Abstract
Selectivity of solid-phase extraction (SPE) was combined with the concentration power of dispersive liquid–liquid microextraction (DLLME) to obtain a sensitive, low solvent consumption method for high-performance liquid chromatography determination of diazinon and chlorpyrifos in rice. In this method, rice samples were extracted by ultrasound-assisted extraction followed by SPE. Then, the SPE eluent was used as a disperser solvent in the next dispersive liquid-liquid microextraction step for further purification and enrichment of diazinon and chlorpyrifos. Under the optimal conditions, the linear range was from 5.0 to 250 μg kg−1 for diazinon and from 2.5 to 250 μg kg−1 for chlorpyrifos. Limits of detection of diazinon and chlorpyrifos were 1.5 and 0.7 μg kg−1, respectively. Limits of quantitation of diazinon and chlorpyrifos were 5.5 and 3.0 μg kg−1, respectively. The precisions and recoveries also were investigated by spiking 10 μg kg−1 concentration in rice. The recoveries obtained were over 90 % with relative standard deviation (RSD%) below 9.0 %. The new approach was utilized to successfully detect trace amounts of diazinon and chlorpyrifos in different Iranian rice samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is the third most popular cereal product consumed worldwide. However, in the last few decades, there is a major concern regarding the possible contamination of foods/feeds with pesticide residues (European community Regulation 396/2005). Pesticides in rice are used to control weeds and pests during cropping stages and for pest management post harvest. Programs of weed control in rice are principally established on the basis of the degree of weed in festation and the conditions of crop seeding (Ferrero et al. 2008). During the wide use of diazinon and chlorpyrifos as organophosphorus pesticides (OPPs) in the Iranian rice, they transfer to the rice to form the OPPs residues. As these OPPs residues have been proved to be acutely toxic to humans and organism (Zhao et al. 2007), it is important to carry out the monitoring of these OPPs residues for the possible risks to human health. However, the OPPs residues always remain at trace level, which are too low to be detected for the common gas chromatography (GC) or high-performance liquid chromatography (HPLC) techniques. In order to ensure the high quality of rice, analytical methodologies for the detection and quantitation of pesticide residues should be sensitive enough to determine residue at very low concentrations. The complex matrix of agricultural products affects analysis precision. Therefore, it is necessary to apply appropriate sample pretreatment techniques for clean-up and extraction of pesticide residues from rice. Solid-phase extraction (SPE) (Zhang et al. 2006), matrix solid-phase dispersion (MSPD) (Dorea and Sobrinho 2004; Tsochatzis et al. 2010), supercritical fluid extraction (SFE) (Aguilera et al. 2005; Kaihara et al. 2002), and solvent extraction (Lee et al. 2009; Pengyan et al. 2006b; Valverde et al. 2009) have been used to achieve this purpose. Although many of these techniques are suitable and effective, but some need lots of time, some are expensive and some require large amounts of organic solvents. Also, some articles review different sample pretreatment methods for pesticide residue analysis in rice and cereals and derivatives (Gonzalez-Curbelo et al. 2012; Pareja et al. 2011).
Recently, a new liquid-liquid microextraction method termed dispersive liquid-liquid microextraction (DLLME) was reported by Rezaee et al. (Rezaee et al. 2006). DLLME has been applied for the analysis of a variety of trace organic pollutants and metal ions in environmental samples (Bidari et al. 2007; Farajzadeh et al. 2007; Rezaee et al. 2009, 2010; Shokoufi et al. 2007; Yamini et al. 2010). The objective of the sample preparation is often not only to isolate the target analytes from the samples and concentrate the analytes, but also simultaneously to reduce or even eliminate the interferences originally present in the sample and to facilitate their determinations at low levels. The main disadvantage of the DLLME is that it is not a selective extraction method. On the other hand, the interferences from matrix co-extractives are often present, especially for the determination of trace analytes in a complex matrix sample. This is the main reason that the most reported applications of DLLME have been focused on simple water samples. Therefore, the exploration of the potential applications of the DLLME technique in more complex matrix samples is desirable. SPE is widely used as a sample clean-up and concentration technique in sample preparations. Assadi and co-workers have reported the combination of SPE with DLLME for the selective determination of chlorophenols in aqueous samples with various matrices (Fattahi et al. 2007). In 2011, combination of SPE with DLLME was used for determination of organophosphorus pesticides in different water samples (Henriques Alves et al. 2011).
