major environmental problem (Mandal et al. 2021).
Regarding adsorption capacity, many studies show the efficiency of water hyacinth biochar (aquatic plant of the genus Eichhornia crassipes) for adsorption of groups that favor hydrogen bonds, electrostatic interactions, and π-π interactions, in which the capacity adsorption capacity increases because of its high surface area (Madikizela 2021; Xu et al. 2020; Ponnam et al. 2020; Okoya et al. 2020; Baharum et al. 2020). Biochar is a byproduct of the pyrolysis process of biomass burning, in low oxygen conditions and the characteristics of the material vary depending on the heating temperature and time to obtain the material (Baharum et al. 2020; Taha et al. 2014). This adsorption capacity has direct links with its physicochemical properties, pore size, functional groups, and ion exchange capacity, and its properties can vary depending on the preparation conditions (Mohan et al. 2014; Gai et al. 2014; Wang and Wang 2019). Consequently, this material presents the possibility of physical adsorption (physisorption), hydrophobic interaction, and efficient electrostatic adsorption, in addition to presenting an increasing number of oxygen-containing functional groups on the surface that allow specific bonds and interactions (Zhou et al. 2017, 2019; Xiang et al. 2019; Suo et al. 2019).
In this way, biochar originating from water hyacinth, which is as useful biomass for various purposes as it is an aquatic plant with rapid growth and availability, was chosen, and its adsorption potential for the pesticides under study was evaluated. This type of biochar has been used in the adsorption of inorganic and organic substances, as well as heavy metals, dyes, among others (Saraswat and Rai 2010; Wanyonyi et al. 2014; Amos et al. 2019).
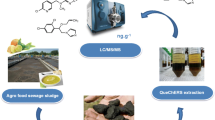
Therefore, the efficiency of water hyacinth biochar was evaluated in comparison with the commercial adsorbent C18 as an adsorbent material for SPE in conjunction with the LC-MS technique. Until the development of this work, no studies were found evaluating the efficiency of this biochar as an adsorbent in SPE for the extraction of the pesticides acetamiprid, ametrine, atrazine, azoxystrobin, carbendazim, chlorpyrifos, deltamethrin, diuron, esfenvalerate, epoxiconazole, flutriafol, haloxyfop, lufenuron, malathion, propanil, thiamethoxan, thiophanate-methyl, and simazine belonging to different chemical groups in beer samples.
Thus, the objective of this study was to develop an SPE method with water hyacinth biochar as an adsorbent for extracting pesticides from Pilsen lager beer using ultra-performance liquid chromatography/mass spectrometry (LC/MS).
Materials and Methods
Chemicals and Reagents
Pesticide standards (purity > 98%) were purchased for Dr. Ehrenstorfer (Augsburg, Alemanha). HPLC grade methanol was obtained from Merck (Darmstadt, Alemanha); ammonium formate was purchased from PanReac (Barcelona, Espanha); C18-bonded silica (50 μm) was obtained from Phenomenex (Torrance, CA), and water hyacinth biochar (Eichhornia crassipes) was produced and supplied by the Petroleum and Biomass Energy Research Group–PEB from the Federal University of Sergipe. Stock solutions (1000 µg/mL) were prepared by weighing 0.0100 g in volumetric flasks 10 mL and dissolved in methanol (Fluka Analytical, Sigma-Aldrich).
Instrumental Conditions
The analyses of the pesticide residues were performed using a Nexera UHPLC system coupled to a quadrupole mass spectrometer equipped with an electrospray ionization (ESI) probe. The chromatographic separation was performed using a C18 column (150 mm × 2.1 mm, 2.5 μm particle size) and a mobile phase consisting of water (A) and methanol (B), both containing 5 mM (v/v) ammonium formate, in gradient mode. The eluent flow rate was 0.5 mL/min, the column temperature was 40 °C, and the injection volume was 5.0 µL. The optimized gradient elution was as follows: (0–1 min) 30% B; (1–15 min) 100% B; (15–18 min) 30% B; (18–20 min) 30% B, when a new injection was made. The mass spectra were acquired using electrospray ionization in positive and negative ion modes, with the following optimized conditions: capillary voltage at 1.2 kV, interface temperature at 350 °C, and DL 250 °C and using nitrogen as nebulizing and drying gas (1.5 mL/min). LabSolution software was used for the qualitative and quantitative analysis.
