Abstract
A method for routine determination of the residues of nine organochlorine pesticides (OCPs), ten organophosphorus pesticides (OPPs), and seven pyrethroid pesticides (PPs) in bee pollens was developed. Bee pollen samples were extracted by petroleum ether followed by solid-phase extraction cleaning and detected by gas chromatography–micro electron capture detection. Range of detection limits are 0.3–3.3 μg/kg for OCPs, 1.0–19.1 μg/kg for PPs and 1.1–19.7 μg/kg for OPPs. Recoveries of OCPs, OPPs, and PPs were in the range of 88.9–122.7 %, 86.8–123.1 %, and 90.8–118.7 %, respectively. The method was applied successfully to analyze real bee pollen samples. The results show a low level of contamination caused by pesticide residues indicating safe supply of bee pollen for consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bee products have been contributing to health protection of people for thousands of years. Bee pollens are collected from blossom and manufactured by bees, which is in turn made up of amino acids, vitamins, and trace elements. It is common practice to spray pesticides to avoid plant diseases and insect pests during the general large-scale cultivation, which causes contamination in surrounding environment in various intensities. Pesticides have been detected in different environmental matrices such as soil, water, and air (Smalling and Kuivila 2008; Wang et al. 2009; Schreck et al. 2008; Simon et al. 1998). Consequently, bee pollens are at the considerable risk of pesticide contamination. Such bee pollens products have been commonly used for many decades, which lead to dose accumulation and cause pesticide poisoning. Great attention has been paid to the safety of bee products in recent years; there are many strict requirements on all the contaminating toxins in bee products (Rial-Otero et al. 2007). Corresponding to these requirements, it is very necessary to monitor and control the pesticide multiresidues in bee pollens.
According to chemical compositions, more common and important synthetic pesticides are organochlorine, organophosphorus, pyrethroid, and carbamate. A number of methods had been developed for the determination of the pesticides, which include gas chromatography–electron capture detector (GC-ECD) for organochlorines (Yavuz et al. 2010; Wu et al. 2011; Rao et al. 2011), GC nitrogen phosphorus detector or GC flame photometric detector (FPD) for organophosphorus (Moinfar and Hosseini M 2009; Lu et al. 2007; Oh 2009), GC-ECD for pyrethroids (Li et al. 2009; Chang et al. 2010), high-performance liquid chromatography (HPLC) and gas chromatography–mass spectroscopy (GC-MS) (Przybylski and Bonnet 2009; Saraji and Esteki 2008) with chemical derivatization for carbamates (Santalad et al. 2009; Wu et al. 2009) were commonly used. However, these methods sometimes show inaccurate quantification caused by the limited detection range, occurrence of false positive, and interferences of unknown substances that are coeluting in the same retention time with analyzed pesticides (Ismail et al. 1993; Fenoll et al. 2007), although GC-MS and HPLC-MS can simultaneously determine two or more kinds of pesticides (Wang et al. 2010; Riederer et al. 2010; Chen et al. 2009a) with disadvantage of costly MS equipment itself and more expensive running cost than other detectors.
Commonly, the pretreatment of the nature product’s samples before determination is required. Conventional methods for cleanup are liquid–liquid extraction (Blasco et al. 2004a), matrix solid-phase dispersion (Sánchez-Brunete et al. 2002), solid-phase extraction (SPE), solid-phase microextraction (Blasco et al. 2004b; Campillo et al. 2006), stir bar sorptive extraction (Blasco et al. 2004b), supercritical fluid extraction (Rissato et al. 2004), and so on. SPE is a common cleanup technique for the determination of samples in complex matrix having advantages of little requirement of organic reagent and time (Aguilar et al. 1997; Herrera et al. 2005; Chen et al. 2009b; Jin et al. 2006).
To the best of our knowledge, no publication has documented the simultaneous analysis method of three kinds of pesticide residues (organochlorine, organophosphorus, and pyrethroid pesticides) in bee pollen with GC-ECD. In this paper, the systematic studies including sample extraction, cleanup by SPE, and simultaneous determination with GC-ECD of the three kinds of pesticide residues in bee pollen were conducted. The proposed method is proved to be rapid, simple, and precise.
Materials and Methods
Chemicals and Reagents
Pesticide Standards
Pesticide reference standards of organochlorine were purchased from the National Institute of Metrology of China, and those of pyrethroid and organophosphate were bought from Dr. Ehrenstorfer (Augsburg, Germany), with purity range of 96–100 %. All pesticides investigated are listed in Table 1.