The application of SPE-DLLME to solid samples had received minor attention. However, for solid samples such as rice SPE-DLLME cannot be used. The main disadvantage of the SPE-DLLME in rice sample is that it is not a suitable extraction technique and also fails because phases do not separate even after centrifugation (because of dirty extracts). Therefore, in the analysis of this sample a first step is necessary before SPE-DLLME. (Fontana et al. 2010; Liu et al. 2009). Ultrasound-assisted extraction (UAE) is considered a good alternative for organic compound extraction from different matrices which provides a more efficient contact between the solid and solvent. One of the advantages of such a combination is that it can be used for complex matrix samples at low levels concentrations. Therefore, the combination of UAS and SPE and DLLME as a novel sample pretreatment method leads to high enrichment factor and can be used successfully in solid matrices for trace analysis. The objective of our study was the development of a sensitive and cost-effective method for the simultaneous determination of diazinon and chlorpyrifos in rice. The developed method was subsequently applied for the assessment of pesticide levels in rice samples from local markets in Mazandaran, Iran.
Experimental
Chemicals and Reagents
Organophosphorus pesticides, diazinon and chlorpyrifos, were purchased from Polyscience (Niles, USA). 0.0100 g of each analyte (OPPs) was dissolved in 10.0 mL methanol to prepare a standard solution of 1000 mg L−1. A fresh standard solution of OPPs (10.0 and 1.0 mg L−1) was prepared in methanol on the first day of every week and stored at 4 °C. Carbon tetrachloride, chloroform, chlorobenzene, carbon tetrachloroethylene, acetone, acetonitrile, methanol, ethanol, and sodium chloride were obtained from Merck (Darmstadt, Germany). The water used was purified on a Nanopure ultra pure water purification system (Nanopure, USA). Since the province of Mazandaran is the major rice producer in Iran, it was considered as the source of real samples, and a number of three packs of Mazandaran rice were purchased from a local market in Iran.
HPLC System
An Agilent 1200 series HPLC system including a quaternary pump and a UV detector were used for separation and determination of the analytes. The separation was performed on Zorbax Eclipse XDB-C18 (250 mm × 4.6 mm ID, 5 μm) column. Water and acetonitrile (35:65) were used as mobile phase in isocratic elution mode. The chromatographic data were collected and recorded using ChemStation software. The direct sample introduction was carried out using a Rheodyne manual injector (Rohnert Park, CA, USA) with a 20 μL loop. Column temperature was kept constant at 25 °C using a thermostatted column compartment. The flow rate was 1.5 mL min−1 and detection was performed at 235 nm. The total running time of the chromatographic system was 13 min (Katsumata et al. 2008; Soodi et al. 2012).
