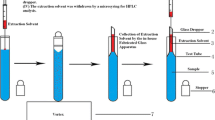
Abstract
A sensitive, rapid, and efficient analytical method based on vortex-assisted liquid–liquid extraction combined with air-assisted liquid–liquid microextraction using high performance liquid chromatography-variable wavelength detection has been developed for the extraction and determination of bisphenol A and bisphenol B in canned doogh samples. In this method, the analytes are partitioned into n-hexane during liquid–liquid extraction and then they are enriched using the following air-assisted liquid–liquid microextraction procedure in a few microliters of an ammonia solution used as an extraction solvent. Under the optimum conditions, limits of detection and quantification were 0.82 and 0.54 and 2.7 and 1.5 ng mL−1, for bisphenol A and bisphenol B, respectively. The extraction recoveries and enrichment factors of the selected analytes were 86 and 81% and 4300 and 4050, respectively. The relative standard deviations were lower than 9% for intra- (n = 6) and inter-day (n = 4) precisions at a concentration of 5 ng mL−1 of each analyte. Finally, some canned doogh samples were effectively analyzed by the proposed method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is an important chemical compound which is extensively used in production of polycarbonates, phenol and epoxy resins, polyesters, lacquer coatings on food cans, flame retardants, and adhesives and as a component of electronic circuits (Erler and Novak 2010; Golub et al. 2010). Consequently, BPA has been detected in various matrices, such as packed foods (Fasano et al. 2015), beverages (Sungur et al. 2014), personal care products (Cacho et al. 2013), paper (Gao et al. 2013), dust (Loganathan and Kannan 2011), and biological samples (Sosvorova et al. 2017). Many studies have demonstrated that BPA is associated with increased incidence of cardiovascular disease, diabetes, reproductive cancers, fertility problems, liver enzyme abnormalities, and other endocrine-related end points (Vandenberg et al. 2009; Meeker et al. 2010; Ehrlich et al. 2011). Bisphenol B (BPB) is a congener used in polymer industry namely in the manufacture of phenolic resins. Similarly to BPA, it presents endocrine-disrupting activities specifically high estrogenic and anti-androgenic activities. Canned beverages are one of the most important foods which are widely used in human’s food chains. Among these beverages, canned milk-based products like canned doogh attracted many attentions. Doogh is prepared from yoghurt, water, and salt and sometimes it is mixed with additives to improve its taste. In some literature, it is considered as the diluted yoghourt. It is a popular drink in Middle East which is packed in glass bottles or in cylindrical metal containers (can). Due to high consumption of the canned doogh, its safety is the main concern. Therefore, the development of a sensitive, accurate, and reliable determination method was a subject for some researches concerning dairy product quality. Determination of bisphenols is frequently performed by means of chromatographic techniques, either gas chromatography coupled with flame ionization detector (Farajzadeh et al. 2015), mass spectrometry (MS) (Fernandez, et al. 2017) or high performance liquid chromatography (LC) coupled to MS (Lee et al. 2017), tandem spectrometry (MS/MS) (Cheng et al. 2017), or fluorescence detectors (Yoon et al. 2003). Due to low levels of BPA and BPB found in canned beverages and the matrix complexity, application of a sample preparation method is required. Liquid–liquid extraction (LLE) (González-Casado et al. 1998) and solid-phase extraction (SPE) (Maragou et al. 2006) are the main techniques used in the treatment of different samples. Some disadvantages associated with LLE such as emulsion formation, use of large volume of samples, and toxic organic solvents make it labor intensive, expensive, time-consuming, and environmentally unfriendly. Although SPE uses much less solvent than LLE, it normally needed an extra step to preconcentrate the analytes into small volumes. On the other hand, SPE is time-consuming and relatively expensive. The drive for “green” methods to overcome these inherent problems of conventional LLE and SPE methods has led to the development of solvent-less (Li et al. 2015) and solvent-minimized microextraction techniques such as solid-phase microextraction (Martins et al. 2011) and liquid-phase microextraction (LPME) (An et al. 2017; Lu et al. 2017). In LPME method, extraction generally occurs from an aqueous solution containing analytes (donor phase) into a small amount of a water-immiscible solvent (acceptor phase). One of the LPME methods is air-assisted liquid–liquid microextraction (AALLME) which was introduced in 2012 (Farajzadeh and Afshar Mogaddam, 2012). In this method, fine organic solvent drops are formed using a syringe to pull in and push out a mixture of an aqueous sample solution and an extraction solvent several times into a test tube in the absence of dispersive solvent.
