Abstract
A fully automated vortex-assisted liquid-liquid microextraction (VALLME) system coupled to gas chromatography-mass spectrometry (GC/MS) was developed for the determination of phthalate esters (PAEs) in liquor samples. The whole analytical procedure, including spiking, extraction, phase separation, extractant collection, and GC/MS quantification, were automatically carried out. The use of a commercially available sample vial and a multipurpose sampler equipped with a highly efficient orbital vortex shaker facilitated the accessibility and automation of the method. Key factors, such as type and volume of the extractant, time and speed for VALLME, agitation speed and time required for phase separation, sample pH, salt effects, and matrix effects, were thoroughly investigated. Under the optimum conditions, linearity was in the range 0.05 to 120 μg L−1. Limits of detection ranged from 0.003 to 0.006 μg L−1. Enrichment factors were in the range 211 to 304. Reproducibility and recoveries were assessed by testing a series of liquor samples spiked with different concentrations of phthalate esters. This work provided an innovative way to automate the VALLME method and couple it on-line with GC/MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters (PAEs) are widely used as plasticizers in industrial and domestic applications for increasing the flexibility and moldability of polymeric materials. Some studies show that PAEs, as well as their metabolites and degradation products, can act as endocrine disrupting compounds, which can cause damage to the kidneys and endocrine and reproductive system and cause male obesity and diabetes (Overturf et al. 1979; Doull et al. 1999; Hoppin et al. 2004; Stahlhut et al. 2007). Because of the weak physical bond between PAEs and polymer chains, they can be easily released into foods, beverages and natural water from various products. Thus, due to their risks and presence during liquor making, PAEs in liquor samples are priority pollutants. However, owing to the low concentrations of PAEs in liquor samples and the complexity of the matrices, reliable pretreatment and a sensitive analytical method are imperative.
Dispersive liquid-liquid microextraction (DLLME), which was introduced by Rezaee and co-workers (Rezaee et al. 2006), represents an important development in the field of sample preparation due to its simplicity, miniaturization, and speed. In DLLME, an appropriate mixture of extraction solvent and disperser solvent is rapidly injected into the aqueous sample. The extraction solvent is dispersed into the aqueous sample as very fine droplets and a cloudy solution is formed. Owing to the extension of the contact surface between the extraction solvent and the aqueous phase, the extraction efficiency is greatly enhanced. However, the main drawback of DLLME is the necessity of using a dispersion solvent which may decrease the partition coefficient of analytes into the extraction solvent (Regueiro et al. 2008). To overcome this, ultrasound energy is commonly used to enhance the dispersion (Cortada et al. 2011; Andruch et al. 2013). However, degradation of the analyte under ultrasound conditions has been reported (Sanchez-Prado et al. 2008).
More recently, a new dispersion solvent-free microextraction technique named vortex-assisted liquid-liquid microextraction (VALLME) was reported (Yiantzi et al. 2010). In VALLME, micro-volumes of extraction solvent were dispersed into the aqueous sample using vortex mixing, and fine droplets and a cloudy solution were dynamically formed. Because of the shorter diffusion distance and larger specific surface area, analytes could rapidly be extracted. After centrifugation, the extraction solvent restores its initial single microdrop shape and is ready for instrumental analysis. VALLME overcomes the main disadvantage of DLLME and has been successfully applied to the extraction of different analytes in various sample matrices (Leng et al. 2012a; Leng et al. 2012b; Leng et al. 2014; Leng et al. 2015).
In our previous work (Leng et al. 2014), VALLME was applied to extract PAEs in liquor samples. However, the reproducibility and linearity of the method were not very satisfactory, and better extraction efficiency for dinoctyl phthalate (DNOP) was required. It is well known that automation and the direct coupling of the sample pretreatment step to the detector could offer a number of advantages, such as minimizing the errors associated with manual handling, reducing sample and reagent consumption, and improving sensitivity and precision (Kocúrová et al. 2013). However, because of the difficulties in (i) developing a suitable vortex agitator for on-line automation system, (ii) automation of phase separation, and (iii) the transfer of the micro-volume collection phase into the measuring system, to our knowledge, no automated VALLME has been reported.