UAE-SPE-DLLME Procedure
One gram of the milled rice were weighted in a 25 mL centrifuge tube and 5.0 mL of acetonitrile was added. Ultrasound assisted extraction was carried out for 20 min using a 40 kHz and 0.138 kW ultrasonic water bath with temperature control (Tecno-Gaz SpA, Italy). Samples were centrifuged for 4 min at 5000 rpm. The extracts were filtered on a filter paper (Whatman No 44) and then the supernatant solution was collected and brought up to 10 mL with deionized water. The final extractant (10 mL) was passed through a C18 sorbent (3 mL syringe barrel, waters, USA), previously activated with 2 mL acetone followed by 2 mL water. After sample loading at flow rate of about 6.7 mL min−1 with the aid of a vacuum pump (Rotavac, Heidolph, Germany), the sorbent was dried. Pesticides were eluted with 1.5 mL acetonitrile and the eluent was collected into the test tube. The elution solvent was used as disperser solvent in the subsequent DLLME procedures. Five-milliliter aqueous solution was placed in a 10 mL screw cap glass test tube with conical bottomed. 1.5 mL acetonitrile (disperser solvent) containing 55.0 μL chlorobenzene (extraction solvent) was injected into the aqueous solution, using a 5.0 mL syringe (gas tight, Hamilton, Reno, NV, USA). A cloudy solution, resulting from the dispersion of the fine chlorobenzene droplets in the aqueous solution was formed in the test tube. In this step, pesticides extracted into fine chlorobenzene droplets in a few seconds. The mixture was then centrifuged for 3 min at 5000 rpm. After this procedure, the dispersed fine chlorobenzene droplets were sedimented at the bottom of the conical test tube (about 25 μL). The sedimented phase was completely transferred to another test tube with conical bottom using 50 μL HPLC syringe. After evaporation of the solvent in a water bath, the residue was dissolved in 30 μL HPLC grade methanol and it was injected into the separation system.
Results and Discussion
SPE-DLLME combined with HPLC-UV was developed for determination of diazinon and chlorpyrifos in rice samples. In order to obtain a high recovery and enrichment factor, effects of different parameters were optimized. Optimization of the variables was performed using one variable at a time method. Compared with the conventional SPE procedure, the SPE-DLLME-HPLC method provided higher purification ability and selectivity and higher enrichment factor.
Ultrasound-Assisted Extraction
In order to enhance recovery and shorten extraction time, we used an ultrasonic-assisted extraction. The optimization of the ultrasound-assisted extraction of pesticides from milled rice was developed with free of pesticide-samples. For this purpose, extraction of spiked samples (100.0 μg kg−1 fortification level) was carried out with different extraction solvents such as acetonitrile, methanol, and acetone. Different amounts of samples (between 1.0 and 5.0 g) were sonicated with the solvents between 5 and 30 min. Results showed that the best recoveries were achieved using acetonitrile as an extracting solvent. Among the different mixtures tested, the extraction of 1.0 g of the milled rice for 20 min with 5 mL of acetonitrile was enough to provide a good extraction of pesticides. Different amounts of water were used for dilution the extracts. Extractants were collected and brought to 10, 15, 20, and 30 mL with deionized water and the best recoveries were obtained using 10 mL solution. This final test portion of 10 mL was passed through the C18 cartridges using the SPE procedure.
Effect of Type and Volume of the Extraction Solvent
In order to achieve the proper formation of the cloudy solution, the suitable disperser and extraction solvents must be selected. The main characteristic that an extraction solvent suitable for DLLME must have is low water solubility and high extraction capability of the analytes. In addition, they need to have higher density than water to facilitate the collection of the extract at the conical bottom of a test tube. Bearing this in mind, carbon tetrachloride (CCl4), carbon tetrachloroethylene (C2Cl4), chlorobenzene (C6H5Cl), and chloroform (CHCl3) were examined as extraction solvents. A series of sample solutions were tested using 1.5 mL methanol, containing different volumes of the extraction solvents to achieve about 25 μL volume of the sedimented phase. Thereby, 200.0, 55.0, 52.0, and 57.0 μL of CHCl3, C6H5Cl, C2Cl4, and CCl4 were used, respectively. The results (Fig. 1) indicate that the C6H5Cl has the highest extraction efficiency in comparison with the other tested solvents. It is probably because of higher solubility of pesticides in C6H5Cl in comparison with the other tested solvents. Therefore, C6H5Cl was selected as the main extraction solvent.