Organic Solvents and Reagents
Petroleum ether, ethyl acetate, and acetonitrile, purchased from Merck (Darmstadt, Germany), were pesticide-free analytical grade. Bulk florisil (for pesticide residue) and activated carbon were used for homemade column. AccuBond SPE ODS-C18 cartridges (500 mg, 3 ml), AccuBond II SPE florisil cartridges (500 mg, 3 ml), and activated carbon cartridges (500 mg, 3 ml) were supplied by Agilent Technologies Co., Ltd of USA.
Instruments
An ultrasonic cleaner (Branson, B8510E, USA) with the working frequency at 40 kHz was used to extract pesticides from bee pollen. Chromatographic analyses were performed on Agilent 7890N gas chromatographic instrument equipped with microelectron capture detector (μECD). An HP-5 column (30 m × 0.25 mm i.d. × 0.25 μm film) was used for chromatographic separation of target pesticides. Nitrogen with the purity of 99.999 % was used as the carrier gas and makeup gas.
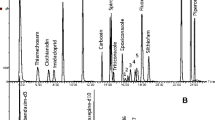
The flow rate of column was held constantly at 1 ml/min and that of the makeup gas was 60 ml/min. The injector temperature was 250 °C, and the injection volume was 1 μl with splitless mode. The detector temperature was 300 °C. The column temperature was programmed as follows: initial temperature was 80 °C (hold 1 min), increased at 8 °C/min to 200 °C (hold 1 min), then increased at 2 °C/min to 250 °C (hold 1 min), followed by a third increase to 280 °C at 10 °C/min (hold 1 min), and a final increase to 290 °C at 1 °C/min. Figure 1 shows the chromatogram of 26 pesticides analyzed by GC-μECD.
GC Chromatograms of 26 pesticides analyzed under the proposed method. The numbers stand for 26 pesticides separately just as shown in Table 1
Pollen Samples
Pollens of pine, water lily, rose, cole flower, Papaver rhoeas, Schisandra chinensis Baill., camellia, and fresh camellia were gathered in Anhui Province in China. Pollens of camellia were collected in March, 2009, while the pollens of pine, cole flower, and Papaver rhoeas were collected in April, 2009. Pollens of Schisandra chinensis Baill. and rose were picked successively in May, 2009. Similarly pollen of water lily and fresh camellia were collected in August, 2009 and March, 2010, respectively. All the pollens were stored in cool and dry place without processing. Collected pollens were grains of different colors with a little sweet taste.
Extraction and Cleanup Procedure
Pollen samples (5 g) were ultrasonically extracted with 50 ml petroleum ether for 20 min. After filtration through filter paper, 25 ml filtrate was concentrated to nearly dryness using a rotary evaporator (Büch, German) in water bath at a temperature of 40 °C. Residue was dissolved by the addition of 2 ml fresh petroleum ether. The dissolved solution was cleaned by passing through activated carbon + C18 column preconditioned with 3 ml petroleum ether for further purification. Adsorbed pesticides were eluted with 10 ml petroleum ether–ethyl acetate (95:5, v/v). Collected extracts were concentrated under a gentle N2 stream. The residues were transferred into a volumetric flask and made up to 2 ml. Finally, the solution is centrifuged for 5 min at a speed of 10,000 rpm, and the supernatant was taken as the test solution.
Recovery Study
Standard solutions of all the pesticides were prepared, as shown in Table 1. Bee pollens were separately spiked with 1, 3, and 5 ml of standard solution of pesticides and placed in a dark place for drying. After 12 h, the spiked samples were analyzed for recovery study. Figure 2 shows the chromatograms of control pollen sample and spiked pollen sample analyzed by GC-μECD.
Results and Discussions
The proposed method by SPE-GC-ECD is rapid, simple, and precise. SPE cleans the sample solution, which ultimately reduces the interference of impurities for detection and consumption of organic solvent. Limit of detection (LOD) shows that GC-ECD has good sensitivity for pesticides analysis. Recoveries indicate the precision and feasibility of method for pollen samples.