In the present work, a fully automated VALLME coupled to gas chromatography-mass spectrometry (GC/MS) was, for the first time, developed for the determination of trace levels of PAEs in liquor samples. With a multipurpose sampler equipped to GC/MS, all VALLME procedures as well as the GC/MS analysis were automatically carried out. In the proposed method, a dual syringe system was used for solvent transfer, extract collection, and sample injection. A highly efficient orbital vortex shaker was used for the vortex extraction and phase separation, and a commercially available sample vial was used in the VALLME that made this method much easier to access. Key factors that affect the performance of the automated VALLME-GC/MS were thoroughly investigated. This work provided an innovative way to automate the VALLME method, as well as its on-line coupling with GC/MS. The fully automated system resulted in significant improvements in sensitivity, repeatability, linear range, and enrichment factors compared with a manual method.
Experimental
Reagents and Standards
Standards of PAEs including dibutyl phthalate (DBP), dimethyl phthalate (DMP), and dinoctyl phthalate (DNOP) were purchased from Merck (Darmstadt, Germany). The stock standard solutions of 1 g L−1 of each compound were prepared in methanol. The standard working solutions were prepared daily by dilution of the stock standard solutions in ultra-pure water from a Milli-Q Integral 3 system (Massachusetts, USA). The stock and working standard solutions were stored at 4 °C. High-performance liquid chromatography (HPLC)-grade dichloromethane (CH2Cl2), chloroform (CHCl3), and carbon tetrachloride (CCl4) were purchased from Rionlon (Tianjing, China). HPLC-grade acetone and methanol were purchased from Tedia (Ohio, USA). Analytical grade sodium chloride (NaCl), hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Kelong (Chengdu, China).
To avoid contamination, all glassware used in the study was soaked in acetone for at least 6 h, then washed with acetone, and dried at 140 °C for at least 4 h.
Instrumentation
The automated VALLME process was carried out with a Gerstel (Mülheim an der Ruhr, Germany) multipurpose sampler (MPS) coupled to GC/MS. The MPS comprised a dual-rail system that was configured with two different syringes. A 250-μL syringe was used for handling standards and reagents in the extraction operations, while a 10-μL syringe was used for the collection and injection of the samples into the GC/MS detector after VALLME. A highly efficient mVorx (Mülheim an der Ruhr, Germany) orbital shaker with a vortex speed ranging from 200 to 3000 rpm was coupled to the MPS for the VALLME and the thereafter phase separation. A commercially available 10-mL conical base vial purchased from Sunda (Ningbo, China) was used, making the proposed method very easy to access. A manipulator was used for transporting the sample vial between the tray and the shaker.
A PHS-3E pH meter purchased from INESA Scientific Instrument (Shanghai, China) was used for measuring sample pH.
Quantification of the extracted PAEs was performed with an Agilent (California, USA) 7890 series gas chromatograph equipped with an Agilent 5975 mass detector. Chromatographic separation was accomplished on a HP-5MS (J&W Scientific, California, USA) capillary column (30 m × 0.25 mm id × 0.25 μm). Helium (>99.999%) was employed as carrier gas at a flow rate of 1.0 mL min−1. The injector temperature was set at 250 °C. The column temperature was programmed as follows: 60 °C, held for 1 min; 20 °C min−1 up to 220 °C, held for 1 min; and 5 °C min−1 up to 280 °C, held for 4 min. All injections were in splitless mode. The mass spectrometer was operated in the electron ionization mode (70 eV), and the analytes were recorded in selective ion monitoring (SIM) mode. After 6 min of solvent delay, the start scan times and masses monitored for each compound were set as follows: DMP, 6.0 min, m/z 163, 77, and 135; DBP, 10.0 min, m/z 149, 223, and 205; and DNOP, 18.0 min, m/z 149, 279, and 167.
Samples and Automated VALLME Procedures
Commercially available liquor samples with alcohol content of 52% (v/v) purchased from the local market (Chengdu, China) were used for this study. After being filtered through a 0.22-μm membrane, the samples were subjected to the automated VALLME-GC/MS analysis.
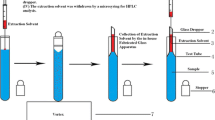
The entire analytical process, including the VALLME, phase separation, collection of extracts, and their final injection into GC/MS, was fully automated using the MPS, which was controlled with the MAESTRO® software. A schematic of the developed automated VALLME procedure is shown in Fig. 1. Initially, 3.0 mL liquor samples were added into a 10-mL screw cap conical base vial and then placed in the tray. After that, 120 μL of CCl4 as extractant were withdrawn into a 250-μL syringe. Then, the syringe was moved to the sample vial and the extractant was injected into the sample. Afterward, the sample vial was transported to the mVorx shaker and vigorously vortexed at 3000 rpm for 10 min. In that case, fine droplets were formed, facilitating mass transfer of the target analytes into the CCl4 phase. After extraction, phase separation was achieved by a mild rate agitation at 200 rpm for 10 min using the mVorx shaker. Thereafter, the sample vial was transferred back to the tray. One microliter of the sedimented extracts was collected using a 10-μL syringe and was then injected into the GC/MS for analysis.
