Abstract
This study aims to optimize bio-oil production through microwave pyrolysis of Triplochiton scleroxylon sawdust (Ayous). After a physicochemical characterization of the sawdust, response surface methodology via centered composite design was used to investigate the influence of pyrolysis factors on bio-oil yield and determine the optimal pyrolysis conditions. The studied pyrolysis factors were microwave power (W), irradiation time (min), and biochar (%) as wave absorber. Finally, the bio-oil produced under optimal conditions was characterized by GC–MS. It emerges from this study that Ayous biomass has physicochemical properties that can be valorized for bio-oil production, with a high volatile matter content (63.2 ± 2%) and low ash content (2.8 ± 0.3%). The optimization study of bio-oil yield shows that all factors have significant effects with a statistical significance level of 5% (p < 0.05) on the measured parameters. The optimal bio-oil yield of 44.82% is obtained at optimal conditions: microwave power of 576 W, irradiation time of 28 min, and a biochar (wave absorber) input of 3.18%. The bio-oil produced under optimal conditions has a pH of 4.6 ± 0.7 and a water content of 25 ± 1.2%. Compound identification of this bio-oil by GC–MS identified families of compounds including alkanes (13.90%), esters (5.88%), alcohols (1.10%), and high molecular weight phenolic compounds (58%). The produced bio-oil can be used as biofuel or in industrial applications. Nevertheless, further processing steps are needed to lower the water content and acidity of the oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many severe environmental, economic, and societal challenges are present as a result of the rapid depletion and rising demand for fossil fuels [1]. According to the International Energy Agency (IEA), the global supply is struggling to keep pace with demand, with many producers bumping up against capacity constraints and Russia’s invasion of Ukraine sharply accentuating market tightness. By the first half of 2022, oil prices had risen to an average of USD 105 per barrel [2]. The search for alternative materials and technologies that can lessen reliance on fossil fuels and their negative effects on the environment is warranted, given the rising fuel cost.
Bio-oil is a second-generation biofuel produced by the thermochemical processing of field crop residues and/or agricultural byproducts from biomass residues. Since biomass is the world’s most plentiful renewable resource, it is a very promising substitute [3,4,5]. Biomass is an environmentally friendly and important energy resource available worldwide, and many suitable technologies related to biomass conversion to energy have been developed. Various thermochemical processes, such as gasification, hydrolysis, liquefaction, and pyrolysis, are used to convert biomass into the desired product [6,7,8,9]. Among these processes, pyrolysis has attracted increasing attention for its ability to convert organic matter. Pyrolysis is the process by which materials are thermally broken down into char, gas, and bio-oil through heat in an inert atmosphere. As microwave-assisted pyrolysis enables uniform, quick internal heating, accelerates kinetic reaction rates, and improves energy efficiency, it has proven to be a fascinating alternative to conventional heating making it viable for commercial applications [10, 11]. Nonetheless, biomass has a low loss tangent value, which indicates that the material can absorb microwaves and convert them into heat energy, making it a poor microwave absorber [12]. To address this issue, adding an absorber material with a higher loss tangent overcomes this drawback, enhancing the absorption of microwave radiation. Microwave absorbers were added to increase the temperature of the pyrolysis reaction in a nitrogen atmosphere [13]. The most widely used absorbers in microwave-assisted pyrolysis processes are charcoal, activated carbon, coke, graphite, and biochar.
The addition of solid carbon-based materials as biochar can garner some perceived benefits, including an increase in the microwave assimilation capacity of bulk materials, heat transmission to surrounding materials, and the supply of a rapid heating rate and sufficient temperature at low microwave power [12,13,14]. The biochar produced from the microwave-assisted pyrolysis of biomass can be reused as a cost-effective microwave absorber [15, 16]. During microwave-assisted biomass pyrolysis, the yield and chemical composition of the products (biochar, gas, and bio-oil) may vary depending on the operating conditions of pyrolysis and the composition of the biomass used [17,18,19]. Bio-oils, the main product of pyrolysis, are complex mixtures consisting of molecules of varying sizes that are produced through the depolymerization and fragmentation reactions of biomass building components, such as cellulose, hemicellulose, and lignin [20]. They are the main volatile products from biomass pyrolysis; they could be potential fuels for diesel engines, gas turbines, boilers, and cooking and could also be used as raw materials for the production of resin, pharmaceutical products, and hydrocarbons by appropriate treatments [21,22,23]. The most important biomass resources used for energy production in forest ecosystems are woody materials.
