Abstract
The present paper describes the validation of ultrasound-assisted emulsification-microextraction method followed by ion mobility spectrometry (IMS) detection for simultaneous determination of two organophosphorus pesticides, diazinon and chlorpyrifos. Ultrasound radiation was applied for accelerating the emulsification of microliter organic solvent in aqueous solutions and enhancing the microextraction efficiency. This preconcentration step combined with IMS detection provided a precise and accurate method for determination of trace amounts of diazinon and chlorpyrifos pesticides. The effect of parameters influencing the extraction efficiency such as sonication time, type of extraction solvent, extraction solvent volume, and salt concentration were investigated and discussed. The enrichment factors found, under optimum conditions, were 230 and 300 for diazinon and chlorpyrifos, respectively, with corresponding LOD of 2.1 and 3.2 μg L−1. The presented method can be applied for the determination of diazinon and chlorpyrifos in the range 6.0–700 and 8.9–750 μg L−1, respectively, with correlation coefficients (R 2) > 0.99. The applicability of the proposed method was evaluated by determination of the residues of the investigated pesticides in rice paddy water gathered from four stations during 60 days after spraying (June 2014), and in storage rice samples in Mazandaran province, Iran.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development and validation of analytical methodologies include optimization of some critical analytical parameters such as accuracy, sensitivity, reproducibility, simplicity, cost effectiveness, flexibility, and speed. Meanwhile, a paradoxical situation emerged during the 1990s, due to the presence of side effects of some analytical procedures developed to analyze different kinds of samples, including environmental samples. These methods generate a large amount of chemical waste, resulting in a great environmental and human impact. In some circumstances, the chemicals employed for analysis are even more toxic than the species being determined.
Taking into account the requirements for determination of many pollutants at trace level from complex matrix, a part of analytical chemistry studies concern on the establishment and development of efficient sample preparation methods. Decrease in the complexity of the matrix and increase in the concentration (preconcentration) of target compounds are the main aspects which are considered in such studies (Sergio et al. 2008; Samadi et al. 2012; Farajzadeh and Afshar Mogaddam 2012; Ramos 2012).
Application of conventional liquid–liquid extraction and solid-phase extraction methods was limited with the disadvantages such as being time-consuming, labor-intensive, and the need for a large amount of organic solvents (Simpson 2000; Aguilar and Cortina 2010). Recent efforts are being placed on the development of miniaturized, efficient, and environment-friendly extraction techniques for the analysis of target chemical species in complex matrix (Jeleń et al. 2012; Kokosa 2013; Spietelun et al. 2014).
Ultrasound-assisted emulsification microextraction was introduced by Regueiro et al. (2008). This technique is known as efficient, simple, rapid, and inexpensive which use a very small volume of extraction solvent. In addition, in this method, the application of water-miscible organic solvents (disperser), an inevitable component in conventional liquid-liquid microextraction procedures, is omitted. In ultrasound-assisted emulsification microextraction, the extraction solvent is emulsified with ultrasound irradiation that boosts mass transfer from the aqueous phase into the organic phase by promoting the formation of a large surface area. This cleanup-preconcentration method has been used for analysis of a variety of compounds such as polycyclic aromatic hydrocarbons (Ozcan et al. 2010), phenolic compounds (Moradi et al. 2013; Reboredo-Rodríguez et al. 2014), organochlorine pesticides (Wei et al. 2011), UV filters (Vila et al. 2016), and heavy metals (Sereshti et al. 2012).
In comparison with conventional organochlorine pesticides, OPPs demonstrate relatively low environmental persistence but a high toxicity to mammalian, which can eventually become a threat to human beings. Some of these pesticides are persistent and enter the food chain and the human body (Engel et al. 2011; London et al. 2012; Hernández et al. 2013; González-Alzaga et al. 2014). Therefore, the monitoring and determination of OPPs in different environmental matrices are important, and form a major subject of many recent investigations (Arjmandi et al. 2010; Lamers et al. 2011; Wu et al. 2014; Jafari et al. 2014; Saraji et al. 2015; Ahmadi et al. 2015). Gas chromatography and high performance liquid chromatography are the common detection techniques used for quantification of organophosphorus residues (Zhao et al. 2011; Hu et al. 2013; Ma and Chen 2014; Zainudin et al. 2015).
