Abstract
The homogeneous ionic liquid microextraction combined with magnetical hollow fiber bar collection was developed for extracting triazine herbicides from water samples. These analytes were separated and determined by high performance liquid chromatography. The triazines were quickly extracted into ionic liquid microdroplets dispersed in solution, and then these microdroplets were completely collected with magnetical hollow fiber bars; the pores of which were impregnated with hydrophobic ionic liquid, which makes the phase separation simplified with no need of centrifugation. Some experimental parameters, such as the type of ionic liquid, ultrasonic immersion time of hollow fiber, pH of sample solution, volume of hydrophilic ionic liquid, amount of ion-pairing agent NH4PF6, NaCl concentration, number of magnetical hollow fiber bar, stirring rate, and collection time were investigated and optimized. When the present method was applied to the analysis of real water samples, the precision and recoveries of six triazine herbicides vary from 0.1 to 9.2% and 73.4 to 118.5%, respectively. The detection limits for terbumeton, ametryn, prometryn, terbutryn, trietazine, and dimethametryn were 0.48, 0.15, 0.15, 0.14, 0.35, and 0.16 μg L−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazine herbicides are widely used to control grasses and broadleaf weeds in agriculture activities around the world [1]. The extensive use of triazine herbicides has resulted in ubiquitous pollution in soil and water [2–5]. According to the European Union (EU) legislation, the maximum residue limit (MRL) for each individual pesticide in drinking water is 0.1 μg L−1 and for total pesticides is 0.5 μg L−1 [6]. Triazine herbicides and their degradation products have caused concern due to their toxicity and persistence in soil, water, and organisms, and they have been classified as a possible human carcinogen [7, 8].
Generally, liquid–liquid extraction (LLE) and solid-phase extraction (SPE) are widely applied to the extraction of the herbicides in environmental samples [9–11]. In recent years, research has been directed toward efficient, economical, and miniaturized sample preparation techniques. Consequently, various microextraction techniques such as solid-phase microextraction (SPME) [12–15], dispersive liquid–liquid micextraction (DLLME) [4, 7], dispersive liquid–liquid microextraction based on solidification of floating organic droplet (DLLME-SFO) [16], single drop microextraction (SDME) [17], and hollow fiber liquid phase microextraction (HF-LPME) [18–20] have been introduced.
Pedersen-Bjergaard and Rasmussen introduced HF-LPME in 1999 [21]. HF-LPME has a large application potential in drug analysis and environmental monitoring [22–24]. Lei et al. proposed a new HP-LPME method [25], in which the hollow fiber was prepared as a magnetic stirring extraction bar for stirring and extraction.
DLLME was proposed by Assadi et al. in 2006 [26]. Due to high surface contact area, the extraction equilibrium can be reached quickly. However, extraction solvents used in conventional DLLME are often highly toxic. In DLLME-SFO, low-density organic solvents with low toxicity were applied. However, additional steps were required, apart from centrifugation, including refrigeration and thaw. In addition, the extraction solvents used in the DLLME-SFO were usually low polar reagents, which make it difficult to extract certain polar analytes and these solvents may damage the reversed-phase chromatographic columns used in high performance liquid chromatography (HPLC).
Ionic liquids (ILs) are organic salts consisting of organic cations and various anions and liquids at room temperature [27]. The important features of ILs include large viscosity, adjustable miscibility and polarity, excellent dissolvability for a wide range of compounds, high thermal stability, and immeasurably low vapor pressure [28–30]. ILs have achieved satisfactory extraction efficiency of the triazine herbicides in various samples [2, 3, 27, 31].
Baghdadi and Shemirani introduced situ IL-DLLME in 2009 [32]. In this method, very small amount of hydrophilic IL (1-hexyl-3-methylimidazolium tetrafluoroborate, [C6MIM][BF4]) was dissolved in sample solution, and then an ion-pairing agent (NaPF6) was added. A cloudy solution was formed as a result of formation of hydrophobic IL (1-hexyl-3-methylimidazolium hexafluorophosphate, [C6MIM][PF6]). Compared with other DLLME methods, the dispersive solvent was not used in situ DLLME, which decreases the consumption of organic solvent. Situ IL-DLLME has been applied to extraction of the analytes in various matrixes [31, 33–35].