In this study, for the first time, vortex-assisted liquid–liquid extraction (VALLE) is combined with AALLME method for the extraction and preconcentration of BPA and BPB. The main goal of this combination was to develop a simple and rapid method for isolation, enrichment, and determination of BPA and BPB from canned doogh samples. This method consists of two steps: (i) extraction of the analytes from the samples by VALLE and (ii) enrichment of the analytes with AALLME procedure. The factors which affect both VALLE and AALLME procedures were investigated and the optimal conditions were selected.
Experimental
Regents and Materials
BPA and BPB with purities higher than 99% were purchased from Sigma-Aldrich (St. Louis, MO, USA). The used extraction solvents in VALLE step including n-hexane, n-heptane, chloroform, and carbon tetrachloride were obtained from Merck (Darmstadt, Germany). Sodium chloride, sodium borate, ammonia, hydrochloric acid (37%), and sodium hydroxide were also provided from Merck. Deionized water was prepared with a Milli-Q water system (Millipore, Billerica, MA, USA). A stock solution of the analytes was prepared in acetonitrile at a concentration of 50 mg L−1 (each analyte). Working standard solutions were daily prepared by dilution of the stock solution with deionized water.
Instrumentation
An Agilent 1100 series liquid chromatograph (Agilent Technologies, USA) equipped with a quaternary pump, a degasser, an injection valve (Rheodyne, Kinesis, USA), and a variable wavelength detector (VWD) was used. Separation was carried out on a Zorbax SB-Aq, analytical column (150 × 4.6 mm i.d., 5 μm particle size) (Agilent, Santa Clara, CA, USA). The analytes were eluted with a mixture of acetonitrile/water (55:45, v/v) at a flow rate of 1.0 mL min−1 using an isocratic elution. The analytes were monitored at 220 nm. All injections were performed manually using a 10-μL sample loop. ChemStation software was used for data acquisition and processing. A Metrohm 654 pH meter (Metrohm, Herisau, Switzerland) equipped with a glass electrode was used for pH adjustments. A Hettich centrifuge, model D-7200 (Germany), was used for accelerating phase separation.
Real Samples
Four canned doogh samples produced by different companies were prepared from local supermarkets (Tabriz, Iran). Another doogh sample packed in glass bottle was obtained from local vendors (Tabriz, Iran). It is plausible to assume that the doogh in a glass bottle is free of the analytes. So it was used as an analyte-free doogh sample (blank doogh) in optimization of the proposed method.
Extraction/Preconcentration Procedure
VALLE Procedure
Initially, 50-mL analyte-free doogh sample spiked with 20 ng mL−1 of each analyte or doogh sample solution was transferred into a 70-mL test tube. Then, 5 mL n-hexane was added to the solution and the mixture was vortexed for 4 min. The mixture was centrifuged at 5000 rpm for 5 min. Then, 4 mL of the upper phase was removed and used in the following AALLME procedure.
AALLME Procedure
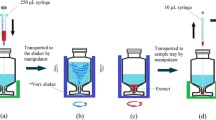
First, 4-mL organic phase obtained from the previous extraction step was transferred into a 10-mL glass test tube with a conical bottom. Then, 14-μL ammoniacal buffer solution (C = 0.5 M, pH = 10) was placed at the bottom of the tube. Then, the mixture was rapidly pulled into a 10-mL glass syringe from the tube and pushed back out into it using a syringe needle. By repeating this action, the solution became increasingly turbid, and ammonia solution was dispersed into the organic phase as fine droplets. During this step (n = 6), the analytes were rapidly extracted into the ammonia solution droplets. Then, the test tube was centrifuged at 5000 rpm for 5 min, and the dispersed fine droplets were settled down in the conical bottom of the tube. Finally, whole of the sedimented phase (10 ± 1 μL) was removed and injected into the separation system.
Calculation of Enrichment Factor and Extraction Recovery
Enrichment factor (EF) is defined as the ratio of the analyte concentration in the sedimented phase (Csed) to its initial concentration of the analyte (C0) in the sample:
Csed is obtained by comparison of the obtained peak areas in two cases: direct injection of the analytes standard solution prepared in mobile phase and injection of the sedimented phase into the high performance liquid chromatography (HPLC). Extraction recovery (ER) is defined as the percentage of the total analyte amount (n0) that is extracted into the sedimented phase (nsed):
where Vsed and Vaq are volumes of the sedimented phase and sample solution, respectively.