As a large number of Asiatic habitants, rice forms the most common food of Iranian peoples. Thus rice is one of the main agricultural productions in Iran, especially in Mazandaran province, North of Iran, with 202,000 ha of rice paddies. This nutrition is always attacked by various pests during cultivation and harvest. Diazinon and chlorpyrifos are two organophosphorus pesticides that frequently are used in Iran rice paddies. They can be absorbed through the digestive system, the skin, and respiratory tract. Diazinon is a very highly toxic organophosphate compound. Chlorpyrifos at low concentrations can interfere with calcium metabolism; in fact, skeletal malformations were mainly happened in a dose-dependent manner. It is noteworthy that the maximum residual limit (MRL) of diazinon and chlorpyrifos varies from country to country. Based on the Iranian National Standard (ISIRI 2011), the MRL values for these organophosphorus pesticides are reported as 0.1 mg kg−1.
Ion mobility spectrometry (Eiceman and Karpas 2005) has been developed as an instrumental analytical technique for detecting and identifying various compounds based upon the mobility of gas-phase ions in an electric field (Gallegos et al. 2015; Cohen et al. 2015). A combination of ion mobility spectrometry with microextraction techniques permits achieving to sensitive environment-friendly detection of trace amounts of a variety of environmentally important organic compounds (Jafari et al. 2012; Holopainen et al. 2013; Karpas 2013; Kalhor et al. 2015; Allafchian et al. 2015). This combination provides some advantages for analytical purposes including high sensitivity and relatively low technical expenditure with high speed data achievement.
In this paper, the combination of dispersive microextraction assisted by ultrasonic irradiation followed by ion mobility spectrometry detection (USAEME-IMS) was investigated for monitoring of organophosphorus pesticides diazinon and chlorpyrifos. The effect of parameters influencing the extraction efficiency such as type and volume of extracting solvent, salt addition, and centrifugation time was investigated and optimized. The applicability of the method was appraised by determination of the analytes in rice and rice paddy water samples. These samples were gathered from Mazandaran province (North Iran). The analysis of the studied OPPs in rice paddy samples was performed from 1 to 60 days after spraying. The rice samples were taken just after cropping and storage the products.
Experimental
Reagents and Chemicals
All the solvents used were HPLC-grade (Merck) and were used as received. Organophophorus pesticides diazinon and chlorpyrifos were obtained from Plant Protection Organization (Tehran, Iran). Rice samples were collected from four rice paddies in Mazandaran province (North Iran).
Preparation of Standard Solutions
The stock standard solutions (1000 mg L−1) of the investigated pesticides were prepared in methanol and were stored in a freezer at −20 °C. The working solutions were made by successive dilution of these solutions with deionized water.
Sample Preparation
The rice paddy water samples were collected after 1, 10, 20, 30, 40, 50, and 60 days of the pesticides spraying. The selected rice paddies were sprayed on 15–20 June 2014. Five replicate samples (each 100 mL) from the depth of 10–15 cm of their basin water of the selected stations were collected. The samples taken from each station were mixed and the water samples were kept in a refrigerator to prevent the OPPs’ decomposition, before applying the preconcentration and determination steps. The analysis of the rice paddy water samples collected before spraying the organophosphorus pesticides ensured that the samples were free from diazinon and chlorpyrifos. The rice samples were collected from four different rice repositories, corresponding to the selected rice paddy sites. The selected rice samples were the products of the same sites and they were obtained just after cropping rice paddies. From each repository, five samples (100 g each) were taken. Four 20 g portions of each sample were grounded and mixed with 25 mL of deionized water. These mixtures were magnetically stirred for 15 min. After centrifugation, the aqueous phase was used for diazinon and chlorpyrifos analysis by the proposed USAEME-IMS procedure. Accuracy of the procedure is checked by the added-found method.
Extraction Procedure
A centrifuge glass vial was filled with 10 mL aqueous sample up to the middle of the conic head of the vial. Appropriate volume of 0.5 μg mL−1 solution of OPPs was added to the vial by a microsyringe (Hamilton). A volume of 50 μL of organic solvent (chloroform) was injected into the water sample. This mixture was irradiated in an ultrasonic water bath, which operated at 37 kHz with an output power of 138 W for 3 min, at 25 ± 3 °C. Then, the emulsion was centrifuged at 3500 rpm for 5 min to separate the phases. After separation of the phases, 2 μL of the extraction solvent was collected with a microsyringe and injected into the IMS system for subsequent pesticides determinations.