Sample Preparation
For the SPE procedure, 100 mL of Pilsen-type lager beer were degassed in an ultrasonic bath for 10 min to eliminate carbon dioxide.
Preparation of Water Hyacinth Biochar
The water hyacinth (Eichhornia crassipes) was collected at the Macela weir (at 10° 40′ 19.83″ S; 37° 25′ 14.60″ W) in the municipality of Itabaiana (Sergipe, Brazil). The water hyacinth was dried at 50 °C for 48 h, and pyrolyzed in a rotary tube furnace at 400 °C for 2 h, followed by treatment with HCl solution to remove impurities and washed with deionized water until reaching neutral pH (Santos et al. 2018). The prepared water hyacinth biochar was studied as adsorbent material in SPE cartridge. The SPE cartridge was performed using a polypropylene cartridge of 1 cm diameter and 7 cm in length. The cartridge was prepared without gaps by compressing a frit on the bottom and then adding 150 mg of biochar and stopcock frit on the upper.
Extraction Procedure
In the optimized SPE procedure, water hyacinth biochar cartridges (150 mg) were conditioned with 5 mL of methanol and 5 mL of water. One hundred milliliters of beer sample were quantitatively transferred to the SPE cartridge. Elution of the sample was performed with 3 mL of methanol:dichloromethane (60:40, v/v), added dropwise using a vacuum manifold system. The sample extract was collected in a volumetric flask and evaporated under a gentle nitrogen stream. The residue was dissolved in 1 mL of methanol, filtered through a 0.22 μm pore size nylon filter, and a 5 µL aliquot was analyzed by LC/MS. The software LabSolution was used for instrument control and data processing. Selected ion monitoring (SIM) mode was used for acquisition.
Method Validation
The linearity was estimated by the construction of calibration curves for the pesticides at ten concentration levels in the range from 0.1 to 500 µg/L in solvent, as well as in a beer sample extract using the optimized SPE procedure. The matrix effects were evaluated using the slope regression coefficients of the previous linearity assays. Accuracy and precision were evaluated in terms of mean recovery and relative standard deviation (RSD). Thus, repeatability was evaluated at three concentration levels (0.5, 2.5, and 5.0 µg/L), and five replicates were carried out. Interday precision was evaluated at one concentration level (2.5 µg/L), and spiked samples were analyzed for a period of three alternate days. The limit of detection (LOD) was determined based on the calibration curve parameters, using the ratio of the standard deviation of the intercept and the slope of the regression line, considering a factor of 3.3. The limit of quantification was fixed as the lowest concentration level of the calibration curve, which presented adequate recovery and precision.
Results and Discussion
Instrumental Conditions for LC-MS Analysis
The selective ion monitoring (SIM) mode was used to obtain maximum selectivity and sensitivity for quantitative analysis of pesticides. Full scans in positive and negative electrospray ionization modes were conducted for the selection of suitable precursor ions. The results showed that almost all of the compounds produced abundant [M + H]+ ions in ESI+ mode, except malathion, esfenvalerate, and deltamethrin, for which the most abundant ion was the [M + NH4]+. Table S1 summarizes the results obtained for the optimized instrumental conditions.
Optimization of Extraction
Initial studies to optimize the SPE extraction method were carried out on samples of Pilsen beer contaminated with 1 µg/mL of the pesticides under study for recovery trials. The extraction procedure was carried out in a cartridge packed with the adsorbent C18 (150 mg), which was conditioned with 2 mL of water and a mixture of methanol/dichloromethane (90:10, v/v), respectively, and as elution solvent 3 mL of this same mixture in which the results were consistent, presenting 15 of the 18 pesticides under study with recovery in the range between 70 and 120%, as established by regulatory agencies such as SANTE (2017).