To examine the effect of extraction solvent volume, different amounts of C6H5Cl (35.0, 55.0, 75.0, 95.0, and 115.0 μL) were evaluated. By increasing the volume of C6H5Cl from 35.0 to 55.0 μL, the extraction efficiency of pesticides increases, while there were decrease for both analytes afterwards (Fig. 2). The dilution effect is responsible for these drops. Therefore, 55.0 μL of C6H5Cl was selected as the optimum volume of the extraction solvent.
Effect of Type and Volume of Disperser Solvent
The elution solvent in the SPE step is used as the disperser solvent in DLLME. The disperser solvent should be miscible with both extraction solvent and aqueous phase. For this purpose, different solvents such as acetonitrile, acetone, ethanol, and methanol were examined. A series of sample solutions were tested using 1.5 mL of each disperser solvent, containing 55.0 μL volume of C6H5Cl (as extraction solvent). The results (Fig. 3) indicated that acetonitrile has the highest extraction efficiency in comparison with the other tested solvents. Thus, acetonitrile was chosen as the disperser or eluent solvent for subsequent experiments.
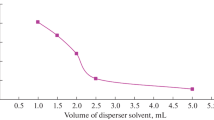
The variation of acetonitrile volume (as disperser solvent) causes changes in the volume of the settled phase. To avoid this problem and also achieving a constant volume of the settled phase, the volume of acetonitrile and C6H5Cl were changed simultaneously. The experimental conditions were fixed and included the use of different acetonitrile volumes (0.5, 1.0, 1.5, and 2.0 mL), each of which contained 37.0, 48.0, 55.0, and 66.0 μL of C6H5Cl, respectively. At this step, the volume of settled phase was relatively constant (25 μL). It was obvious from Fig. 4, that 1.5 mL of acetonitrile has the highest extraction efficiency than that of the others. Therefore, 1.5 mL was selected as the optimum volume of acetonitrile.
Effect of the Flow Rate of the Sample Solution
Flow rate of sample loading is a very important parameter in this kind of study. This is because the flow rate not only affects the retention of analytes on the sorbent but also controls the time of analysis. The flow rate influence of the sample solutions from the solid-phase cartridge on the pesticides recovery was investigated in the range of 0.65–8.6 mL min−1. It was found that in the range of 0.65–6.7 mL min−1, the pesticides recovery by the cartridge was not affected considerably by the sample solution flow rate (Fig. 5). According to the results, 6.7 mL min−1 was used as the best sample flow rate.
Effect of Salt Addition
The influence of ionic strength was evaluated at 0–8 % (w/v) NaCl levels while other parameters were kept constant. The experimental results show that salt addition had no significant effect on the extraction efficiency of the analytes. This is probably due to the opposite effects of the addition of salt. One is to increase the volume of the sedimented phase and dilution effect, this reduces the extraction efficiency; another is the salting out effect, that increases the extraction efficiency. It is to be noted that by increasing the salt concentration, the volume of the sedimented phase increased, due to the decrease in the solubility of the extraction solvent. Therefore, all the following experiments were carried out without addition of salt.
Analytical Performance
The figures of merit of the proposed method are shown in Table 1. The optimum experimental conditions were used to assess the applicability of the proposed method for quantitative determination of the target analytes by HPLC-UV. A series of experiments were designed for obtaining linear ranges, precision, detection limits, and other characteristics of the method. The calibration curves showed a satisfactory linearity within the concentration range: 5.0–250.0 μg kg−1 for diazinon and 2.5.0–250.0 μg kg−1 for chlorpyrifos and the coefficient of determination (r2) 0.9984 for diazinon and 0.9993 for chlorpyrifos. The intra-day precisions, obtained by performing five replicates at the three concentration levels (10, 25, and 50 μg kg−1) of the analytes were shown in Table 2. Based on signal-to-noise ratio (S/N) of 3, the limit of detections (LODs) was 1.5 μg kg−1 for diazinon and 0.7 μg kg−1 for chlorpyrifos. Based on signal-to-noise ratio (S/N) of 10, the limit of quantitations (LOQs) was 5.5 μg kg−1 for diazinon and 3.0 μg kg−1 for chlorpyrifos. It is remarkable that the studied pesticides can be determined at very low concentrations (5 μg kg−1) by the proposed method, clearly below the minimum MRL stated by the EU legislation (10 μg kg−1).