Choice of Detector
Sensitivities of μECD, FPD, and MS have been compared in our study. Literatures showed that LOD of most pesticides of MS was higher than 0.01 mg/kg, such as aldrin (Nguyen et al. 2010), while that of μECD was <0.002 mg/kg (Chen et al. 2009b). Experiments indicated that the sensitivity of organophosphorus pesticides with μECD was higher than FPD having LOD more than 0.02 mg/kg. An appropriate purification method can exclude the interferences in estimation of pesticides with a reasonable and stable recovery, which give confidence to use a detector with high sensitivity like μECD. FPD is an optional detector for organophosphorus pesticides and cannot detect other compounds without phosphorus and sulfur. μECD is a commonly used detector for most pesticides. Due to simultaneous determination of different kinds of pesticides, μECD is superior to FPD. Moreover, to use MS is expensive as compared to μECD, which again make this detector convenient.
Study of Extraction Process
According to the appendix Q of Chinese Pharmacopoeia, petroleum ether was used for the extraction of organochlorine and pyrethroid pesticides, whereas ethyl acetate was used for organophosphorus pesticides. In the preliminary examination, the bee pollen samples were separately extracted by petroleum ether and ethyl acetate, then concentrated and analyzed by GC. The sample in which pesticides were not detected was used as a control sample. The control sample added with pesticides standard solution was extracted by petroleum ether, ethyl acetate, acetonitrile, petroleum ether–ethyl acetate (1:1), respectively. The extractive rates were compared and optimized. Results indicates that too many impurities existed in the samples after extraction by ethyl acetate and acetonitrile, and the baseline in chromatogram was too fluctuant for most pesticides to be integrated reasonably. Extractive rates of petroleum ether–ethyl acetate (1:1) were beyond 150 % due to the matrix effects. Extractive rates of petroleum ether were between 101.5 and 119.7 %. Peaks of impurities were relatively fewer, and the baseline was smooth in chromatogram. As a conclusion, petroleum ether was selected as extraction solvent.
Study of Purification Method
Purification step was optimized by comparison of different solid-phase extraction adsorbents such as C18, florisil, activated carbon, activated carbon combined with C18, and activated carbon combined with florisil. Solid-phase extraction adsorbents involved in commercial and self-made models. The self-made florisil column contains 5 g florisil and 0.5 g sodium sulfate anhydrous, which requires 100 ml eluant. Recovery rates of pesticides in bee pollen purified by homemade florisil column were all favorable, but too much organic solvent was used for elution. In case of commercial columns, the effect of activated carbon combined with C18 showed the best performance. The peaks of impurities in the test with activated carbon combined with C18 column were less than that with C18 column only. In addition, the recovery rates in the test with activated carbon combined with C18 column were higher than those with florisil column. Furthermore, less amount of organic solvent was used for elution in the test by activated carbon combined with C18 solid-phase extraction adsorbents.
In this study, the estimated pesticides were medium- and nonpolar compounds. Different organic solvents or the mixtures with different polarities were tested, such as petroleum ether–ethyl acetate (1:1) and (95:5). The results indicate that petroleum ether–ethyl acetate (95:5) can elute most of the pesticides.
Method Validation
Results of the validation of this method are presented in Table 2. The stock standard solutions of each pesticide were diluted into the solutions of seven concentrations with ethyl acetate. Correlation coefficients ranged from 0.9966 to 0.9999, which showed good linear correlation at the chromatographic condition. The standard mixture, containing about 10 ng/ml pesticides, was injected six times with the relative standard deviation between 0.5 and 9.2 %. The LODs and limits of quantification (LOQs) were defined as three times of signal/noise ratio (S/N = 3) and ten times signal/noise ratio (S/N = 10), respectively.
Recovery Results and Fittingness of Methods
The recovery study was conducted with spiked with three concentrations standard solutions in pine pollen. The average recovery values are listed in Table 3. The formula of recovery was shown as: recovery(%) = (concentration of spiked sample/concentration of standard spiked in sample) × 100 %. Recoveries of target pesticides ranged from 78.7 to 101.5 %, 81.3 to 113.7 % and 85.7 to 97.3 % at the low, medium, and high concentrations, respectively. Relative standard deviation (RSD%) was <7.0 %, indicating a good precision and accuracy of the established method.
Experiments were carried out to verify the method for pollens of water lily, rose, Papaver rhoeas, cole, Schisandra fructus, camellia, and fresh camellia. Recovery rates of all above pollens except cole were between 70 ∼ 120 % which show that the methods can be applied for the determination of pesticides in most real bee pollen samples. GC chromatogram of cole pollen showed impure peaks, which were high enough to interfere with separation of target peaks of pesticides. This might be attributed to the fact that cole pollen had more oil, which could not be effectively removed by pretreatment of the proposed methods. This problem requires further research work on this type of pollens.