Equipments
Detection and quantification of organophophorus pesticides were carried out using a laboratory designed ion mobility spectrometer with a 63Ni ionization source working in positive mode. A GC injection port was equipped with a heating element and a digital temperature controller was used for introducing the sample in the IMS. The carrier gas was passed through the port and carries the analyte vapor to the IMS cell. The drift length was 10 cm and the applied electric field was adjusted at 550 V cm−1. The shutter grid was made of two series of parallel wires biased to a potential, creating an orthogonal field relative to the drift field, to block ion passage to the drift tube. The grid potential was removed for a short period of time by the pulse generator, to admit an ion pulse to the drift region. In this work, the period of time was selected 120 μs. The IMS cell was held in a thermostated oven in which temperature was controlled within ±2 °C. The optimized experimental conditions for obtaining the ion mobility spectra of the compounds are listed in Table 1. Deionized water with a resistivity of at least 18.2 MΩ was produced by a M-UV-3+ Zolalan water purification system (Iran). An Elmasonic ultrasonic bath (Germany), which worked at a 37-kHz and 138-W power, was used for emulsification of the extraction organic solvent. The separation of emulsion was assisted by a centrifuge Heraeus (Labofuge 300).
Results and Discussion
Optimization of the Extraction Procedure
Selection of a suitable extraction solvent and its volume, sufficient centrifugation time and an appropriate ionic strength are the main parameters to be optimized in ultrasound assisted emulsification microextraction procedures (Ozcan et al. 2010; Wei et al. 2011; Sereshti et al. 2012; Moradi et al. 2013; Reboredo-Rodríguez et al. 2014; Vila et al. 2016). The optimization of these variables was performed using one-variable-at-a-time method. The ion mobility spectrometry peak height was used as the following signal to evaluate the influence of the investigated parameters on the extraction efficiency of OPPs.
Extraction Solvent
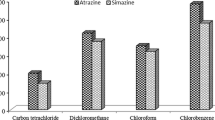
The selection of an appropriate extraction solvent is a key step for approaching to an efficient USAEME procedure. The extraction solvent should have low water solubility, high extraction capability towards target analytes, and also it should be compatible with IMS detection. Considering these criteria, dichloromethane, chloroform, and carbon tetrachloride were examined as extraction solvents. The experiments were carried out using 10 mL water solution containing 0.5 μg mL−1 of diazinon and chlorpyrifos pesticides. Preliminary experiments showed that the examined solvents can form emulsions after being irradiated by ultrasonic waves. It was observed that the extraction efficiency as a function of the extraction solvent varies as CHCl3 > CH2Cl2 > CCl4 (Fig. 1). Thus, chloroform was selected as the appropriate extraction solvent for following the preconcentration experiments of the analytes.
Variation of the USAEME efficiency of diazinon (gray columns) and chlorpyrifos (black columns) as a function of the type of extraction solvent. Experimental conditions: aqueous phase pH 7, extraction solvent volume 70 μL, time of ultrasonic irradiation 4 min, initial concentration of the pesticides 0.5 μg mL−1, centrifugation time 5 min
Extraction Solvent Volume
The volume of extraction solvent influences directly the enrichment factor of microextraction procedures. The enrichment factor can be enhanced by using low organic to aqueous phase ratio. However, this advantage can be compensated by further reduction in the extraction solvent due to the saturation of the organic phase by analytes.
In order to optimize the volume of chloroform, the extraction of the investigated OPPs (0.5 μg mL−1 each) from 10 mL of sample solutions by addition of extraction solvent in the range 30–80 μL was realized (Fig. 2). The results showed an enhancement in the extraction efficiency by increasing the solvent volume up to 50 μL. A further increase in the solvent volume reduced the extraction efficiency. Based on these results, a volume of 50 μL of chloroform was used for the following experiments.