Results and Discussion
In this study, a VALLE–AALLME method combined with HPLC–VWD was used for the preconcentration and determination of BPA and BPB in canned doogh samples. The extraction efficiency of the developed method is affected by various parameters including (i) type and volume of extraction solvent in two steps, (ii) ionic strength, (iii) extraction numbers, and (iv) centrifuging rate and time. These parameters were carefully investigated using the “one-variable-at-a-time” approach and the optimum conditions were selected.
Optimization of Parameters in VALLE Procedure
Selection of Extraction Solvent
In the present work, the target analytes are extracted from the samples using VALLE method and the extracted analytes are preconcentrated in the following AALLME method. In this step, selection of a suitable extraction solvent is the main parameter. The extraction solvent should have extraction capability of the target analytes from doogh sample and low solubility in the extraction solvent used in AALLME method. According to these requirements, four extraction solvents with different polarities including chloroform, n-hexane, n-heptane, and carbon tetrachloride were tested. For this purpose, 50 mL of the doogh sample spiked with the target analytes at a concentration of 25 ng mL−1 (each analyte) was transferred into a 70-mL test tube and 5 mL of each solvent was added to the solution, separately. After vortexing for 6 min, the mixture was centrifuged at 5000 rpm for 5 min. Then, 4.0 mL of the organic phase was transferred into a 10-mL conical bottom glass test tube and 14 μL of the ammoniacal buffer (C = 0.5 M, pH = 10) was placed at the bottom of the tube. After performing aspiration/dispersion cycles for five times, the cloudy solution was centrifuged at 5000 rpm for 5 min. The sedimented phase (10 ± 1 μL) was collected and injected to the separation system. Comparison of the peak areas obtained with the different extraction solvents (Fig. 1) shows that n-hexane is the most effective extraction solvent and gives the highest extraction efficiencies for the target analytes among the four solvents investigated. Therefore, n-hexane was selected as the extraction solvent for the following experiments.
Selection of the extraction solvent in VALLE step. Extraction condition: sample, 50 mL blank dough sample spiked with the analytes (each analyte, 25 ng mL−1); extraction solvent volume, 5 mL; vortex time, 6 min; centrifugation rate, 5000 rpm; and centrifugation time, 5 min. Air-assisted liquid–liquid extraction: the extract volume obtained from the previous step, 4 mL; extraction numbers, five times, extraction solvent, ammoniacal buffer solution (C = 0.5 M, pH = 10); extraction solvent volume, 14 μL; centrifugation rate, 5000 rpm; and centrifugation time, 5 min. The error bars indicate standard deviation of three repeated determinations
Optimization of Extraction Solvent Volume
The volume of the extraction solvent is another important parameter which can affect the extraction efficiency of the method. Generally, the extraction solvent volume is taken as small as it can be in order to reduce toxicity hazards for the environment. On the other hand, it should be taken as much as possible to extract the maximum possible amounts of the analytes. To study the effect of the extraction solvent volume, the volume of n-hexane was changed over the range of 4–8 mL (at 1-mL intervals). In the cases of 4, 5, 6, 7, and 8 mL n-hexane, the volume of the collected phase was 3.3, 4.2, 5.2, 6.1, and 7.4 mL, respectively. In all cases, except 4 mL of the extraction solvent in which 3.3 mL collected phase was obtained, 4 mL of the collected phase was used in the next AALLME method. In the case of 4 mL, all collected phase (3.3 mL) was removed and diluted to 4 mL with pure n-hexane and then it was used. Thus, in the following studies, 5 mL was used as an optimal volume of extraction solvent.
Optimization of Vortexing Time
A vortex agitator is used to swirl the fluids and create vortex emulsification between the two immiscible liquid phases. This action usually converts one of the two liquid phases as fine droplets. In this study, vortex agitation was used to accelerate extraction of the analytes into n-hexane. To investigate, the vortexing duration, different times including 1.0, 2.0, 4.0, 6.0, and 8.0 min were subjected for extraction of the analytes into the extraction solvent. The obtained results in Fig. 2 show that, when the vortexing time increases from 1.0 to 4.0 min, the analytical signals of all analytes increase. However, the extraction efficiency had no noticeable enhancement when the vortexing time increases from 4.0 to 8.0 min. Thus, 4.0 min was selected as the optimum vortexing time.