The main aim of the work is to develop an extraction method with low solvent consumption, short extraction time, and simple phase separation. Therefore, homogeneous ionic liquid microextraction combined with magnetical hollow fiber bar collection (HILME-MHFBC) was developed for the extraction of triazine herbicides in environmental water samples. In the present method, the extraction equilibrium can be reached quickly, and then the extraction phase containing analytes was collected with magnetical hollow fiber in short time. The present method has both features of DLLME and HF-LPME. Compared with DLLME, the phase separation was simplified with no need of centrifugation. Compared with HF-LPME, the extraction time was shorter. In the present method, very small amount of IL was used as extraction solvent. Although the ILs have large viscosity and do not have strong affinity with the hollow fiber, which makes the adsorption of ILs with hollow fiber difficult, in the present work, the difficulty was successfully overcome with the help of magnetical hollow fiber bar in sample treatment and the ultrasound in the bar preparation. Hollow fiber is cheap and commercially available, and the magnetical hollow fiber bar is easy to be prepared. The bar can be used to both stir the sample solution and collect the extraction phase.
The effect of various experimental parameters was investigated and optimized. The developed method was successfully applied to the extraction of triazines in real water samples.
Materials and methods
Reagents and materials
Six triazine herbicides, including terbumeton, ametryn, prometryn, terbutryn, trietazine, and dimethametryn were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The structures of these herbicides are shown in Fig. 1. Stock standard solutions for these herbicides at a concentration level of 200 μg mL−1 were prepared in acetonitrile and stored at 4 °C. Working and mixed working standard solutions were prepared every week by diluting the stock standard solutions with acetonitrile. Chromatographic grade acetonitrile and methanol were purchased from Fisher Scientific Company (Pittsburgh, PA, USA). 1-Butyl-3-methylimidazolium tetrafluoroborate ([C4MIM][BF4]), 1-octyl-3-methylimidazolium tetrafluoroborate ([C8MIM][BF4]), 1-butyl-3-methylimidazolium hexafluorophosphate ([C4MIM][PF6]), 1-octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6]), and ammonium hexafluorophosphate (NH4PF6, 99%) were obtained from Chengjie Chemical Co. Ltd. (Shanghai, China). All other reagents were of analytical grade and purchased from Beijing Chemical Factory (Beijing, China). Pure water was obtained with a Milli-Q water purification system (Millipore Co., USA).
Accurel Q3/2 polypropylene hollow fiber membrane (600 mm inner diameter, 200 mm wall thickness, and 0.2 mm pore size) was purchased from Membrana GmbH (Wuppertal, Germany). The stainless steel wire (505 μm outer diameter) just fit into the hollow fiber membrance.
Apparatus
Chromatographic separation and determination of herbicides were carried out on a 1100 series liquid chromatograph (Agilent Technologies Inc., USA) equipped with a diode-array detector (DAD) and a quaternary gradient pump. Eclipse XDB-C18 column (3.5 μm, 4.6 mm × 150 mm, Agilent, USA) was used.
The KQ3200E ultrasonic cleaner was purchased from Kunshan Ultrasonic Instrument Co., Ltd. (Kunshan, China). The Delta320 pH meter was purchased from Mettler-Toldedo Instruments (Shanghai) Ltd (Shanghai, China). A homemade multi-position magnetic stirrer was used in this experiment.