Application of the Method to the Real Samples
A low level of α- and γ-HCH were detected in water lily and rose. The water or sediments in which the water lily resided might be polluted slightly by α- and γ-HCH, which degraded slowly. Any residual pesticides were not detected in other pollens. Therefore, the pollens on sale could be eaten safely as a whole.
Conclusions
In this study, a multiresidue determination method was proposed for determining three kinds of pesticide residues in bee pollens. Method involves the dissolution of bee pollens in petroleum ether, using a SPE column (activated carbon + C18) for cleanup, then determination by GC-μECD. Beauty of this method is that a number of OCPs (9), OPPs (10), and pyrethroids (7) can be determined simultaneously. Additionally, this method is very simple, rapid, consuming small amount of organic solvent, and consequently produces little hazardous waste. Due to high accuracy and precision, the method may be easily implemented in routine determinations of pesticide residues in bee pollens.
References
Aguilar C, Borrull F, Marce RM (1997) J Chromatogr A 771:221–231
Blasco C, Lino CM, Picó Y et al (2004a) J Chromatogr A 1049:155–160
Blasco C, Fernández M, Picó Y et al (2004b) J Chromatogr A 1030:77–85
Campillo N, Peñalver R, Aguinaga N et al (2006) Analytica Chim Acta 562:9–15
Chang QY, Feng T, Song SJ et al (2010) Microchim Acta 171:241–247
Chen H, Chen RW, Feng R et al (2009a) Chromatographia 70:165–172
Chen F, Xue XF, Zhao J (2009b) Agric China 60:5–8
Fenoll J, Hellin P, Martinez CM et al (2007) Food Chem 105:711–719
Herrera A, Pérez-Arquillué C, Conchello P et al (2005) Anal Bioanal Chem 381:695–701
Ismail SMM, Ali HM, Habiba RA (1993) J Agric Food Chem 41:610–615
Jin Z, Lin ZG, Chen MY et al (2006) Chinese J Chromatogr 24:440–446
Li HP, Lin CH, Jen JF (2009) Talanta 79:466–471
Lu JW, Xia J, Miao S et al (2007) Chinese Pharm J 42:227–231
Moinfar S, Hosseini M RM (2009) J Hazard Mat 169:907–911
Nguyen TD, Lee KJ, Lee MH et al (2010) Microchem J 95:43–49
Oh CH (2009) Bull Environ Contam Toxicol 82:639–643
Przybylski C, Bonnet V (2009) Anal Bioanal Chem 394:1147–1159
Rao MM, KumarMeena A, Galib (2011) Environ Monit A 181:267–271
Rial-Otero R, Gaspar EM, Moura I (2007) Talanta 71:503–514
Yavuz H, Guler GO, Aktumsek A (2010) Environ Monit A 168:277–283
Riederer AM, Smith KD, Barr DB et al (2010) Arch Environ Contam Toxicol 58:908–917
Rissato SR, Galhiane MS, Knoll FRN et al (2004) J Chromatogr A 1048:153–159
Sánchez-Brunete C, Albero B, Miguel E et al (2002) J AOAC Int 85:128–133
Santalad A, Srijaranai S, Burakham R et al (2009) Anal Bioanal Chem 394:1307–1317
Saraji M, Esteki N (2008) Anal Bioanal Chem 391:1091–1100
Schreck E, Geret F, Gontier L et al (2008) Talanta 77:298–303
Simon D, Helliwell S, Robards K (1998) Anal Chim Acta 360:1–16
Smalling KL, Kuivila KM (2008) J Chromatogr A 1210:8–18
Wang DL, Weston DP, Lydy MJ (2009) Talanta 78:1345–1351
Wang DL, You J, Lydy MJ (2010) Arch Environ Contam Toxicol 59:382–392
Wu QH, Zhou X, Li YM et al (2009) Anal Bioanal Chem 393:1755–1761
Wu JW, Liu YG, Zhao RH et al (2011) J Nat Med 65:406–409
Acknowledgment
We are grateful to the Institute for food and drug control of Jiangsu Province for support of this research.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, Y., Sun, C. et al. Simultaneous Determination of Organochlorine, Organophosphorus, and Pyrethroid Pesticides in Bee Pollens by Solid-Phase Extraction Cleanup Followed by Gas Chromatography Using Electron-Capture Detector. Food Anal. Methods 6, 1508–1514 (2013). https://doi.org/10.1007/s12161-012-9539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9539-7