Optimization of vortexing time. Extraction conditions are the same as used in Fig. 1, except 5 mL n-hexane was selected as the suitable extraction solvent in VALLE step. The error bars indicate standard deviation of three repeated determinations. VALLE step. The error bars indicate standard deviation of three repeated determinations
Salt Addition
In most extraction methods, a salt is usually added to an aqueous solution for enhancing the extraction efficiency. The salt addition can decrease the solubility of the analytes in the aqueous phase and at the same time can reinforce partitioning of the analytes into the organic phase (Kojima and Davis, 1984; Korenman et al. 2010). To evaluate the effect of salt addition, sodium chloride (0–10%, w/v, at 2.5% intervals) was added to increase the ionic strength sample solution, whereas the other experimental conditions were kept constant. According to the obtained results, the analytical signals decrease gradually in the presence of NaCl. It could be because of an increase in the viscosity of the aqueous phase due to addition of NaCl, which leads to a decrease in the diffusion coefficients of the analytes. Therefore, all experiments were performed without salt addition.
Optimization of AALLME Procedure
Selection of the Extraction Solvent
In this method, the extraction solvent should have the following requirements: (i) immiscible with n-hexane, (ii) ability to form a cloudy solution during suction and aspiration process, (iii) good chromatographic behavior, and (iv) extraction capability for the analytes. On the other hand, considering this fact that the target analytes have acidic nature, three alkaline solutions including ammoniacal, borate, and phosphate buffers at a constant concentration (0.5 M) and a constant pH of 10 were selected to investigate as the extraction solvent type. All experiments were carried out with 14 μL of the buffers. From the obtained results in Fig. 3, it was found that ammoniacal buffer gives the relatively high analytical signals among the tested solvents. Therefore, it was selected for the further experiments.
Selection of extraction solvent in AALLME procedure. Extraction conditions are the same as in Fig. 3, except 4.0 min was used as the vortexing time. The error bars indicate standard deviation of three repeated determinations
Optimization of Ammoniacal Buffer Concentration and Volume
Concentration of the buffer is another important factor that could affect the extraction efficiency. For this purpose, the concentration of ammoniacal buffer varied from 0.10 to 1.50 M. The obtained data (Fig. 4) show that when ammoniacal buffer concentration increases from 0.10 to 0.50 M, extraction efficiencies also increase and then remain nearly constant at higher concentrations. Thus, 0.50 M was chosen as the optimum concentration of ammoniacal buffer.
Optimization of ammoniacal buffer concentration in AALLME procedure. Extraction conditions are the same as used in Fig. 3, except the ammoniacal buffer was used at the extraction solvent. The error bars indicate standard deviation of three repeated determinations
The volume of the extractant used can affect different parameters including volume of the sedimented phase, the repeatability of results, and extraction efficiencies. By changing the volume of the extraction phase (with keeping the sample size constant), the volume ratio of sample to extractant phase varies, and hence, ERs and EFs of the analytes may also change. To study the effect of the extraction solvent volume, some experiments involving different volumes of the ammoniacal buffer (14, 20, 25, 35, and 40 μL) were performed using the same extraction procedure. The results showed that analytical signals are decreased rapidly owing to the decreased EFs due to dilution effect at high volumes. It is noted that by increasing the volume of ammoniacal buffer from 14 to 40 μL, the volume of the sedimented phase increased from 10 to 36 μL. As a result, at low volumes of the extractive phase, high analytical signals could be obtained. Therefore, 14 μL was selected as the optimum volume for the extraction solvent.
Optimization of Extraction Numbers
In an AALLME method, the repeatedly aspiration/dispersion cycles of the aqueous solution and extraction solvent mixture are defined as the extraction numbers. It is predicted that by increasing extraction numbers, similar to batch extraction, the ER and EF could be increased. Therefore, to reach the equilibrium status, the extraction numbers should be optimized. In the present work, evolution of the extraction numbers was studied by aspirating/dispersion of the mixture of the extraction solvent (ammoniacal buffer) and n-hexane extract obtained from the previous VALLE step in the range of one to eight times. The results in Fig. 5 indicate that by increasing the extraction numbers, the analytical signals increase till sixth cycles, and then remain constant. Hence, extraction number of six was selected for the following studies. It should be noted that this step is very brief and takes less than 1 min.