Table 3 compare the proposed method with other extraction methods for the determination of the target analytes in rice samples. The quantitative results of the proposed method are better than of solid-phase extraction (SPE) (Pengyan et al. 2006a), supercritical fluid extraction (SFE) (Skopec et al. 1993), solvent extraction (Uddin et al. 2011), and accelerated solvent extraction (Cho et al. 2007) methods. The quantitative results of the proposed method are better with quick, easy, cheap, effective, rugged, and safe method (QuEChERS) (Grande-Martinez et al. 2016) without using very sensitive detector MS/MS. The comparison of extraction time of the proposed method with supercritical fluid extraction (Skopec et al. 1993) and QuEChERS (Herrmann and Poulsen 2015) for the extraction of the target analytes indicates that this novel method needs less time compared with them. In comparison with QuEChERS method (Grande-Martinez et al. 2016) for the determination of the target analytes in rice, the evaporation of the final extraction phase (25 μL) in the proposed method is easier compare with QuEChERS method which the final extraction phase is 3 mL. This procedure may cause the loss of the analytes and is time-consuming. Finally, the proposed method has great potential to determine the selected analytes at trace levels in rice samples.
To evaluate applicability of the proposed method in rice samples, three different rice samples (Tarome Hashemi, Neda, and Khazar rice) were extracted (three replicate) using the UAE-SPE-DLLME. In Tarome Hashemi and Khazar rice samples, diazinon and chlorpyrifos were not detected. However, in Neda rice 2.5 μg kg−1 of chlorpyrifos was detected and diazinon was not detected. In order to assess matrix effect, the rice samples were spiked with the standards of the pesticides at the concentration of 10.0 μg kg−1, and three replicate experiments with the whole analysis process were performed and the results are given in Table 4. As can be seen, the relative recoveries ranged from 90 to 95 %, which indicated that the method was reliable and could be used for the determination of trace pesticides in rice samples. Figure 6 shows the typical chromatograms of the extracted pesticides from Neda rice sample before and after spiking with 10.0 μg kg−1 of pesticides. As illustrated, the chromatograms confirm the presence of chlorpyrifos in Neda rice samples.
Conclusion
UAE-SPE-DLLME provides an efficient and selective methodology for the determination of diazinon and chlorpyrifos in rice samples. The method was successfully applied to determination these pesticides in rice samples; satisfied recoveries and reproducibility of the method were obtained. To our knowledge, the proposed method is the first application of a UAE-SPE-DLLME procedure to the simultaneous determination of diazinon and chlorpyrifos in rice samples. The satisfactory results obtained prove that this method could be a suitable alternative to previously reported methods.
References
Aguilera A, Rodriguez M, Brotons M, Boulaid M, Valverde A (2005) Evaluation of supercritical fluid extraction/aminopropyl solid-phase “in-line” cleanup for analysis of pesticide residues in rice. J Agric Food Chem 53:9374–9382
Bidari A, Zeini Jahromi E, Assadi Y, Milani Hosseini MR (2007) Monitoring of selenium in water samples using dispersive liquid–liquid microextraction followed by iridium-modified tube graphite furnace atomic absorption spectrometry. Microchem J 87:6–12
Cho SK, Abd El-Aty AM, Park YS, Choi JH, Khay S, Kang CK, Park BJ, Kim SJ, Jae-Han Shim JH (2007) A multiresidue method for the analysis of pesticide residues in polished rice (Oryza sativa L.) using accelerated solvent extraction and gas chromatography and confirmation by mass spectrometry. Biomed Chromatogr 21:602–609
Dorea HS, Sobrinho LL (2004) Analysis of pesticide residues in rice using matrix solid-phase dispersion. J Braz Chem Soc 15:690–694
European community Regulation 396/2005, Brussels, Belgium. http://ec.europa.eu/food/plant/protection/pesticides/database-pesticide-en.htm. Accessed 5 June (2009).