Preparation of magnetical hollow fiber bar
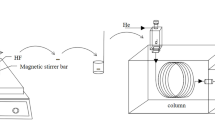
Because the diameter of the beaker was 3.0 cm, the hollow fiber and steel wire were cut manually into 2.2 and 1.8 cm in length, respectively, ultrasonically cleaned in acetone for 5 min, and dried in air before use. The stainless steel wire was inserted into the hollow fiber first, and then the two ends of fiber piece were sealed up with heat. The stainless steel wire in the fiber was used as the magnetic stirrer, by means of which the bar can stir in the magnetic field. The bar was fully immersed in hydrophobic ionic liquid [C4MIM][PF6], and the pores of the fiber wall were impregnated with [C4MIM][PF6] in an ultrasonic cleaner for 10 min. The bar was immersed in water in a beaker on a magnetically stirring apparatus for a certain time in order to remove the excess ionic liquid outside the pores. The prepared bar was referred to as magnetical hollow fiber bar (MHFB), as shown in Fig. 2a
Sample preparation
In this study, four water samples, including commercial water (Sample 1), tap water (Sample 2), river water (Sample 3), and ground water (Sample 4), were used. Commercial water was purchased from a local market (Changchun, China). Tap water was obtained in the laboratory (Changchun, China). River water and ground water were collected in Tonghua, China. Except for the experiments described in Section 3.2.3, which were performed with Samples 1–4, all other experiments were performed with Sample 1. All real samples were filtered through a 0.45 μm membrane using a Millipore YT30 142HW device.
Extraction procedure
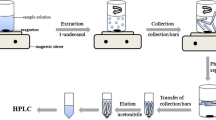
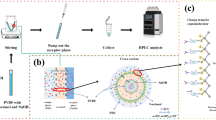
In the HILME, hydrophilic ILs were used as extraction solvents and the hydrophobic ILs were formed in the presents of [PF6]−. In the collection of extract, the MHFBs were used and wall pores of MHFB were impregnated with hydrophobic ILs. An aliquot of 10 mL of water sample was added into a 25-mL beaker and adjusted to pH 4.0 with 0.1 mol L−1 HCl. A total of 2.5000 g NaCl was added into the sample solution and stirred to dissolve. Then 10 μL of [C8MIM][BF4] was added into the mixture. The IL was completely dissolved after seconds. A total of 0.1000 g NH4PF6 was added into the sample solution, and a cloudy solution was formed as a result of formation of fine droplets of a new forming hydrophobic IL [C8MIM][PF6]. Due to high surface contact area, the extraction equilibrium can be reached quickly. After 1 min, nine MHFBs were added into the extraction system and the beaker was sealed with parafilm and the system was stirred for 15 min at a stirring rate of 750 rpm to collect the extraction phase [C8MIM][PF6]. The [C8MIM][PF6] fine droplets containing target analytes were collected with the MHFBs. After collection of the extract, the nine MHFBs were taken out from the sample solution (aqueous phase) and eluted using 800 μL of methanol for 5 min. Then the resulting solution was evaporated just to dryness under a gentle stream of nitrogen. The residue was dissolved in 150 μL of acetonitrile and then filtered with 0.22 μm polyterafluoroethylene (PTFE) filter. The resulting solution was referred to as the analytical solution. Twenty microliters of the analytical solution was injected into the HPLC system for analysis. A schematic diagram of the present method is given in Fig. 2b.
HPLC analysis
The mobile phase of HPLC consisted of water (A) and acetonitrile (B) at a flow rate of 0.5 mL min−1. The gradient condition was as follows: 0–6 min, 30–50% B; 6–11 min, 50–50% B; 11–21 min, 50–65% B; 21–24 min, 65–80%B; 24–26 min, 80–90% B; 26–28 min, 90–90% B, and 28–29 min, 90–30% B. The temperature of the column was maintained at 30 °C. The detection wavelength was set at 222 nm. The injection volume of analytical solution was 20 μL.
Results and discussion
Optimization of experimental parameters
In order to achieve an adequate extraction performance, the effects of some significant parameters, including the type of ionic liquid, the ultrasonic immersion time of hollow fiber, the pH of sample solution, the volume of [C8MIM][BF4], the amount of ion-pairing agent NH4PF6, the NaCl concentration, the number of MHFB, the stirring rate, and the collection time, on the recoveries of the analytes were investigated in detail. All the experiments were performed in triplicate and the concentration of triazines in spiked samples was 50 μg L−1.