Variation of the USAEME efficiency of diazinon (gray columns) and chlorpyrifos (black columns) as a function of the volume of extraction solvent (chloroform). Experimental conditions: aqueous phase pH 7, time of ultrasonic irradiation 4 min, initial concentration of the pesticides 0.5 μg mL−1, centrifugation time 5 min
Ionic Strength
In general, a salting-out effect of salt added to the aqueous phase improves the transfer of an organic analyte to the extraction solvent and decreases its solubility in the aqueous phase. In contrast an increase in salt concentration may diminish the extraction efficiency in USAEME procedures due to increase in viscosity and density of the aqueous phase which reduce the emulsification efficiency. In addition in USAEME procedures, ultrasonic irradiation becomes inefficient in high salt concentration solutions, due to its absorption and distribution in such media.
The effect of the ionic strength of the aqueous sample on the microextraction process has been evaluated by the addition of sodium chloride in the range 0–10 % w/v. The results revealed that this parameter does not alter significantly the extraction efficiency of the procedure. This may be a result of the contrary effects of the salt concentration in the aqueous phase on the extraction procedure, as described above.
Sonication Time
The influence of sonication time on the extraction recovery was investigated by varying the irradiation time of the mixture of extraction solvent/water solutions containing the analytes in the range 1–10 min. The variation of IMS area peak as a function of ultrasonic irradiation time is shown in Fig. 3. It was found that extraction recovery increases by sonication time in the range 1–3 min. This can be attributed to the formation of smaller size droplets by sonication time, resulting more efficient mass transfer of the analytes into the organic phase. An increase in the sonication time beyond 3 min did not affect the extraction efficiency. Based on these results, a time of 3 min was selected in order to be sure for sufficient irradiation time and an efficient extraction procedure.
Method Validation
Figure 4 shows the ion mobility spectrum of a water samples spiked separately with 400 and 600 μg L−1 of diazinon and chlorpyrifos, respectively. Water samples spiked with diazinon and chlorpyrifos were used to investigate the linearity, repeatability, limit of detection (LOD) and limit of quantification (LOQ) of the proposed method, under determined optimum experimental conditions (Table 2). The values of LOD and LOQ were calculated by using the equations 3Sy/xm and 10Sy/xm, respectively. In these relations, “Sy/x” is the average signal of three replicate blank experiments and “m” notifies the slope of the calibration curve (Fashi et al. 2015).
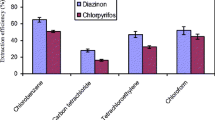
In order to assess repeatability, the peak height of five replicate analyses for 500 μg L−1 of the studied OPPs were used and expressed as percentage relative standard deviation (RSD%). It was found that intra-day and inter-day RSD% for the determination of the investigated OPPs were ≤7.3 % and 8.9 %., respectively. It was found that the calibration graphs were linear in the range 6.0–700 and 8.0–750 μg L−1 for diazinon and chlorpyrifos, respectively, with R 2 > 0.99. The limit of detection was determined as 2.1 and 3.1 μg L−1 for diazinon and chlorpyrifos. The enrichment factor (EF) was calculated by EF = Corg/C0 in which Corg and C0 are the concentration of the OPPs in the organic phase and their initial concentration respectively. This parameter was calculated to be 230 for diazinon and 310 for chlorpyrifos.
Analysis of the Studied OPPs in Rice Paddy Water and Rice Samples
The reliability and applicability of the proposed UASEME-IMS method were assessed by using it for the determination of diazinon and chlorpyrifos in rice paddy water and rice samples. The samples were collected from four agricultural sites in Mazandaran province (North Iran).
The rice paddy water samples were collected after 1, 10, 20, 30, 40, 50, and 60 days of the pesticide spraying. The selected rice paddies were sprayed on 15–20 June 2014. Five replicate samples (each 100 mL) from the depth of 10–15 cm of their basin water of the selected stations were collected. The samples taken from each station were mixed and the water samples were kept in a refrigerator to prevent the OPPs’ decomposition, before applying the preconcentration and determination steps. The analysis of the rice paddy water samples collected before spraying the organophosphorus pesticides ensured that the samples were free from diazinon and chlorpyrifos. The rice samples were collected from four different rice repositories, corresponding to the selected rice paddy sites. The selected rice samples were the product of the same sites and they were the production of just cropping rice paddies. From each repository, five samples (100 g each) were taken. Four 20 g portions of each sample were grounded and mixed with 25 mL of deionized water. These mixtures were magnetically stirred for 15 min. After centrifugation, the aqueous phase was used for diazinon and chlorpyrifos analysis by the proposed USAEME-IMS procedure. For instance, Fig. 5 shows the ion mobility spectrum of a rice paddy water sample before spraying and after 10 days of spraying.