Next, the extraction efficiency was evaluated when using water hyacinth biochar as an adsorbent for the SPE method. Optimizations were carried out in terms of varying the proportion of the elution solvent so that it was possible to carry out the desorption process of the analytes bound to the biochar surface and, subsequently, provide results as satisfactory as when using the commercial adsorbent C18.
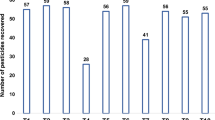
The results were evaluated based on recovery tests, verifying the efficiency of the method. The extraction procedure using water hyacinth biochar was optimized according to the steps mentioned above and under conditions that were suitable when using C18 as an adsorbent. Based on the results presented in Fig. S1, it was possible to observe the efficiency of the alternative material for extracting the analytes.
In order to evaluate improvements in the extraction process, the eluting strength of the medium was increased for better desorption of the analytes, by changing the proportion of solvents used. When using the mixture of methanol/dichloromethane solvents in the proportion 60:40, (v/v), results showed an increase in recovery percentages above 70% for 12 of the 18 pesticides belonging to different chemical groups, and, therefore, the results obtained with biochar provided a similar extraction efficiency as When using C18, ensuring the suitability of this alternative material as a promising adsorbent for SPE.
Even changing the eluent, some analytes such as flutriafol, atrazine, haloxyfop, and thiophanate-methyl continued to show recovery values below 70% in the presence of biochar, and this may be indicative of groups present in these compounds or some steric hindrance that hindered the adsorption of these analytes to surface of the material, in addition to the possibility that these analytes were better adsorbed when using C18 as an adsorbent due to its surface area of 500 m2/g which is superior to that of water hyacinth biochar, which generally has an area around 116 m2/g (Liu et al. 2023; Kostiainen and Kauppila 2009; Rebane et al. 2019; Silicycle 2023). The low recovery for thiophanate-methyl may also be related to the possibility that is has degraded to its metabolite, which is carbendazim (Dong et al. 2018). On the other hand, it was possible to observe that even using a complex matrix such as beer, biochar provided adequate efficiency for extracting pesticides.
Validation of the SPE Method
Based on the optimized method and as a way to guarantee reliable results for its intended purpose, the method was validated based on pre-established parameters. The linearity based on the method producing results proportional to the concentration of the analyte was evaluated from the curve in the solvent at concentrations of 0.25; 0.5; 1.0; 2.5; 5; 10; 25; 50; 75 and 500 µg/L. The sensitivity of the method was evaluated using the coefficient of determination and the results showed linearity with r2 above 0.99, as can be seen in Table S2.
The selectivity of the method was evaluated based on the matrix effect through the ratio of the curve prepared in the extract in relation to that in the solvent. The results presented in Table S2 showed the presence of a matrix effect. Therefore, the analytical curves used for quantification were constructed using pesticide-free matrix extracts.
The accuracy of the method was evaluated in quintuplicate with three concentration levels. The results were adequate for the concentration levels evaluated, with the exception of simazine, thiamethoxam, flutriafol, haloxyfop, and thiophanate-methyl, which showed recovery below 70%, but with relative standard deviation (RSD) from 20%.
Repeatability was evaluated based on intraday precision through tests carried out in quintuplicate throughout a working day and intermediate precision on three non-consecutive days in which they were accurate with RSD < 20%.
The detection limit (DL) of the method was evaluated based on the calibration curve through the relationship S/N in 3 and the limit of quantification through S/N in 10. The results showed DL varying between 0.05 and 0.08 µg/L and the LQ 0.1–0.25 µg/L. The values studied are in agreement with agricultural crops, as there are no maximum residue limits established for beer (Table S3). Therefore, it was possible to validate the SPE method using water hyacinth biochar as an alternative adsorbent, capable of providing satisfactory, reliable, and consistent results when compared with materials and extraction methods proposed in the literature.