Type of ionic liquid
Characteristics of ionic liquids, such as solubility in water, the viscosity, extraction capacity, and chromatographic behavior, play a key role in influencing the recovery and enrichment factor. In this study, the effects of hydrophobic ionic liquids, including [C4MIM][PF6], [C8MIM][PF6], which were impregnated in the pores of MHFBs wall, and hydrophilic ionic liquids, including [C4MIM][BF4], [C8MIM][BF4]), which were used as extraction solvents, on the recoveries of the analytes were investigated. The recoveries obtained with [C4MIM][PF6]-[C4MIM][BF4] (C4-C4), [C4MIM][PF6]-[C8MIM][BF4] (C4-C8), [C8MIM][PF6]-[C4MIM][BF4] (C8-C4), and [C8MIM][PF6]-[C8MIM][BF4] (C8-C8) are shown in Fig. 3a. When the pores of the fiber wall were impregnated with [C8MIM][PF6], the chromatographic peaks of terbumeton and ametryn obtained by C8–C4 and C8–C8 were affected by chromatographic peak of [C8MIM][PF6] and other interference peaks. When [C4MIM][BF4] was used, there was no formation of a cloudy solution after NH4PF6 was added. The main reason may be due to the high solubility of [C4MIM][PF6] in water. Therefore, the recoveries obtained with C4–C4 and C8–C4 were relatively low. The retention time of [C4MIM][PF6] was shorter than that of [C8MIM][PF6]. When the pores of fiber wall were impregnated with [C4MIM][PF6], the chromatographic peaks of target analytes were not affected by other peaks and recoveries obtained with C4–C8 were higher than those obtained with C4–C4. Therefore, [C4MIM][PF6]-[C8MIM][BF4] was selected in the following experiments.
Effect of type of ionic liquid (a), pH of sample solution (b), volume of [C8MIM][BF4] (c), and amount of NH4PF6 (d) on the recoveries of triazine herbicides. Ultrasonic immersion time of hollow fiber, 10 min; NaCl concentration, 25%; number of magnetical hollow fiber bar, 9; stirring rate, 750 rpm; collection time, 15 min; extraction time, 1 min; spiked concentration, 50 μg L−1; C4–C4: [C4MIM][PF6]-[C4MIM][BF4], C4–C8: [C4MIM][PF6]-[C8MIM][BF4], C8–C4: [C8MIM][PF6]-[C4MIM][BF4], C8–C8: [C8MIM][PF6]-[C8MIM][BF4]
Ultrasonic immersion time of hollow fiber
Immersion time is an important factor which affects whether the pores of the fiber wall are fully impregnated with the hydrophobic ionic liquid. Due to the high viscosity of the ionic liquid, the diffusion of the ionic liquid is slow. Thus, in order to accelerate the impregnation of ionic liquid into the pores, ultrasonic immersion was applied. The effect of ultrasonic immersion time was studied in the range of 10 to 40 min. The results shown in Fig. S1 in the Electronic Supplementary Material (ESM) indicate that the recoveries change slightly from 10 to 30 min and then decrease slightly when the ultrasonic immersion time is 40 min. Therefore, 10 min was selected as ultrasonic immersion time.
pH of sample solution
The effect of pH value of sample solution in the range of 2.0–10.0 on the recovery was investigated. The results shown in Fig. 3b indicate that the extraction efficiencies reached maximum values when the pH value of the sample solution was adjusted to 4.0. The herbicides are weakly basic and can be hydrolyzed in excessively low pH value. The pKa values of the six triazines are 4.59 for terbumeton, 3.71 for ametryn, 3.76 for prometryn, 4.03 for terbutryn, 2.78 for trietazine, and 3.69 for dimethametryn (pKa is obtained from SciFinder scholar database). Therefore, pH 4.0 is enough to keep all the studied triazines in the molecule form. In addition, it was found that with the increase of pH value, especially when the sample solution was alkaline, the efficiency of collecting the [C8MIM][PF6] extraction droplets dispersed in the sample solution was not very high and the repeatability became poor. The main reason may be that the strong interaction between of [C8MIM][PF6] and OH-1 existed in the high pH value. Therefore, we carried out all subsequent experiments at pH 4.0.
Volume of [C8MIM][BF4]
The effect of the volume of [C8MIM][BF4] ranging from 4 to 16 μL was investigated. The results shown in Fig. 3c indicate that the recoveries of the herbicides increase when the volume of [C8MIM][BF4] increases from 4 to 10 μL and then slowly decreases with the volume increase. The main reason may be that the difficulty of collecting the [C8MIM][PF6] droplets increase with the increase of the volume of [C8MIM][BF4], so the extraction efficiency decreased when the volume of [C8MIM][BF4] was larger than 10 μL. Therefore, 10 μL of [C8MIM][BF4] was selected for further experiments.