The results of contamination of the selected rice paddy water samples with diazinon and chlorpyrifos after 1, 10, 20, 30, 40, 50, and 60 days are given in Table 3. A graphical representation of these values was drawn by plotting the variation of mean values of the determined pesticides as a function of the sampling interval (Fig. 6). These results showed that the amounts of diazinon and chlorpyrifos were decreased with time. However, they have persisted in rice paddy waters for 60 days, which can be attributed to their high persistency in the ambient environment (Bondarenko et al. 2004).
Variation of the mean concentration of diazinon (black triangle) and chlorpyrifos (black circle) of the rice paddy water samples taken from the selected stations as a function of the sampling time with respect to the OPP spraying time. The values are taken from Table 3
To investigate the diazinon and chlorpyrifos residues in the rice samples, on one hand, and verify the presence of a possible matrix effect, the rice samples were analyzed by applying the standard addition method (Table 4). This analysis revealed that the presented method is free from matrix effect. It was found that the average amounts of diazinon and chlorpyrifos residues in the rice samples were 8.5 (±3.9) and 15.5 (±4.2) μg kg−1. It is worth noting that, although the average residual amount of both diazinon and chlorpyrifos in the examined rice samples is lower than that reported by the Institute of Standard and Industrial Research of Iran (ISIRI 2011).
Comparison of the Presented Method with Some Other Related Reported Methods
The main characteristics of some of the methods established along with those found in this work for determination of diazinon and chlorpyrifos are given in Table 5. The linear range of USAEME-IMS is better than that of SPE-GC-MS and SPE-GC-ECD. This parameter is comparable for the presented method and that of UADLLME-SFO-GC-FID. Although the limit of detection for USAEME-IMS is close to that of SPE-GC-MS and SPE-GC-ECD, a better LOD is presented by the methods SBDLLME-GC-FID and UADLLME-SFO-GC-FID. These methods provide comparable limit of detection with the proposed method. The enrichment factor of USAEME-IMS is superior to that of UADLLME-SFO-GC-FID, and is inferior to the calculated LOD of more sophisticated method SBDLLME-GC-FID.
Conclusion
A method based on ultrasound-assisted emulsion liquid-liquid microextraction combined with ion mobility spectrometry (USAEME-IMS) for determination of organophosphorus pesticides diazinon and chlorpyrifos was developed. The presented method was very simple, rapid, inexpensive, and accurate. The relatively wide linear range for determination of the studied analytes was another advantage of the presented method. The proposed USAEME-IMS method was successfully used for monitoring the concentration variation of diazinon and chlorpyrifos in rice samples and water samples collected from rice paddies located in Mazandaran province (North Iran).
References
Aguilar M, Cortina JL (2010) Solvent extraction and liquid membranes. CRC Press, Florida
Ahmadi K, Abdollahzadeh Y, Asadollahzadeh M, Hemmati A, Tavakoli H, Torkaman R (2015) Chemometric assisted ultrasound leaching-solid phase extraction followed by dispersive-solidification liquid–liquid microextraction for determination of organophosphorus pesticides in soil samples. Talanta 137:167–173
Allafchian AR, Majidian Z, Ielbeigi V, Tabrizchi M (2015) A novel method for the determination of three volatile organic compounds in exhaled breath by solid-phase microextraction–ion mobility spectrometry. Anal Bioanal Chem
Arjmandi R, Tavakol M, Shayeghi M (2010) Determination of organophosphorus insecticide residues in the rice paddies. Int J Environ Sci Technol 7:175–182
Bondarenko GJ, Haver DL, Kabashima JN (2004) Persistence of selected organophosphate and carbamate insecticides in water from a coastal watershed. Environ Toxicol Chem 23:2649–2654
Cohen G, Rudnik DD, Laloush M, Yakir D, Karpas Z (2015) A novel method for determination of histamine in tuna fish by ion mobility spectrometry. Food Anal Methods 8:2376–2382
Eiceman GA, Karpas Z (2005) Ion mobility spectrometry. Taylor and Francis, London
Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS (2011) Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 119:1182–1188
Farajzadeh MA, Afshar Mogaddam MR (2012) Air-assisted liquid–liquid microextraction method as a novel microextraction technique; application in extraction and preconcentration of phthalate esters in aqueous sample followed by gas chromatography–flame ionization detection. Anal Chim Acta 728:31–38
Farajzadeh MA, Khorram P, Alizadeh Nabil AA (2016) Development of a green liquid–liquid microextraction method using a solid disperser performed in a narrow-bore tube for trace analysis of some organophosphorus pesticides in fruit juices. J Food Compos Anal. doi:10.1016/j.jfca.2015.04.012
Fashi A, Yaftian MR, Zamani A (2015) Determination of melamine in dairy products using electromembrane–LPME followed by HPLC. Food Chem 188:92–98
Gallegos J, Garrido-Delgado R, Arce L, Medina LM (2015) Volatile metabolites of goat cheeses determined by ion mobility spectrometry. Potential applications in quality control. Food Anal Methods 8:1699–1709
González-Alzaga B, Lacasaña M, Aguilar-Garduño C, Rodríguez-Barranco M, Ballester F, Rebagliato M, Hernández AF (2014) A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett 230:104–121
Hernández AF, Parrón T, Tsatsakis AM, Requena M, Alarcón R, López-Guarnido O (2013) Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology 307:136–145
Holopainen S, Luukkonen V, Nousiainen M, Sillanpää M (2013) Determination of chlorophenols in water by headspace solid phase microextraction ion mobility spectrometry (HS-SPME-IMS). Talanta 114:176–182
Hu C, He M, Chen B, Hu B (2013) A sol–gel polydimethylsiloxane/polythiophene coated stir bar sorptive extraction combined with gas chromatography-flame photometric detection for the determination of organophosphorus pesticides in environmental water samples. J Chromatogr A 1275:25–31
ISIRI, Institute of Standard and Industrial Research of Iran (2011) Pesticides—maximum residue limit of pesticides—cereals. Iranian National Standard No. 13120
Jafari MT, Saraji M, Yousefi S (2012) Negative electrospray ionization ion mobility spectrometry combined with microextraction in packed syringe for direct analysis of phenoxyacid herbicides in environmental waters. J Chromatogr A 1249:41–47
Jafari MT, Saraji M, Sherafatmand H (2014) Polypyrrole/montmorillonite nanocomposite as a new solid phase microextraction fiber combined with gas chromatography–corona discharge ion mobility spectrometry for the simultaneous determination of diazinon and fenthion organophosphorus pesticides. Anal Chim Acta 814:69–78
Jeleń HH, Majcher M, Dziadas M (2012) Microextraction techniques in the analysis of food flavor compounds: a review. Anal Chim Acta 738:13–26
Kalhor H, Hashemipour S, Yaftian MR, Shahdousti P (2015) Determination of carbamazepine in formulation samples using dispersive liquid–liquid microextraction method followed by ion mobility spectrometry. Int J Ion Mobil Spectrom
Karpas Z (2013) Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res Int 54:1146–1151
Kokosa JM (2013) Advances in solvent-microextraction techniques. Trends Anal Chem 43:2–13
Lamers M, Anyusheva M, La N, Nguyen VV, Streck T (2011) Pesticide pollution in surface- and groundwater by paddy rice cultivation: a case study from northern Vietnam. Clean: Soil, Air, Water 39:356–361
London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, Harari R, Kootbodien T, Kromhout H, Little F, Meijster T, Moretto A, Rohlman DS, Stallones L (2012) Neurobehavioral and neurodevelopmental effects of pesticide exposures. NeuroToxicology 33:887–896
Ma G, Chen L (2014) Determination of chlorpyrifos in rice based on magnetic molecularly imprinted polymers coupled with high-performance liquid chromatography. Food Anal Methods 7:377–388
Moradi M, Yamini Y, Seidi S, Ghambarian M, Esrafili A (2013) Ultrasound-assisted emulsification microextraction using low density solvent for analysis of toxic nitrophenols in natural waters. Int J Environ Anal Chem 93:199–212
Ozcan S, Tor A, Aydin ME (2010) Determination of polycyclic aromatic hydrocarbons in waters by ultrasound-assisted emulsification-microextraction and gas chromatography–mass spectrometry. Anal Chim Acta 665:193–199