Farajzadeh MA, Bahram M, Jonsson JA (2007) Dispersive liquid-liquid microextraction followed by high-performance liquid chromatography-diode array detection as an efficient and sensitive technique for determination of antioxidants. Anal Chim Acta 591:69–79
Fattahi N, Samadi S, Assadi Y, Milani Hosseini MR (2007) Solid-phase extraction combined with dispersive liquid-liquid microextraction-ultra preconcentration of chlorophenols in aqueous samples. J Chromatogr A 1169:63–69
Ferrero A, Tinarelli A, Capri E, Karpouzas DG (eds) (2008) Pesticide risk assessment in Rice paddies: theory and practice. Elsevier, Amsterdam, p. 1
Fontana AR, Lana NB, Martinez LD, Altamirano JC (2010) Ultrasound-assisted leaching-dispersive solid-phase extraction followed by liquid–liquid microextraction for the determination of polybrominated diphenyl ethers in sediment samples by gas chromatography–tandem mass spectrometry. Talanta 82:359–366
Gonzalez-Curbelo MA, Herrera-Herrera AV, Ravelo-Perez LM, Hernandez-Borges J (2012) Sample-preparation methods for pesticide-residue analysis in cereals and derivatives. Trends Anal Chem 38:32–51
Grande-Martínez A, Arrebola-Liebanas FJ, Martinez-Vidal JL, Hernandez-Torres ME, Garrido-Frenich A (2016) Optimization and validation of a multiresidue pesticide method in rice and wheat flour by modified QuEChERS and GC–MS/MS. Food Anal Methods 9:548–563
Henriques Alves AC, Boavida Gonçalves MMP, Serrano Bernardo MM, Mendes BS (2011) Determination of organophosphorous pesticides in the ppq range using a simple solid-phase extraction method combined with dispersive liquid–liquid microextraction. J Sep Sci 34:2475–2481
Herrmann SS, Poulsen ME (2015) Clean-up of cereal extracts for gas chromatography–tandem quadrupole mass spectrometry pesticide residues analysis using primary secondary amine and C18. J Chromatogr A 1423:47–53
Kaihara A, Yoshii K, Tsumura Y, Ishimitsu S, Tonogai Y (2002) Multi-residue analysis of 18 pesticides in fresh fruits, vegetables and rice by supercritical fluid extraction and liquid chromatography-electrospray ionization mass spectrometry. J Health Sci 48:173–178
Katsumata H, Matsumoto T, Kaneco S, Suzuki T, Ohta K (2008) Preconcentration of diazinon using multiwalled carbon nanotubes as solid-phase extraction adsorbents. Microchem J 88:82–86
Lee SJ, Park HJ, Kim W, Jin JS, El-Aty AMA, Shim JH, Shin SC (2009) Multiresidue analysis of 47 pesticides in cooked wheat flour and polished rice by liquid chromatography with tandem mass spectrometry. Biomed Chromatogr 23:434–442
Liu X, Li J, Zhao Z, Zhang W, Lin K, Huang C, Wang X (2009) Solid-phase extraction combined with dispersive liquid–liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices. J Chromatogr A 1216:2220–2226
Pareja L, Fernandez-Alba AR, Cesio V, Heinzen H (2011) Analytical methods for pesticide residues in rice. Trends Anal Chem 30:270–291
Pengyan L, Qingxue L, Yusong M, Jinwei L, Xuan J (2006a) Analysis of pesticide multiresidues in rice by gas chromatography-mass spectrometry coupled with solid phase extraction. Chin J Chromatogr 24:228–234
Pengyan L, Qingxue L, Yusong M, Jinwei L, Xuan J, Chin J (2006b) Analysis of pesticide multiresidues in rice by gas chromatography-mass spectrometry coupled with solid phase extraction. J Chromatogr A 24:228–238
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 1116:–9