Cameroon has the second largest forest in Africa, with more than 18 million hectares of exploitable dense forest, representing 40% of the country’s surface area. Triplochiton scleroxylon, commonly known as Ayous (light white wood), is the first and most exploited species in Cameroon. It is a prominent biomass resource, producing approximately 1.5 million m3 of forest residue annually [24, 25]. The first and second-generation processing industries generate sawdust of approximately one million cubic meters annually [23]. Of all this wood waste, only 20,000 m3 are recovered to produce 600 tons of charcoal per year; many of the scraps are burned on the forest side, and others are abandoned in sawmills [26]. This inefficient management of wood waste poses environmental, economic, and social challenges and highlights the need for effective strategies to optimize wood waste utilization, particularly from Triplochiton scleroxylon. Little attention has been paid to the valorization of this type of biomass in the literature. Indeed, only two studies have been conducted on their valorization into bioethanol and syngas [27,28,29]. No study has been found in the literature on the valorization of Triplochiton scleroxylon sawdust into bio-oil by pyrolysis. Thus, this study aims to fill this gap by assessing the potential of Triplochiton scleroxylon sawdust for bio-oil production, thereby making a significant contribution to the literature on the valorization of wood waste and forest resources. This study aims to optimize bio-oil production through microwave-assisted pyrolysis of Triplochiton wood sawdust. Firstly, the chemical and thermal characteristics of the wood sawdust are determined. Subsequently, the effects of parameters such as microwave power, irradiation time, and wave absorber on product yield during microwave-assisted pyrolysis are investigated. Finally, the bio-oil produced under optimal conditions is analyzed using gas chromatography and mass spectrometry (GC‒MS).
Materials and Methods
Determination of the Chemical Composition of Triplochiton scleroxylon Sawdust
The substrate used in this study was Triplochiton scleroxylon sawdust, commonly known as Ayous, collected from a sawmill in the Ngaoundere region in Cameroon. Particles with a mean size of 200 µm were obtained by sieving the sawdust used in the studies. To reduce the amount of water in the bio-oil produced, the sawdust was dried for 24 h at 105 °C in an oven. The extraction of extractable substances such as resins, fatty acids, and solvent-soluble phenolic compounds was performed with a toluene/ethanol mixture. The biomass, once freed from its extractables, is then used to determine the contents of other macromolecules such as cellulose, lignin, and hemicellulose. The basic chemical composition of these macromolecules was determined using the Tappi T204 om-88 and T22 om-88 standards.
Thermogravimetric (TG) Analysis
Thermogravimetric analysis (TGA) is an essential technique for characterizing the thermal properties of biomass during heating. It allows for the determination of thermal stability, decomposition stages, and the composition of volatile and non-volatile constituents in biomass. The TG was performed using a SESTSYS Evolution TGA 16/18 thermal analyzer. Approximately 5 mg of Triplochiton scleroxylon sawdust sample was heated in an alumina crucible under air atmosphere with a flow rate of 50 ml/min at a heating rate of 10 °C/min from room temperature to 1000 °C. The weight loss of the sample was recorded continuously, and the thermogravimetric curves were analyzed to determine the thermal decomposition characteristics of the biomass. The onset temperature, maximum degradation rate, and final residue were determined from the TG and derivative thermogravimetric (DTG) curves.