Amount of NH4PF6
The effect of amount of NH4PF6 on the recoveries of the herbicides was studied when the volume of [C8MIM][BF4] was 10 μL. The results are shown in Fig. 3d. As can be seen, the recoveries of the herbicides increase with the increase of the amount of NH4PF6 from 0.0500 to 0.1000 g and then decrease slightly with further increase of amount of NH4PF6, so the amount of NH4PF6 selected was 0.1000 g. That is, to say, the molar ratio of [C8MIM][BF4] to NH4PF6 was 1:15. The NH4PF6 was used as both ion-pairing and salting-out reagent. Because the ratio of NH4PF6 to [C8MIM][BF4] (15:1) was too high, the salting-out effect can play some roles in improving recoveries. However, to prove the formation of [C8MIM][PF6], the analytical solution of [C8MIM][BF4] and [C8MIM][PF6] was prepared by dissolving the ILs in acetonitrile and the analytical solution of the blank sample was prepared by the present extraction method. The chromatograms obtained with analytical solutions are shown in Fig. S2 (see ESM). The results shown in Fig. S2 indicate that [C8MIM][PF6] was formed, which should play a most significant role in the extraction of the analytes, and NH4PF6 should be a kind of ion-pairing reagent.
NaCl concentration
In most traditional extraction methods, the use of proper salt can decrease the solubility of target analytes in aqueous phase, accelerate the mass transfer rate, and improve the extraction efficiency [1, 36]. In this study, NaCl was used to adjust the ionic strength. The effect of NaCl concentration in the range of 0–35% (w/v) on the recoveries of the herbicides was investigated. The results shown in Fig. 4a indicate that the recoveries of the herbicides increase with the increase of NaCl concentration from 0 to 25% and then change slightly or decrease slightly with the further increase of NaCl concentration. The salt out effect was enhanced with the increase of the NaCl concentration. However, when the NaCl concentration was too high, the ion exchange between [PF6]−1 in [C8MIM][PF6] and Cl−1 in solution occurred, and the resulting [C8MIM][Cl] is soluble, which may lead to poor extraction performance [37]. In additional, the viscosity of sample solution increases with the increase of NaCl concentration, which is not beneficial to the diffusion and mass transfer of target analytes. Finally, 25% NaCl (i.e., 2.5000 g NaCl) was chosen in the following experiments.
Effect of NaCl concentration (a), number of magnetical hollow fiber bar (b), stirring rate (c), and collection time (d) on the recoveries of triazine herbicides. Type of ionic liquid, [C4MIM][PF6]-[C8MIM][BF4]; ultrasonic immersion time of hollow fiber, 10 min; pH of sample solution, 4; volume of hydrophilic ionic liquid [C8MIM][BF4], 10 μL; amount of NH4PF6, 0.1000 g; extraction time, 1 min; spiked concentration, 50 μg L−1
Number of magnetical holloe fiber bar
The effect of the number of MHFB on the recoveries of the herbicides was studied. As can be seen from Fig. 4b, the recoveries of the herbicides increase with the increase of the number of MHFB from 5 to 9 and then decrease. Too many MHFBs were not beneficial to the elution efficiency. In order to collect the extraction phase completely and elute conveniently, nine MHFBs were selected in the following experiment.
Stirring rate
A homemade multi-position magnetic stirrer was used in this experiment. The effect of the stirring rate on the recoveries of the herbicides was studied when the collection time was 15 min. The results are shown in Fig. 4c. As can be seen, there is no obviously change from 500 to 1000 rpm when the collection time is 15 min. However, when the stirring rate was 1000 rpm, the agitation was too violent. Finally, 750 rpm was selected for further experiments.
Collection time
In this study, the [C8MIM][PF6] fine droplets containing target analytes was collected by the magnetically stirring collection bars. The effect of collection time was studied in the range of 5 to 30 min. The results shown in Fig. 4d indicate that the recoveries of the herbicides increase from 5 to 15 min and then change slightly. To ensure sufficient collection, 15 min was selected as the collection time.