Rezaee M, Yamini Y, Shariati S, Esrafili A, Shamsipur M (2009) Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography-UV detection as a very simple, rapid and sensitive method for the determination of bisphenol A in water samples. J Chromatogr A 1216:1511–1514
Rezaee M, Yamini Y, Faraji M (2010) Evolution of dispersive liquid-liquid microextraction. J Chromatogr A 1217:2342–2357
Shokoufi N, Shemirani F, Assadi Y (2007) Fiber optic-linear array detection spectrophotometry in combination with dispersive liquid–liquid microextraction for simultaneous preconcentration and determination of palladium and cobalt. Anal Chim Acta 597:349–356
Skopec ZV, Clark R, Harvey PMA, Wells RJ (1993) Analysis of organophosphorus pesticides in rice by supercritical fluid extraction and quantitation using an atomic emission detector. J Chromatogr Sci 31:445–449
Soodi M, Garshasbi A, Eskandari S (2012) Development a HPLC method for simultaneous determination of Azinphose methyl, Diazinon, Phosalone and Chlorpyrifos residues in fruit. J Pharm Health Sci 4:79–87
Tsochatzis ED, Menkissoglu-Spiroudi U, Karpouzas DG, Tzimou-Tsitouridou R (2010) A multi-residue method for pesticide residue analysis in rice grains using matrix solid-phase dispersion extraction and high-performance liquid chromatography-diode array detection. Anal Bioanal Chem 397:2181–2190
Uddin R, Iqbal S, Farhanullah Khan M, Parveen Z, Ahmed M, Abbas M (2011) Determination of pesticide residues in rice grain by solvent extraction, column cleanup, and gas chromatography-electron capture detection. Bull Environ Contam Toxicol 86:83–89
Valverde A, Aguilera A, Rodriguez M, Brotons M (2009) Evaluation of a multiresidue method for pesticides in cereals using supercritical fluid extraction and gas chromatographic detection. J Environ Sci Health Part B 44:204–213
Yamini Y, Rezaee M, Khanchi A, Faraji M, Saleh A (2010) Dispersive liquid–liquid microextraction based on the solidification of floating organic drop followed by inductively coupled plasma-optical emission spectrometry as a fast technique for the simultaneous determination of heavy metals. J Chromatogr A 1217:2358–2364
Zhang WG, Chu XG, Cai HX, An J, Li CJ (2006) Simultaneous determination of 109 pesticides in unpolished rice by a combination of gel permeation chromatography and Florisil column purification, and gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 20:609–617
Zhao EC, Zhao WT, Han LJ, Jiang SR, Zhou ZQ (2007) Application of dispersive liquid–liquid microextraction for the analysis of organophosphorus pesticides in watermelon and cucumber. J Chromatogr A 1175:137–140
Acknowledgment
Financial support from Yadegar -e- Imam Khomeini (RAH) Shahre Rey Branch, Islamic Azad University, Tehran, Iran for the support during the period of this research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There is no funding for this study.
Conflict of Interest
No conflict exists; author Faezeh khalilian declares that she has no conflict of interest. Author Mohammad Rezaee declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
No humans are involved in this study.
Rights and permissions
About this article
Cite this article
Khalilian, F., Rezaee, M. Ultrasound-Assisted Extraction Followed by Solid-Phase Extraction Followed by Dispersive Liquid–Liquid Microextraction for the Sensitive Determination of Diazinon and Chlorpyrifos in Rice. Food Anal. Methods 10, 885–891 (2017). https://doi.org/10.1007/s12161-016-0653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0653-9