Experimental Procedure
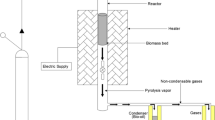
A modified home multimode microwave (Moulinex) was used for the microwave-assisted pyrolysis studies. The microwave oven operates at 2450 MHz and can generate 850 W of microwave power at its highest level. The vacuum pyrolysis apparatus utilized in this work is illustrated in Fig. 1.
The condensation system, attached to the microwave pyrolysis reactor, collected the condensation product (bio-oil) for additional study. Vapors produced during pyrolysis were condensed using ice water cooled and maintained within a temperature range of 0 to 5 C by a pump. The whole installation is inerted under a vacuum system via a pump. The vacuum flow is variable to evacuate noncondensable gas and limit secondary reactions. This noncondensable gas entrained by the vacuum system passes through the gas washing device. In a preliminary study, bio-oil production experiments were carried out. For every experiment, 100 g of Triplochiton scleroxylon sawdust was introduced into a 500-ml reactor.
A kinetic study on the degradation of biomass through microwave-assisted pyrolysis was conducted to understand the underlying processes and determine the experimental domain of irradiation time. The research aimed to elucidate the reaction mechanisms involved in the breakdown of biomass components under microwave irradiation, providing insights into the optimal conditions for maximizing product yields. The first-order kinetics of biomass degradation by pyrolysis described by Cano-Pleite et al. [30] in Eq. (1) was used to describe the degradation kinetics of Ayous sawdust.
where Y(t) is the bio-oil yield; a, b, and c are kinetics parameters; and t is the irradiation time.
The yield of Triplochiton scleroxylon sawdust bio-oil produced was determined using Eq. (2).
Microwave-Assisted Pyrolysis of Triplochiton scleroxylon Sawdust
Using the central composite design (CCD), MINITAB version 2018 software was used to optimize bio-oil production (response). Three factors were considered: microwave power \(\left({X}_{1}\right)\), irradiation time \(\left({X}_{2}\right)\), and wave absorber \(\left({X}_{3}\right)\). The factor and their levels are presented in Table 1.
The experimental domains of factors were established using different approaches. For the microwave power and wave absorber, a literature review and preliminary studies were conducted to identify the most relevant value ranges for the experiments [12, 13, 15]. Conversely, for the time factor, the experimental domain was based on the results of the kinetic study. This approach allowed for the precise definition of the value ranges to be explored for each factor, ensuring the robustness and relevance of the experimental design.
The biochar used as a wave absorber was produced by microwave pyrolysis of sawdust at 500 W for 15 min. The centered composite design used consisted of three parts with a total of 17 experiments distributed as follows: a 23 factorial design (08 experiments), three experimental points located at the center of the study domain (03 experiments), and axial points (+ α and − α) (6 experiments), which are the experimental points located on the axes of each of the factors and constitute the star design. The central composite design being a response surface methodology, a second-order polynomial regression model was used to relate the bio-oil yield to the studied factors. The equation of the model is presented in Eq. (3).
where Yi is the bio-oil or biochar yield predicted; a0 is the intercept; a1, a2, and a3 are the linear coefficient of the independent variable; a11, a22, and a33 are the quadratic coefficients of the independent variable; a12, a13, and a23 are the different interaction coefficients between the input factors x1, x2, and x3; and e is the error of the model.
The CDD results were analyzed using analysis of variance (ANOVA) with a 95% confidence level. The coefficient of determination R2, the adjusted coefficient of determination R2, and the lack of fit test were used to assess the statistical significance of the regression coefficients and the quality and efficiency of the yield bio-oil model.
Bio-Oil Characterization
The bio-oil total acid and moisture content were determined according to NF EN 14104 and ASTM 95–83, respectively. The bio-oil heating value was determined using an adiabatic bomb calorie meter (PARR, Instrument Company, Moline, IL, 61,265 USA).