Results and Discussion
Effects of several key factors, such as the type and volume of the extractant, vortex speed and time for extraction, agitation speed and time for phase separation, sample pH, and salt addition, on the performance of the proposed method were investigated. Real commercial liquor samples described in “Samples and Automated VALLME Procedures” section were used for the optimization of the experimental factors. Blank tests were performed in each set of experiments, and the results showed that the analytical processes of the proposed method were free from PAE contamination.
Effect of Extractant
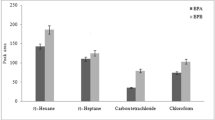
Generally, an ideal extractant used in the proposed method should possess a higher density than the liquor, a low solubility in the liquor, high extraction efficiency for the analytes, and good gas chromatographic behavior. Based on these considerations, CH2Cl2, CHCl3, and CCl4 were investigated as potential extractants. As shown in Fig. 2, CCl4 gave better extraction recovery for PAEs when compared with the other tested solvents. Consequently, carbon tetrachloride was selected as the optimum extractant and used in subsequent experiments.
Effect of the extractant on the automated VALLME-GC/MS method. Three measurements were performed for each set of experiments. Extraction conditions: sample volume, 3.0 mL; extraction solvent volumes, 250 μL; vortex speed and time for extraction, at 3000 rpm for 10 min; agitation speed and time for phase separation, at 200 rpm for 10 min; no salt addition; liquor samples were spiked with 5.0 ng of each PAE
Effect of Volume of Extractant
The volume of extraction solvent also plays a very important role, which may affect the enrichment factor. In order to investigate the effect of the volume of the extractant on the extraction, different volumes of CCl4, ranging from 120 to 300 μL, were studied. The results were shown in Fig. 3. As expected, by increasing the volume of the extractant, the peak areas decreased. Owing to the complexity of the matrix effect of the real liquor sample (52 alc vol−1), extractant volumes lower than 120 μL were avoided, since the amount of the remaining sedimented extract was unstable and too small to be collected. It is worth noting that collection of the micro-volume extract phase has been considered as one of the most difficult steps for the automation of DLLME/VALLME (Kocúrová et al. 2013). With the MPS in the proposed method, micro-volumes of extract can be accurately collected and injected into the GC/MS detector using a 10-μL syringe. Hence, lower extractant volumes can be used in VALLME, resulting in a dramatic improvement in the extraction enrichment factor when compared with manual procedures (Leng et al. 2014). Thus, 120 μL of CCl4 was considered the optimum volume.
Effect of the volume of extractant on the automated VALLME-GC/MS. Three measurements were performed for each set of experiments. Method. Extraction conditions: sample volume, 3.0 mL; extractant, CCl4; vortex speed and time for extraction, at 3000 rpm for 10 min; agitation speed and time for phase separation, at 200 rpm for 10 min; no salt addition; liquor samples were spiked with 5.0 ng of each PAE
Effect of Speed and Time of Vortex Shaken
A vortex shaker that can be coupled to an on-line system has been considered as a key requirement for the automation of VALLME. With the mVorx vortex shaker integrated in the MPS, VALLME could automatically be carried out. The vortex speed and its duration of extraction are known to affect the performance of the proposed method. Effects of different vortex speeds (1000, 1200, 1400, 1600, 1800, 2000, 2200, 2400, 2600, 2800, and 3000 rpm) and extraction times (1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 min) were simultaneously and thoroughly investigated using a 102 full factorial experiment. The results for each of the selected PAEs are shown in Fig. 4. It can be seen that all PAEs gave similar response trends when different vortex speeds and extraction times were used. Due to the limit of the vortex shaker, vortex speeds higher than 3000 rpm were avoided. The highest peak area was obtained when VALLME was carried out at 3000 rpm for 10 min. Extraction times >10 min were also investigated but no significant improvement on the extraction efficiency could be observed. Therefore, VALLME carried at 3000 rpm for 10 min was considered to be optimum.
Effects of vortex speed and extraction time of VALLME on the automated VALLME-GC/MS. Three measurements were performed for each set of experiments. Extraction conditions: sample volume, 3.0 mL; extractant, 120 μL CCl4; agitation speed and time for phase separation, at 200 rpm for 10 min; no salt addition; liquor samples were spiked with 5.0 ng of each PAE. Identification: a DNOP, b DBP, and c DMP.