There are some studies in the literature related to the determination of pesticides in beer samples, as shown in Table S4 (Hengel and Shibamoto 2002; Silva et al. 2019; Pires et al. 2021). Compared with the SPE and QuEChERS methods with different adsorbents (Oasis HLB, 500 mg) or steps for the extraction procedure, this proposed method needed a small amount of adsorbent (150 mg) and minimal sample handling. The number of chemical classes of pesticides studied in the proposed method (11 chemical classes) is higher than the methods described in the literature (7–8 chemical classes). Furthermore, excellent values were obtained in the recovery study, and lower values of detection and quantification values for the studied analytes. Moreover, water hyacinth biochar is much cheaper than most other adsorbents such as Oasis HLB. It can reduce the cost of extraction step. Consequently, the proposed method is sensitive and accorded with the principles of green chemistry, since the proposed method employed environmental-friendly biochar derived from water hyacinth as adsorbent.
Application to Real Samples
The SPE method developed with water hyacinth biochar as an alternative adsorbent was applied to 13 Pilsen-type lager beer labels, sold in São Cristóvão, Sergipe, Brazil. However, no residues of the pesticides under study were found in these samples above the detection limit of the method.
Conclusion
An SPE method was developed using water hyacinth biochar as an adsorbent to evaluate the presence of residues of the pesticides acetamiprid, ametrine, atrazine, azoxystrobin, carbendazim, chlorpyrifos, deltamethrin, diuron, esfenvalerate, epoxiconazole, flutriafol, haloxifop, lufenuron, malathion, propanil, thiamethoxam, methyl thiophanate, and simazine, in Pilsen-type lager beers. The method was validated, proving to be linear, accurate, and precise for the present study, and after validation, it was applied to different beer labels, but no residues of these analytes under study were detected. Therefore, it was possible to develop a sustainable and economical method with water hyacinth biochar that was suitable for extracting pesticide residues from beer matrix.
Data Availability
No datasets were generated or analyzed during the current study.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Alavanja MC, Ross MK, Bonner MR (2013) CA: Cancer J Clin 63:120–142. https://doi.org/10.3322/caac.21170
Amos SP, Naicker D, Mdlulli PS, Madikizela LM (2019) Envir Foren 20:129–138. https://doi.org/10.1080/15275922.2019.1597780
Augusto F, Hantao LW, Mogollón NGS, Braga SCGN (2013) Trend Anal Chem 43:14–23. https://doi.org/10.1016/j.trac.2012.08.012
Baharum NA, Nasir HM, Ishak MY, Isa NM, Hassan MA, Aris AZ (2020) Arab J Chem 13:6106–6121. https://doi.org/10.1016/j.arabjc.2020.05.011
Birniwa AH, Abubakar AS, Hug AO, Mahmud HNME (2020) J Macromol Sci Part A 58:206–217. https://doi.org/10.1080/10601325.2020.1840921
Demir O, Ulusoy HI, Ozer ET, Osman B (2020) Microchem J 158:105317. https://doi.org/10.1016/j.microc.2020.105317
Dong BZ, Yang YP, Pang NN, HU JY (2018) J Food Chem 260:66–72. https://doi.org/10.1016/j.foodchem.2018.03.062