Method validation
Under the optimal experimental conditions, a series of experiments for examining the linearity, limits of detection (LODs), limits of quantification (LOQs), precision, accuracy, and repeatability were performed to validate the performances of the present method.
Limits of detection and quantification
The working curves were constructed by plotting the peak areas measured versus the concentrations of the analytes in spiked samples. The results obtained are listed in Table 1. Good linearities were achieved for all of the analytes. The limits of detection (LODs) and quantification (LODs) were calculated on the basis of signal-to-noise (S/N) ratio of 3 and 10, respectively. The LODs and LOQs were in the range of 0.14–0.48 and 0.46–1.59 μg L−1, respectively.
Precision and recovery
The precision of the present method was evaluated by measuring the relative standard deviations (RSDs) of the intra- and inter-day tests. The intra-day precision was determined by analyzing samples five times in one day. The inter-day precision was achieved by analyzing the samples once a day in five consecutive days. The concentration of the analytes in spiked samples was at a spiking concentration of 50 μg L−1. The results are presented in Table 2. The recoveries and RSDs are in the range of 100.2–112.3% and 1.3–8.0%, respectively. The precision and recovery of the present method should be satisfactory.
Analysis of real water samples
In order to evaluate the practical applicability, the present method was applied to the determination of the residues of the triazines in four real water samples. No residues of the triazines in these samples were detectable. The samples at two spiked levels were analyzed, and the results are listed in Table 3. It can be seen that good recoveries (73.4–118.5%) and RSDs (0.1–9.2%) for the determination of triazine herbicides in real water samples were obtained. The chromatograms of blank and spiked river water samples are shown in Fig. 5.
Comparison with other methods
A comparison of the present method with existing standard methods adopted by China (Chinese standard) [38], US Environmental Protection Agency (EPA) (EPA method 523 and EPA method 619) [39, 40], and Association of Official Analytical Chemists (AOAC) [41] and other similar methods reported in the literature [4, 19, 20, 42] is shown in Table 4. Compared with the standard methods, the amounts of sample and extraction solvent were much lower and the procedure was much simpler. The LODs obtained by the present method were comparable with those obtained by China and EPA method 523 standards and higher than those obtained by EPA method 619. The recoveries and RSDs obtained by the present method were more satisfactory than those obtained by EPA method 619 and AOAC official method. The present method is a combination of HF-LPME and DLLME; compared with HF-LPME method, the extraction time of the present method is shorter. Compared with DLLME, the extraction phase (IL) was directly collected with magnetical hollow fibers, the phase separation was simplified with no need of centrifugation, and smaller amount of extraction solvent was required in the present method. The linearity, LODs, RSDs, and recoveries of the analytes obtained by the present method are closely comparable with those obtained by HF-LPME and DLLME. Therefore, it can be concluded that the present methods are suitable for the determination of triazine herbicides in water samples.
Conclusion
The developed HILME-MHFBC was proved to be effective for extraction of triazine herbicides from environmental water samples. The small amount of IL was used as extraction solvent, and the prepared magnetical hollow fiber bar was used as collector of the extract. The analytical performance of the present method should be satisfactory. It could be considered that this method is very promising and can be applied to the extraction of pesticides from other complex matrices by varying the extraction parameters.
References
Yang X, Yu R, Zhang S, Cao B, Liu Z, Lei L, et al. Aqueous two-phase extraction for determination of triazine herbicides in milk by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;972:111–6.
Wu L, Song Y, Hu M, Yu C, Zhang H, Yu A, et al. Ionic-liquid-impregnated resin for the microwave-assisted solid-liquid extraction of triazine herbicides in honey. J Sep Sci. 2015;38:2953–9.
Zhao G, Song S, Wang C, Wu Q, Wang Z. Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent. Anal Chim Acta. 2011;708:155–9.
Wang C, Ji S, Wu Q, Wu C, Wang Z. Determination of triazine herbicides in environmental samples by dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. J Chromatogr Sci. 2011;49:689–94.