Pengyan LIU, Qingxue LIU, Yusong MA, Jinwei LIU, Xuan JIA (2006) Analysis of pesticide multiresidues in rice by gas chromatography–mass spectrometry coupled with solid phase extraction. Chin J Chromatogr 24:228–234
Ramos L (2012) Critical overview of selected contemporary sample preparation techniques. J Chromatogr A 1221:84–98
Reboredo-Rodríguez P, Rey-Salgueiro L, Regueiro J, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2014) Ultrasound-assisted emulsification–microextraction for the determination of phenolic compounds in olive oils. Food Chem 150:128–136
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R (2008) Ultrasound-assisted emulsification–microextraction of emergent contaminants and pesticides in environmental waters. J Chromatogr A 1190:27–38
Samadi S, Sereshti H, Assadi Y (2012) Ultra-preconcentration and determination of thirteen organophosphorus pesticides in water samples using solid-phase extraction followed by dispersive liquid–liquid microextraction and gas chromatography with flame photometric detection. J Chromatogr A 1219:61–65
Saraji M, Jafari MT, Sherafatmand H (2015) Sol–gel/nanoclay composite as a solid-phase microextraction fiber coating for the determination of organophosphorus pesticides in water samples. Anal Bioanal Chem 407:1241–1252
Sereshti H, Entezari Heravi Y, Samadi S (2012) Optimized ultrasound-assisted emulsification microextraction for simultaneous trace multielement determination of heavy metals in real water samples by ICP-OES. Talanta 97:235–241
Sergio A, Garrigues S, De la Guardia M (2008) Green analytical chemistry. Trends Anal Chem 27:497–511
Sharafi K, Fattahi N, Mahvi AH, Pirsaheb M, Azizzadeh N, Noori M (2015) Trace analysis of some organophosphorus pesticides in rice samples using ultrasound-assisted dispersive liquid–liquid microextraction and high-performance liquid chromatography. J Sep Sci. doi:10.1002/jssc.201401209
Simpson NJ (2000) Solid-phase extraction: principles, techniques, and applications. CRC Press, Florida, p 2000
Spietelun A, Marcinkowski Ł, de la Guardia M, Namieśnik J (2014) Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta 119:34–45
Vila M, Lamas JP, Garcia-Jares C, Dagnac T, Llompart M (2016) Ultrasound-assisted emulsification microextraction followed by gas chromatography–mass spectrometry and gas chromatography–tandem mass spectrometry for the analysis of UV filters in water. Microchem J 124:530–539
Wei SY, Leong MI, Li Y, Huang SD (2011) Development of liquid phase microextraction based on manual shaking and ultrasound-assisted emulsification method for analysis of organochlorine pesticides in aqueous samples. J Chromatogr A 1218:9142–9148
Wu L, Song Y, Xu X, Li N, Shao M, Zhang H, Yu A, Yu C, Ma Q, Lu C, Wang Z (2014) Medium-assisted non-polar solvent dynamic microwave extraction for determination of organophosphorus pesticides in cereals using gas chromatography–mass spectrometry. Food Chem 162:253–260
Zainudin BH, Salleh S, Mohamed R, Yap KC, Muhamad H (2015) Development, validation and determination of multiclass pesticide residues in cocoa beans using gas chromatography and liquid chromatography tandem mass spectrometry. Food Chem 172:585–595
Zhang L, Wang Y, Sun C, Yang S, He H (2013) Simultaneous determination of organochlorine, organophosphorus, and pyrethroid pesticides in pee pollens by solid-phase extraction cleanup followed by gas chromatography using electron-capture detector. Food Anal Methods 6:1508–1514
Zhao WJ, Sun XK, Deng XN, Huang L, Yang MM, Zhou ZM (2011) Cloud point extraction coupled with ultrasonic-assisted back-extraction for the determination of organophosphorus pesticides in concentrated fruit juice by gas chromatography with flame photometric detection. Food Chem 127:683–688
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hamideh Kalhor declares that she has no conflict of interest. Siamak Hashemipour declares that he has no conflict of interest. Mohammad Reza Yaftian declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Kalhor, H., Hashemipour, S. & Yaftian, M.R. Ultrasound-Assisted Emulsification-Microextraction/Ion Mobility Spectrometry Combination: Application for Analysis of Organophosphorus Pesticide Residues in Rice Samples. Food Anal. Methods 9, 3006–3014 (2016). https://doi.org/10.1007/s12161-016-0492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0492-8