Bio-Oil Analysis Using GC‒MS
The composition of the bio-oil sample was determined by gas chromatography and mass spectrometry (GC‒MS QP 2010 Plus, Shimadzu) using an Rtx-5 ms column (30 m × 0.25 mm ID, 0.25 µm film thickness). The bio-oil samples were diluted by approximately 10% in hexane according to the solvent compatibility in the column, and only 0.4 µl was injected into the GC. The GC oven temperature was increased from 60 °C for 3 min to 100 °C at a rate of 3 °C/min and then to 300 °C at a rate of 5 °C/min. The final oven temperature of 300 °C was held constant for approximately 5 min. The flow rate of helium gas was maintained at 1.2 ml/min. The MS conditions were as follows: mode electron ionization (EI), ion source temperature 225 °C, ionization energy 70 eV, and a full scan range of 50 to 500.
The compounds that showed more than 90% similarity were identified, and a comparison was made between their individual mass spectra and those obtained from the National Institute of Standards and Technology (NIST).
Results and Discussion
Characteristics of Raw Materials
The results of the immediate analysis and chemical composition of wood sawdust provide valuable insights into its suitability as a feedstock for bio-oil production via pyrolysis. The proximate analysis and chemical composition results of Triplochiton scleroxylon sawdust are presented in Table 2. The results presented are based on the analysis of the dried Triplochiton scleroxylon sawdust.
With a moisture content of 16.77%, wood sawdust is likely to require pretreatment, such as drying to reduce this moisture content before microwave pyrolysis, thus optimizing the energy efficiency of the process. The high water content of the biomass is a constraint for bio-oil production, according to the work of Westerhof et al. [31]. The water content of biomass is lower than 5% [32].
The low ash content of 2.85% is favorable as it minimizes the formation of solid residues during pyrolysis, which could disrupt operations and reduce the quality of the produced bio-oil. The volatile matter represents a significant portion of wood sawdust composition, at 63.23%, indicating that it can readily decompose during pyrolysis to produce volatile compounds, including bio-oil. Additionally, wood sawdust exhibits abundant cellulose composition, with high contents of cellulose (46.66%) and hemicellulose (30.34%), suggesting that it can be effectively converted into sugars and volatile compounds during pyrolysis, thereby contributing to bio-oil yield production. The cellulose content in Triplochiton scleroxylon sawdust is close to that reported in the literature [27, 28]. The significant difference between hemicellulose and lignin content can be explained by the high fiber content variability depending on the tree’s age, origin, and height. Combining these characteristics, it is reasonable to conclude that Triplochiton scleroxylon sawdust holds strong potential as a feedstock for bio-oil production through pyrolysis, offering a promising pathway to valorize this wood by-product into a valuable energy and chemical resource.
Thermogravimetric (TG) Analysis of Triplochiton scleroxylon Sawdust
Thermogravimetric analysis (TGA) of biomass before pyrolysis is used to understand its thermal decomposition behavior under different temperature conditions. Figure 2 presents the results of TGA and DTG of Triplochiton scleroxylon sawdust.
The pyrolysis of biomass usually consists of three stages: dehydration and devolatilization, which lead to biochar formation and the slow transformation of the formed biochar [33, 34]. The initial breakdown stage for Triplochiton scleroxylon sawdust occurs at < 150 °C and is associated with very little mass loss attributed to the evaporation of water and light volatiles [34]. The main weight loss occurs between 300 and 400 °C, with one distinct DTG peak at 360 °C corresponding to the steep weight loss. This stage is primarily associated with cellulose degradation between 300 and 400 °C [35,36,37]. Above 400 °C, a slow weight loss was observed, which can be attributed to lignin degradation and the slow transformation of biochar [37].
Kinetic Study of the Pyrolysis of Triplochiton scleroxylon Sawdust
To optimize the bio-oil production, an initial experiment was carried out to determine the optimal duration of operation. One hundred grams (100 g) of biomass was used, and constant microwave power and wave absorber were maintained at 575 W and 20%, respectively. Figure 3 illustrates the impact of irradiation time on bio-oil yield.