Dusek M, Jandovská V (2023) Food Addit Contam Part A Chem Anal Control Expo. Risk Assess 40(5):655–666. https://doi.org/10.1080/19440049.2023.2202773
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) PLoS ONE 9:113888. https://doi.org/10.1371/journal.pone.0113888
Hack M, Nitz S, Parlar H (1997) J Agric Food Chem 45:1375–1380. https://doi.org/10.1021/jf9605411
Hakme E, Nielsen IK, Madsen JF, Storkehave LM, Pedersen MSE, Schulz BL, Poulsen ME, Hobley TJ, Duedahl-Olesen L (2024) Food Addit Contam Part Chem Anal Control Expo Risk Assess 41(1):45–59. https://doi.org/10.1080/19440049.2023.2282557
Haq N, Iqbal M, Hussain A, Shakeel F, Ahmad A, Alsarra IA, Alajmi MF, Mahfooz A, Abouzadeh M (2023) J Sep 10:253. https://doi.org/10.3390/separations10040253
Hengel MJ, Shibamoto T (2002) J Agric Food Chem 50:3412–3418. https://doi.org/10.1021/jf020089n
Jha RR, Singh N, Kumari R, Patel DK (2018) J Sep Sci 41:1625–1634. https://doi.org/10.1002/jssc.201701155
Jones RD, Kavanagh TE, Clarke BJ (1988) J Am Soc Brew Chem 46:43–50. https://doi.org/10.1094/ASBCJ-46-0043
Kaczynski P, Iwaniuk P, Hrynko I, Tuniewski S, Tozowicka B (2024) Food Control 160:110356. https://doi.org/10.1016/j.foodcont.2024.110356
Kostiainen R, Kauppila TJ (2009) J Chromat A 1216:685–699. https://doi.org/10.1016/j.chroma.2008.08.095
Liu Q, Shi JB, Jiang GB (2012) Trend Anal Chem 37:1–11. https://doi.org/10.1016/j.trac.2012.03.011
Liu Y, Ji X, Gao Z, Wang Y, Zhu Y, Zhang Y, Zhang Y, Sun H, Li W, Duan J (2023) J Clean Prod 411:137258. https://doi.org/10.1016/j.jclepro.2023.137258
Madikizela LM (2021) J Environ Manag 295:113153. https://doi.org/10.1016/j.jenvman.2021.113153
Mandal A, Kumar A, Singh N (2021) J Eviron Manag 295:113104. https://doi.org/10.1016/j.jenvman.2021.113104
Mega JF, Neves E, Andrade CJ (2011) Revista Citino 1(1):34–42
Mohan D, Sarswat A, Ok YS Jr PC (2014) Biores Tech 160:191–202. https://doi.org/10.1016/j.biortech.2014.01.120
Mostafalou S, Abdollahi M (2013) Toxic Appl Pharm 268:157–177. https://doi.org/10.1016/j.taap.2013.01.025
Okoya AA, Adegbaju OS, Akinola OE, Akinyele AB, Amuda OS (2020) Cur J Appl Sci Tech 1–11. https://doi.org/10.9734/cjast/2020/v39i230491
Pérez-Lucas G, Navarro S (2023) J Agric Food Chem 71(4):1820–1836. https://doi.org/10.1021/acs.jafc.2c07830
Pires NA, Oliveira MLG, Gonçalves JA, Faria AF (2021) J Agric Food Chem 69:4533–4541. https://doi.org/10.1021/acs.jafc.0c07004
Ponnam V, Katari NK, Mandapati RN, Nannapanesi S, Tondepu S, Jonnalagadda SB (2020) J Env Sci Health Part B 1–10. https://doi.org/10.1080/03601234.2019.1707008
Rebane R, Kruve A, Liigand J, Gornischeff A, Leito I (2019) Rapid Commun Mass Spec 33:1834–1843. https://doi.org/10.1002/rcm.8545
SANTE (2017) Commission of the European Communities. Document n° SANTE/11813/2017. Guidance document on analytical quality control and method validation procedures for pesticides and analysis in food and feed. https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11813_2017-fin.pdf. Accessed 30 july 2023
Santos LO, Silva FFS, Santos LC, Carregosa ISC, Jr Wisniewski A (2018) J Braz Chem Soc 29:151–158. https://doi.org/10.21577/0103-5053.20170124