Optimization of extraction numbers. Extraction conditions are the same as used in Fig. 4, except the concentration of ammoniacal buffer which was 0.50 M. The error bars indicate standard deviation of three repeated determinations
Optimization of Centrifugation Rate and Time in Both Steps
Centrifugation is an essential parameter in the present procedure, and hence, its optimization from the views of rate and time is necessary. The effect of time and speed of centrifuging were examined at the ranges of 3–10 min and 2000–5000 rpm, respectively. The results showed that these parameters had no obvious effect on the extraction efficiency in high centrifuging time and speed. However, 5 min and 4000 rpm were selected as centrifuging time and rate, respectively, in this study.
Quantitative Analysis
Some quantitative characteristics of the proposed VALLE–AALLME–HPLC–VWD method were obtained under the optimized conditions according to “FAO food and nutrition” guideline (Fao 1998). To validate performance of the method, calibration curves were obtained by spiking the target analytes directly into the analyte-free doogh sample and performing the proposed method on them. Quantitative parameters including linear range (LR), coefficients of determination, limit of detection (LOD), limit of quantification (LOQ), relative standard deviation (RSD), EF, and ER were investigated and the results are summarized in Table 1. The LOD is defined as the lowest concentration level of a substance that can be detected by an analytical procedure. It is typically determined to be in a range in which the signal measured (as peak height) to noise ratio is equal to 3 (S/N = 3). The LOQ is calculated experimentally, being concentration of the analyte which shows a signal-to-noise ratio of 10 (S/N = 10). The LODs were 0.82 and 0.54 ng mL−1 and the LOQs were 2.7 and 1.5 ng mL−1, for BPA and BPB, respectively, which are satisfactorily low for an HPLC–DAD method. The good linearities were obtained for the calibration graphs with coefficients of determination higher than 0.9988. Precision of the method was determined by analyzing the blank doogh spiked with 5 ng mL−1 (each analyte) at a same day and at four different days. The RSDs were in the range of 5–9 for intra- (n = 6) and inter-day precisions (n = 4), which indicate that the method is satisfactorily repeatable. The mean experimental results obtained from the specified concentration express the accuracy of an analytical method. The excellent way to investigate the accuracy of an analytical method is its application to Certified Reference Materials (CRMs). Unfortunately, there is no CRM for the studied analytes. Thus, the accuracy of the method was evaluated by standard addition method using six replicate determinations at a concentration of 3 ng mL−1 for each analyte. The obtained deviations from mean values were less than 10% for the target analytes which is acceptable for the developed method. The EFs and ERs for the selected analytes were 4300 and 4050 and 86 and 81% for BPA and BPB, respectively. Generally, the proposed method can be considered as a sensitive and repeatable method with high EFs and ERs and low LODs and LOQs.
Real Sample Analysis
To evaluate the applicability of the proposed method, determination of the selected analytes in different commercial canned doogh samples produced by different companies was performed using the proposed method. Figure 6 shows the typical HPLC chromatograms of the selected samples after performing the presented method. The results show that BPA was found (17 ± 2 ng mL−1, n = 3) in one sample. Other samples were free of the studied analytes. The influence of matrix effect on the detection response of the analytes is a well-known subject in analysis. This can result in an enhanced or decreased analyte signal in the extract obtained in the presence of matrix compared to the signal obtained in the absence of the matrix. In order to evaluate the matrix effect, the samples were spiked with the analytes at three levels (5, 10, and 25 ng mL−1 of each analyte), and the proposed method was applied on them (three replications for each concentration). The recoveries obtained for the analytes in doogh samples in comparison with the recoveries obtained for the deionized water spiked at the same three concentration levels are given in Table 2. In both cases, the method was performed, and the obtained extractive phase was injected into the separation system. The results demonstrate that the matrices of real samples have no significant effect on efficiency of the proposed method. Therefore, there was no need to perform any additional treatments. Selectivity of the developed method was studied by analysis of four blank doogh samples obtained from different brands. The results showed that no interfering peak in retention times belong to the target analytes and the developed method is selective for the analytes in real samples.