Hernandez F, Portoles T, Ibanez M, Bustos-Lopez MC, Diaz R, Botero-Coy AM, et al. Use of time-of-flight mass spectrometry for large screening of organic pollutants in surface waters and soils from a rice production area in Colombia. Sci Total Environ. 2012;439:249–59.
EC Drinking Water Guideline, 98/83/CE, Brussels, November 1998.
Nagaraju D, Huang SD. Determination of triazine herbicides in aqueous samples by dispersive liquid-liquid microextraction with gas chromatography-ion trap mass spectrometry. J Chromatogr A. 2007;1161:89–97.
Abbas HH, Elbashir AA, Aboul-Enein HY. Chromatographic methods for analysis of triazine herbicides. Crit Rev Anal Chem. 2014;45:226–40.
Pinto GMF, Jardim ICSF. Use of solid-phase extraction and high-performance liquid chromatography for the determination of triazine residues in water: validation of the method. J Chromatogr A. 2000;869:463–9.
Tran AT, Hyne RV, Doble P. Determination of commonly used polar herbicides in agricultural drainage waters in Australia by HPLC. Chemosphere. 2007;67:944–53.
Retamal M, Costa C, Suárez JM, Richter P. Multi-determination of organic pollutants in water by gas chromatography coupled to triple quadrupole mass spectrometry. Int J Environ Anal Chem. 2013;93:93–107.
Passeport E, Guenne A, Culhaoglu T, Moreau S, Bouye JM, Tournebize J. Design of experiments and detailed uncertainty analysis to develop and validate a solid-phase microextraction/gas chromatography-mass spectrometry method for the simultaneous analysis of 16 pesticides in water. J Chromatogr A. 2010;1217:5317–27.
Hernandez F, Beltran J, Lopez FJ, Gaspar JV. Use of solid phase microextraction for the quantitative determination of herbicides in soil and water samples. Anal Chem. 2000;72:2313–22.
Huang SD, Huang HI, Sung YH. Analysis of triazine in water samples by solid-phase microextraction coupled with high-performance liquid chromatography. Talanta. 2004;64:887–93.
Perreau F, Einhorn J. Determination of frequently detected herbicides in water by solid-phase microextraction and gas chromatography coupled to ion-trap tandem mass spectrometry. Anal Bioanal Chem. 2006;386:1449–56.
Sanagi MM, Abbas HH, Ibrahim WA, Aboul-Enien HY. Dispersive liquid-liquid microextraction method based on solidification of floating organic droplet for the determination of triazine herbicides in water and sugarcane samples. Food Chem. 2012;133:557–62.
Ye C, Zhou Q, Wang X. Improved single-drop microextraction for high sensitive analysis. J Chromatogr A. 2007;1139:7–13.
Shen G, Lee HK. Hollow fiber-protected liquid-phase microextraction of triazine herbicides. Anal Chem. 2002;74:648–54.
Megersa N. Hollow fiber-liquid phase microextraction for trace enrichment of the residues of atrazine and its major degradation products from environmental water and human urine samples. Anal Methods. 2015;7:9940–8.
Peng J, Lü J, Hu X, Liu J, Jiang G. Determination of atrazine, desethyl atrazine and desisopropyl atrazine in environmental water samples using hollow fiber-protected liquid-phase microextraction and high performance liquid chromatography. Microchim Acta. 2007;158:181–6.
Pedersen-Bjergaard S, Rasmussen KE. Liquid-liquid-liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem. 1999;71:2650–6.
Saleh A, Yamini Y, Faraji M, Shariati S, Rezaee M. Hollow fiber liquid phase microextraction followed by high performance liquid chromatography for determination of ultra-trace levels of Se(IV) after derivatization in urine, plasma and natural water samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1758–64.
Huang SP, Huang SD. Dynamic hollow fiber protected liquid phase microextraction and quantification using gas chromatography combined with electron capture detection of organochlorine pesticides in green tea leaves and ready-to-drink tea. J Chromatogr A. 2006;1135:6–11.
Ho TS, Halvorsen TG, Pedersen-Bjergaard S, Rasmussen KE. Liquid-phase microextraction of hydrophilic drugs by carrier-mediated transport. J Chromatogr A. 2003;998:61–72.