Phase separation in VALLME, which was usually achieved by centrifugation in manual operations, was also considered as one of the biggest challenges for automation. The most commonly used ways to achieve phase separation in an automated system have been elution after microcolumn retention or static separation, which were considered either tedious or time-consuming. In the proposed method, a mild agitation was applied for the phase separation after VALLME. Different times (1 to 30 min) and speeds of agitation (200 to 1000 rpm) were studied. Due to the limitation of the vortex shaker, agitation speed lower than 200 rpm was avoided. The results indicated that phase separation was not achieved when agitation speeds higher than 700 rpm were used, since sample turbulence occurred during the agitation. The time needed for phase separation increased with increasing agitation speed from 200 to 600 rpm, which was unexpected. However, this can be explained by the fact that agitation in the proposed method was achieved using a micro-extent orbital shaker. In this case, sample will be affected by both centrifugal force and turbulence, yielding a compromise result. Therefore, an agitation at 200 rpm for 10 min was selected as optimum.
Effect of Salt Addition and Sample pH
In liquid phase extraction, salt is often added into the sample solution to enhance the extraction efficiency, since the increase in ionic strength can decrease the solubility of analytes in aqueous samples and enhance their partitioning into the organic phase. In order to study the effect of salt addition on the performance of the proposed method, samples with different salinities (from 0 to 5%, m/v), realized by adding different amounts of NaCl, were subjected to the same VALLME-GC/MS procedure. The results demonstrated that the peak area of the analytes decreased when the sample salinity increased. This may be because the increase in ionic strength may also lead to a decrease in CCl4 solubility in the aqueous phase, which may further increase the volume of sedimented phase and decrease the enrichment factors of the analytes. Furthermore, an increase in the salt content of liquor sample may increase the viscosity and density of the solution, forming a physical barrier across the sample/extractant interface, thus reducing the diffusion rates of analytes into the microdrop. Consequentially, no salt addition was used in this study.
Sample pH is another important factor that potentially affects the performance of the proposed method. Effects of different sample pH, ranging from 3 to 10, on the proposed VALLME-GC/MS method were investigated. The pH of sample with original value of 5.9 was adjusted using 0.1 mol L−1 HCl and NaOH. Negligible effects on the peak area of the analytes were observed within the studied pH range. Thus, there is no need for pH adjustment for the proposed method.
Analytical Figures of Merit
Under the optimum conditions, chromatograms of PAEs in liquor samples described in “Samples and Automated VALLME Procedures” section before and after spiking with 5.0 ng of each PAE are shown in Fig. 5. The performance of the automated VALLME-GC/MS method is summarized in Table 1. The limits of detection (LOD) and the limits of quantitation (LOQ) were calculated according to the IUPAC recommendation (Currie 1995) as the concentration yielding a peak area over the blank signal by a three- and tenfold standard deviations, respectively. LODs of 0.006 μg L−1 for DMP and 0.003 μg L−1 for DBP and DNOP were obtained. Enrichment factors, defined as the ratio between the concentration of analyte in the sediment phase (C sed) and the initial concentration of analyte (C 0) in the sample, were obtained from three-replicate analysis of 5.0 ng of spiked liquor samples, varied from 211 to 304. Five-point calibration curves, using the optimum parameters for the proposed method, were evaluated and coefficients of determination (R 2) ranged from 0.9711 to 0.9906. Good linearity was found in the concentration range from 0.05 to 120 μg L−1. The relative standard deviations (RSDs) were <3.1% for all analytes, demonstrating good repeatability of the method.
Application to Real Liquor Samples
To investigate the applicability of the established method, six different commercial liquor samples (L1 to L6) purchased from the local market (Chengdu, China) were subjected to the proposed automated VALLME-GC/MS analysis. The results are shown in Table 2. The results indicated that DBP was present in all the samples with concentrations ranging from 14.7 ± 0.59 to 74.2 ± 3.0 μg L−1, whereas BMP and DNOP were not systematically present in all the samples. Furthermore, in the recovery experiments, samples with different alcohol contents were spiked with 5.0 ng of PAEs and then subjected to the same procedures. The recoveries of PAEs in all the real liquor samples were in the range of 85.7 to 96.1%, indicating no significant interferences or matrix effects.