The bio-oil yield data as a function of time were fitted to an empirical model that follows a sigmoidal curve, typical of many pyrolysis processes. The fitted equation is as follows:
The results show that the bio-oil yield increases rapidly at the beginning (5 to 15 min) and then stabilizes as time progresses (15 to 35 min). This trend is typical of pyrolysis, where the decomposition of volatile components occurs quickly at first, followed by a phase of reduced production as the more resistant components decompose.
When the irradiation time increased from 5 to 15 min, the bio-oil yield rose sharply from 12.45 to 36.65%. The high value of parameter b (15.72) indicates a fast reaction in the early stages of the process. This corresponds well with the nature of microwave heating, which provides uniform and rapid heating to the biomass [10].
The second part of the curve (15 to 35 min) corresponds to a stable stationary phase, where the yield of bio-oil reaches its maximum. Beyond this point, increasing the time does not significantly impact bio-oil production. The parameter c (5.63) and the stabilization of the yield indicate a limit to the amount of bio-oil that can be produced from the biomass under the given conditions. Thus, extending the irradiation time beyond this optimal period does not enhance bio-oil yield. This suggests that a properly chosen irradiation time can accelerate depolymerization and defragmentation processes, leading to the efficient production of condensable vapors. However, excessive irradiation offers no additional benefits and may unnecessarily increase energy consumption.
Optimization of Bio-Oil Yield
Experimental Models
Table 3 presents the bio-oil and biochar yields obtained using central composite design (CCD) as the experimental design.
The wood sawdust pyrolysis yield through microwave pyrolysis ranges from 36.33 to 43.98% for bio-oil (runs 9 and 12) and from 32.34 to 37.01% for biochar (runs 17 and 11). These results underscore the influence of the studied factors on the yield of the produced bio-oil and biochar. A study by Demirbas and Balat [37] indicated that quick pyrolysis may convert 40–75 weight percent of biomass into bio-oil, which lends support to this finding. The design was generated to evaluate and optimize microwave power, irradiation time, and wave absorber process variables.
A quadratic polynomial equation was proposed for the estimation of bio-oil and biochar yields. The regression equation obtained between the independent variables (encoded), bio-oil, and biochar yields is shown in Eqs. (5) and (6).
where X1, X2, and X3 are the microwave power (W), irradiation time (min), and the quantity of wave absorber (%), respectively.
Statistical Analysis of the Models
Analysis of variance (ANOVA) was performed to evaluate the quality of the model, and the p-value was used to check it. The ANOVA results for the response surface quadratic polynomial model are summarized in Table 4. In this table, the regression coefficients and significant probabilities of linear X1, microwave power; X2, irradiation time; and X3, absorbent; quadratic (X12, X22, and X32); and interaction effects of process variables (X1X2, X1X3, and X2X3) are shown. Probability values (p-values) < 0.05 indicated a significant effect on the bio-oil or biochar yields, while values greater than 0.05 indicated that the model terms were not significant.
The regression coefficients (Table 4) show that the linear effects (X1, X2, and X3) are all significant at the 0.05 significance level except X1 and X3 for bio-oil and biochar yields, respectively. The quadratic effect, microwave power (X1*X1), and irradiation time (X2*X2) were regarded as the most significant terms in the bio-oil production model because of the lower values of p-value. These terms are not significant for the biochar production model. As for the interaction terms, only the X1*X3 interaction is significant.
After fitting the regression models, the results show that the coefficients of determination R2 are 0.92 for the bio-oil yield and 0.91 for the biochar yield, respectively. These high values of R2 indicate that the fitted models explain approximately 92% and 91% of the total variance in the corresponding experimental data. Additionally, the adjusted coefficients of determination (R2 adjusted) for the bio-oil and biochar yields are 0.83 and 0.87, respectively. These values confirm that the models effectively capture the variation in the experimental data, accounting for the number of independent variables in each model. Thus, the factors included in the models, along with their second-order interactions, are significantly related to the observed yields in microwave pyrolysis experiments, for both bio-oil and biochar production.