Comparison of the Proposed Method with Others
The efficiency of the method for the selected analytes was compared with other methods reported in the literature in terms of features such as LOD, RSD, LR, extraction time, and ER. The results are summarized in Table 3. The LODs are comparable or better than those of the mentioned techniques while very sensitive detection system such as MS was used. The RSDs for this method are better than other mentioned methods. Short extraction time and comparable ERs are other characteristics of the proposed method compared to others. All these results reveal that the present method is a sensitive, rapid, and repeatable technique that can be used as an alternative method for the extraction, preconcentration, and determination of the target analytes in a relatively complex matrix such as doogh sample.
Conclusion
In this study, a VALLE–AALLME method has been successfully developed for the extraction of BPA and BPB from different doogh samples. The extracted analytes have been determined using HPLC–VWD. The method provided high EFs and ERs for the analytes. Therefore, low LODs and LOQs were obtained by the proposed method. Comparison of this method with other methods showed that the method is simple, easy, and efficient for the target analytes. The satisfactory results obtained make this method as a suitable potential alternative for the previously established methods.
Abbreviations
- AALLME:
-
Air-assisted liquid–liquid microextraction
- BFA:
-
Bisphenol A
- BFB:
-
Bisphenol B
- EF:
-
Enrichment factor
- ER:
-
Extraction recovery
- HPLC:
-
High performance liquid chromatography
- VALLE:
-
Vortex-assisted liquid–liquid extraction
- VWD:
-
Variable wavelength detector
References
An J, Trujillo-Rodríguez MJ, Pino V, Anderson JL (2017) Automated direct-immersion solid-phase microextraction using cross linked polymeric ionic liquid sorbent coatings for the determination of water pollutants by gas chromatography. J Chromatogr A 1500:1–23
Asati A, Satyanarayana GNV, Panchal S, Thakur RS, Ansari NG, Patel DK (2017) Ionic liquid based vortex assisted liquid–liquid microextraction combined with liquid chromatography mass spectrometry for the determination of bisphenols in thermal papers with the aid of response surface methodology. J Chromatogr A 1509:35–42
Cacho JI, Campillo N, Viñas P, Hernández-Córdoba M (2013) Stir bar sorptive extraction with EG-Silicone coating for bisphenols determination in personal care products by GC-MS. J Pharm Biomed Anal 78–79:255–260
Cheng Y, Nie X, Wu H, Hong Y, Yang B, Liu T, Zhao D, Wang JF, Yao GH, Zhang F (2017) A high-throughput screening method of bisphenols, bisphenols digycidyl ethers and their derivatives in dairy products by ultra-high performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 950:98–107
Cunha SC, Almeida C, Mendes E, Fernandes JO (2011) Simultaneous determination of bisphenol A and bisphenol B in beverages and powdered infant formula by dispersive liquid-liquid microextraction and heart-cutting multidimensional gas chromatography-mass spectrometry. Food Addit. Contam Part B 28:513–526
Cunha SC, Ferreira AR, Fernandes JO (2012) Determination of bisphenol A and bisphenol B in canned seafood combining QuEChERS extraction with dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Anal Bioanal Chem 404:2453–2463
Ehrlich S, Williams PL, Missmer SA, Berry KF, Petrozza J, Hauser R (2011) Urinary bisphenol A and implantation failure among women undergoing in vitro fertilization. Fertil Steril 96:S6–S7
Erler C, Novak J (2010) Bisphenol A exposure: human risk and health policy. J Pediatr Nurs 25:400–407
Farajzadeh MA, Afshar Mogaddam MR (2012) Air-assisted liquid-liquid microextraction method as a novel microextraction technique; application in extraction and preconcentration of phthalate esters in aqueous sample followed by gas chromatography-flame ionization detection. Anal Chim Acta 728:31–38
Farajzadeh MA, Abbaspour M, Afshar Moghaddam MR, Ghorbanpour H (2015) Determination of some synthetic phenolic antioxidants and bisphenol A in honey using dispersive liquid-liquid microextraction followed by gas chromatography-flame ionization detection. Food Anal Methods 8:2035–2043
Fasano E, Cirillo T, Esposito F, Lacorte S (2015) Migration of monomers and plasticizers from packed foods and heated microwave foods using QuEChERS sample preparation and gas chromatography/mass spectrometry. LWT Food Sci Technol 64:1015–1021