Conclusions
VALLME, which overcomes some of the main disadvantages of DLLME, is a simple, fast, and highly efficient method for the extraction of trace analytes, with good sensitivity and ease of automation. In this work, a fully automated VALLME coupled to GC/MS method was developed. With a MPS and vortex shaker that were coupled to a GC/MS, the whole analytical procedure, including spiking, extraction, phase separation, extractant collection, and GC/MS quantification, were automatically carried out. It is also worth noting that the VALLME was carried out in a commercially available sample vial that made this method very easy to use. This work provides an innovative way to automate the VALLME method and couple it on-line with GC/MS.
References
Andruch V, Burdel M, Kocurova L, Sandrejova J, Balogh IS (2013) Application of ultrasonic irradiation and vortex agitation in solvent microextraction. TrAC Trends Anal Chem 49:1–19
Cortada C, Vidal L, Canals A (2011) Determination of geosmin and 2-methylisoborneol in water and wine samples by ultrasound-assisted dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry. J Chromatogr A 1218:17–22
Currie LA (1995) Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl Chem 67:1699–1723
Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M (1999) A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new US EPA risk assessment guidelines. Regul Toxicol Pharmacol 29:327–357
Hoppin JA, Ulmer R, London SJ (2004) Phthalate exposure and pulmonary function. Environ Health Perspect 112:571–574
Kocúrová L, Balogh IS, Andruch V (2013) Solvent microextraction: a review of recent efforts at automation. Microchem J 110:599–607
Leng G, Yin H, Li S, Chen Y, Dan D (2012a) Speciation analysis of mercury in sediments using vortex-assisted liquid-liquid microextraction coupled to high-performance liquid chromatography-cold vapor atomic fluorescence spectrometry. Talanta 99:631–636
Leng G, Lui G, Chen Y, Yin H, Dan D (2012b) Vortex-assisted extraction combined with dispersive liquid-liquid microextraction for the determination of polycyclic aromatic hydrocarbons in sediment by high performance liquid chromatography. J Sep Sci 35:2796–2804
Leng G, Chen W, Zhang M, Huang F, Cao Q (2014) Determination of phthalate esters in liquor samples by vortex-assisted surfactant-enhanced-emulsification liquid-liquid microextraction followed by GC-MS. J Sep Sci 37:684–690
Leng G, Chen W, Wang Y (2015) Speciation analysis of mercury in sediments using ionic-liquid-based vortex-assisted liquid-liquid microextraction combined with high-performance liquid chromatography and cold vapor atomic fluorescence spectrometry. J Sep Sci 38:2684–2691
Overturf ML, Druilhet RE, Liehr JG, Kirkendall WM, Caprioli RM (1979) Phthalate-esters in normal and pathological human kidneys. Bull Environ Contam Toxicol 22:539–542
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R (2008) Ultrasound-assisted emulsification-microextraction of emergent contaminants and pesticides inenvironmental waters. J Chromatogr A 1190:27–38
Rezaee M, Assadi Y, Hosseinia MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 1116: 1–9
Sanchez-Prado L, Barro R, Garcia-Jares C, Llompart M, Lores M, Petrakis C, Kalogerakis N, Mantzavinos D, Psillakis E (2008) Sonochemical degradation of triclosan in water and wastewater. Ultrason Sonochem 15:689–694
Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH (2007) Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environ Health Perspect 115:876–882
Yiantzi E, Psillakis E, Tyrovola K, Kalogerakis N (2010) Vortex-assisted liquid-liquid microextraction of octylphenol, nonylphenol and bisphenol-A. Talanta 80:2057–2062
Acknowledgments
The authors gratefully acknowledge the financial support of the Fundamental Research Funds for the Central Universities (ZYGX2014J092, ZYGX2013Z006) and National Natural Science Foundation of China (51408101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Fundamental Research Funds for the Central Universities (ZYGX2014J092, ZYGX2013Z006) and National Natural Science Foundation of China (51408101).
Conflict of Interest
Geng Leng declares that he has no conflict of interest. Wen-Jin Chen declares that she has no conflict of interest. Wen-Bo Xu declares that he has no conflict of interest. Yong Wang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Leng, G., Chen, WJ., Xu, WB. et al. Fully Automated Vortex-Assisted Liquid-Liquid Microextraction Coupled to Gas Chromatography-Mass Spectrometry for the Determination of Trace Levels of Phthalate Esters in Liquor Samples. Food Anal. Methods 10, 3071–3078 (2017). https://doi.org/10.1007/s12161-017-0874-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0874-6