Lei L, Kang MQ, Li N, Yang X, Liu ZL, Wang ZB, et al. Determination of sex hormones in cosmetic products by magnetically stirring extraction bar liquid–liquid microextraction coupled with high performance liquid chromatography. Anal Methods. 2014;6:3674.
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A. 2006;1116:1–9.
Li N, Zhang R, Nian L, Ren R, Wang Y, Zhang H, et al. Extraction of eight triazine and phenylurea herbicides in yogurt by ionic liquid foaming-based solvent floatation. J Chromatogr A. 2012;1222:22–8.
Liu J, Jiang GB, Chi YG, Cai YQ, Zhou QX, Hu JT. Use of ionic liquids for liquid-phase microextraction of polycyclic aromatic hydrocarbons. Anal Chem. 2003;75:5870–6.
Vidal L, Psillakis E, Domini CE, Grane N, Marken F, Canals A. An ionic liquid as a solvent for headspace single drop microextraction of chlorobenzenes from water samples. Anal Chim Acta. 2007;584:189–95.
Martin-Calero A, Ayala JH, Gonzalez V, Afonso AM. Ionic liquids as desorption solvents and memory effect suppressors in heterocyclic aromatic amines determination by SPME-HPLC fluorescence. Anal Bioanal Chem. 2009;394:937–46.
Wu L, Hu M, Li Z, Song Y, Yu C, Zhang Y, et al. Determination of triazine herbicides in fresh vegetables by dynamic microwave-assisted extraction coupled with homogeneous ionic liquid microextraction high performance liquid chromatography. Anal Bioanal Chem. 2015;407:1753–62.
Baghdadi M, Shemirani F. In situ solvent formation microextraction based on ionic liquids: A novel sample preparation technique for determination of inorganic species in saline solutions. Anal Chim Acta. 2009;634:186–91.
Yao C, Anderson JL. Dispersive liquid-liquid microextraction using an in situ metathesis reaction to form an ionic liquid extraction phase for the preconcentration of aromatic compounds from water. Anal Bioanal Chem. 2009;395:1491–502.
Gao S, Jin H, You J, Ding Y, Zhang N, Wang Y, et al. Ionic liquid-based homogeneous liquid-liquid microextraction for the determination of antibiotics in milk by high-performance liquid chromatography. J Chromatogr A. 2011;1218:7254–63.
López-Darias J, Pino V, Ayala JH, Afonso AM. In-situ ionic liquid-dispersive liquid-liquid microextraction method to determine endocrine disrupting phenols in seawaters and industrial effluents. Microchim Acta. 2011;174:213–22.
Su R, Li X, Liu W, Wang X, Yang H. Headspace Microextraction of Sulfonamides from Honey by Hollow Fibers Coupled with Ultrasonic Nebulization. J Agric Food Chem. 2016;64:1627–34.
Gao S, You J, Zheng X, Wang Y, Ren R, Zhang R, et al. Determination of phenylurea and triazine herbicides in milk by microwave assisted ionic liquid microextraction high-performance liquid chromatography. Talanta. 2010;82:1371–7.
Chinese Standard GB/T 21925-2008, Detemination of herbicide residues in water LC/MS method, 2008.
EPA Method 523, Determination of triazine pesticides and their degradates in drinking water by gas chromatography/mass spectrometry (GC/MS), 2011.
EPA Method 619, The determination of triazine pesticides in municipal and industrial wastewater.
AOAC Official Method 992.14, pesticides in finished drinking water liquid chromatographic method with ultraviolet detector, 1997.
Peng JF, Liu JF, Hu XL, Jiang GB. Direct determination of chlorophenols in environmental water samples by hollow fiber supported ionic liquid membrane extraction coupled with high-performance liquid chromatography. J Chromatogr A. 2007;1139:165–70.
Acknowledgements
This work was supported by Scientific and Technological Project of the General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (2012IK162 and 2013IK162).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 475 kb)
Rights and permissions
About this article
Cite this article
Wang, K., Jiang, J., Kang, M. et al. Magnetical hollow fiber bar collection of extract in homogenous ionic liquid microextraction of triazine herbicides in water samples. Anal Bioanal Chem 409, 2569–2579 (2017). https://doi.org/10.1007/s00216-017-0201-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0201-5