Fernandez MAM, André LC, Cardeal ZL (2017) Hollow fiber liquid-phase microextraction-gas chromatography-mass spectrometry method to analyze bisphenol A and other plasticizer metabolites. J Chromatogr A 1481:31–36
Gao L, Zou J, Liu H, Zeng J, Wang Y, Chen X (2013) Determination of bisphenol A in thermal printing papers treated by alkaline aqueous solution using the combination of single-drop microextraction and HPLC. J Sep Sci 36:1298–1303
Golub MS, Wu KL, Kaufman FL, Li LH, Moran-Messen F, Zeise L, Alexeeff GV, Donald JM (2010) Bisphenol A: developmental toxicity from early prenatal exposure. Birth Defects Res B Dev Reprod Toxicol 89:441–466
González-Casado A, Navas N, Del Olmo M, Vílchez JL (1998) Determination of bisphenol A in water by micro liquid-liquid extraction followed by silylation and gas chromatography-mass spectrometry analysis. J Chromatogr Sci 36:565–569
Kojima I, Davis SS (1984) The effect of salt concentration on the distribution of phenol between aqueous sodium chloride and carbon tetrachloride. Int J Pharm 20:203–207
Korenman YI, Mokshina NY, Zykov AV (2010) Distribution coefficients of vitamin B2 in hydrophilic organic solvent-aqueous salt solution systems. Russ J Phys Chem A 84:415–418
Lee C, Kim CH, Kim S, Cho SH (2017) Simultaneous determination of bisphenol A and estrogens in hair samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B 1058:8–13
Li G, Liu S, Sun Z, Xia L, Chen G, You J (2015) A simple and sensitive HPLC method based on pre-column fluorescence labelling for multiple classes of plant growth regulator determination in food samples. Food Chem 170:123–130
Loganathan SN, Kannan K (2011) Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch Environ Contam Toxicol 61:68–73
Lu S, Wu D, Li G, Lv Z, Gong P, Xia L, Sun Z, Chen G, Chen Z, You J, Wu Y (2017) Facile and sensitive determination of N-nitrosamines in food samples by high performance liquid chromatography via combining fluorescent labeling with dispersive liquid-liquid microextraction. Food Chem 234:408–415
Maragou NC, Lampi EN, Thomaidis NS, Koupparis MA (2006) Determination of bisphenol A in milk by solid phase extraction and liquid chromatography-mass spectrometry. J Chromatogr A 1129:165–173
Martins J, Esteves C, Simões T, Correia M, Delerue-Matos C (2011) Determination of 24 pesticide residues in fortified wines by solid–phase microextraction and gas chromatography–tandem mass spectrometry. J Agric Food Chem 59:6847–6855
Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Ye X, Trisini AT, Hauser R (2010) Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxic 30:532–539
Report of a Joint FAO/IAEA Expert consultation (1998) Validation of analytical methods for food control. Food and Agriculture Organization of the United Nations 68:1–19
Sosvorova LK, Chlupacova T, Vitku J, Vlk M, Heracek J, Starka L, Saman D, Simkova M, Hampl R (2017) Determination of selected bisphenols, parabens and estrogens in human plasma using LC–MS/MS. Talanta 174:21–28
Sungur S, Köroğlu M, Özkan A (2014) Determinatıon of bisphenol a migrating from canned food and beverages in markets. Food Chem 142:87–91
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95
Yoon Y, Westerhoff P, Snyder SA, Esparza M (2003) HPLC-fluorescence detection and adsorption of bisphenol A, 17 beta-estradiol, and 17alpha-ethynyl estradiol on powdered activated carbon. Water Res 37:3530–3537
Acknowledgments
Authors are grateful to Research Council of the Sarab Branch of Islamic Azad University for financial support.
Funding
Jalil Khandaghi has received research grants from Sarab Branch of Islamic Azad University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jalil Khandaghi declares that he has no conflict of interest. Roya Amini declares that she has no conflict of interest. Mohammad Reza Afshar Mogaddam declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Amini, R., Khandaghi, J. & Mogaddam, M.R.A. Combination of Vortex-Assisted Liquid–Liquid Extraction and Air-Assisted Liquid–Liquid Microextraction for the Extraction of Bisphenol A and Bisphenol B in Canned Doogh Samples. Food Anal. Methods 11, 3267–3275 (2018). https://doi.org/10.1007/s12161-018-1260-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1260-8