Saraswat S, Rai JPN (2010) Int J Min Proces 94:203–206. https://doi.org/10.1016/j.minpro.2010.02.006
Shahryari T, Singh P, Raizada P, Davidyants A, Thangavelu L, Silvamani S, Naseri A, Vahidipour F, Ivanets A, Bandegharaei AH (2022) Col Surf A: Physic Eng Asp 641:128528. https://doi.org/10.1016/j.colsurfa.2022.128528
SILICYCLE (2023) Silica SPE cartridges and well plates. https://www.silicycle.com. Accessed 19 july 2023
Silva AR, Navickiene S, Santos LFS (2019) J AOAC Inter 102. https://doi.org/10.5740/jaoacint.18-0172
STATISTA (2022) Alcoholic drinks. https://www.statista.com/outlook/100000000/100/alcoholicdrinks/worldwide#marketvolume. Accessed 20 december 2022
Suo F, You X, Ma Y, Li Y (2019) Chemosphere 235:918–925. https://doi.org/10.1016/j.chemosphere.2019.06.158
Taha SM, Amer ME, Elmarsafy AE, Elkady MY (2014) J Env Chem Eng 2:2013–2025. https://doi.org/10.1016/j.jece.2014.09.001
Tang J, Zhu W, Kookana R, Katayama A (2013) J Biosci Bioeng 116:653–659. https://doi.org/10.1016/j.jbiosc.2013.05.035
Valverde MTG, Lucena R, Cárdenas S, Valcárcel M (2014) Trend Anal Chem 62:37–45. https://doi.org/10.1016/j.trac.2014.06.015
Wang J, Wang S (2019) J Clean Prod 227:1002–1022. https://doi.org/10.1016/j.jclepro.2019.04.282
Wanyonyi CW, John W, Onuari JM, Shindu PM (2014) Energy Procedia 50:862–869. https://doi.org/10.1016/j.egypro.2014.06.105
Wen YY, Chen L, Li JH, Liu DY, Chen LX (2014) Trend Anal Chem 59:26–41. https://doi.org/10.1016/j.trac.2014.03.011
Wierucka M, Bizuik M (2014) Trend Anal Chem 59:50–58. https://doi.org/10.1016/j.trac.2014.04.007
Xiang YJ, Xua ZY, Wei YY, Zhou YY, Yang X, Yang Y, Yang J, Zhang JC, Luo L, Zhou Z (2019) J Envir Manag 237:128–138. https://doi.org/10.1016/j.jenvman.2019.02.068
Xu Z, Xing Y, Ren A, Ma D, Li Y, Hu S (2020) J Radioanal Nuc Chem 324:1317–1327. https://doi.org/10.1007/s10967-020-07160-2
Zhou YY, Liu XC, Xiang YJ, Wang P, Zhang JC, Zhang FF (2017) Biores Tech 245:266–273. https://doi.org/10.1016/j.biortech.2017.08.178
Zhou YY, He YZ, Xiang YJ, Meng SJ, Liu XC, Yu JF, Yang J, Zhang JC, Qin PF, Luo L (2019) Sci Total Envir 646:29–36. https://doi.org/10.1016/j.scitotenv.2018.07.267
Acknowledgements
The authors also wish to thank the CLQM (Multi-User Chemistry Laboratories Center), at the Federal University of Sergipe, for analytical support. Thanks are also made to Prof. Dr. Alberto Wisniewski Jr for the collaboration with this work, and M.Sc. Ingred Suellen Carvalho Carregosa for the pyrolysis process and product recovery.
Author information
Authors and Affiliations
Contributions
Roseane dos Santos Nascimento: investigation, methodology, validation, writing—original draft. Luís Fabrício Santana Santos: supervision. Sandro Navickiene: supervision, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dos Santos Nascimento, R., Santana Santos, L.F. & Navickiene, S. Effectiveness of Water Hyacinth Biochar as a Potential Adsorbent in Solid-Phase Extraction Together with Liquid Chromatography-Mass Spectrometry for Determination of Pesticide Residues in Lager Beer. Food Anal. Methods 17, 1183–1188 (2024). https://doi.org/10.1007/s12161-024-02639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-024-02639-0