The refitted models of the bio-oil and biochar yield are written as follows (Eqs. (7) and (8)):
Influence of the Parameters on the Bio-Oil Yield and Optimal Conditions
Figure 4 presents the response surface and the contour plots illustrating the relationships between the independent and dependent variables for various fixed parameters.
bio-oil yield factor interactions.
Figure 4a illustrates the interaction effects between the wave absorber and microwave power. This indicates that at high microwave power, the bio-oil yield increases linearly with decreasing wave absorber but decreases with increasing wave absorber. Moreover, high microwave power can quickly increase the upper and middle biomass temperatures to pyrolysis conditions, demonstrating a fast pyrolysis system [13]. The decrease in bio-oil yield with increasing wave absorber at low microwave power is mainly due to the self-heating of the absorber by an insufficient amount of microwave energy [15]. From Fig. 4b, the bio-oil yield is influenced by the microwave power and time interaction. The contour plot (Fig. 4f) shows several optimal conditions. For irradiation times greater than 26 min, we obtain an efficiency of 41%, while for irradiation times longer than 28 min, there is a maximum bio-oil yield of 43% for a range of powers ranging from 500 to 675 W. An increase in microwave power increases the bio-oil yield because increasing microwave power leads to a rise in the power density [38]. It has been reported that for short irradiation time, the pyrolysis process may fail to proceed, resulting in a reduced bio-oil yield [39]. The elevated power of microwave irradiation was shown to be more selective in favor of the bio-oil in the lower layer.
Optimizing the mathematical model of the bio-oil yield equation based on the studied factors yields a maximum bio-oil yield of 44.82% under the following conditions: microwave power of 576 W, irradiation time of 28 min, and wave absorber of 3.18%.
Physicochemical Properties of Bio-Oil
Table 5 presents the physicochemical characterization results on the bio-oil produced under the optimal pyrolysis conditions.
The characterization results reveal that the bio-oil exhibits an acidic pH of 4.6, attributable to organic acids. The most widely used value to indicate the mean pH of bio-oil is 2.5, typically the pH of bio-oil obtained from wood pyrolysis [40]. The total acid number is measured at 47 mg KOH/g of bio-oil, suggesting a significant acidity that may influence its stability and oxidation potential [41]. Sınağ et al. [42] reported that hemicellulose and lignin degradation during pyrolysis may lead to a high concentration of acidic chemicals. The water content of the bio-oil was 25%; during pyrolysis, the lignocellulosic biomass lost free water, which was released by evaporation, and the reaction water resulting from the decomposition of biopolymers, thus favoring a high water content. The variation in water content is 15–30% for pyrolysis condensable vapor, depending on the feedstock and process conditions [43]. A high water content may require additional drying or purification steps to enhance its quality. However, despite these characteristics, the bio-oil displays a heating value of 19.17 MJ/kg, nearly half that of crude or heavy fuel oil. Bio-oil contains a lower proportion of carbon and hydrogen, with higher heating values [44]. The reason for this reduced heating value is that bio-oil contains oxygenated molecules. Wang et al. [35] performed a pyrolysis experiment on corn straw in a fluidized bed and reported that the calorific value of bio-oil was 15–17 MJ/kg.
GC‒MS Analysis of Bio-Oil
Bio-oil is dark brown and contains hundreds of organic compounds, such as acids, alcohols, ketones, aldehydes, phenols, ethers, esters, sugars, furans, alkenes, nitrogen compounds, and miscellaneous oxygenates [45], as well as solid particles [46, 47]. GC‒MS analysis was subsequently conducted to determine the chemical composition of the bio-oil. GC‒MS analysis of bio-oil derived from the pyrolysis of Triplochiton scleroxylon sawdust revealed a significant variety of chemical components (Table 6). The spectra obtained during GC‒MS analysis are presented in Fig. 5.
This study analyzed only substances with peak areas greater than 0.5%, disregarding those with lower peak areas. The compounds identified by GC/MS were alkanes (13.90%), esters (5.88%), alcohols (1.10%), and phenolic compounds (58%) of low and high molecular weights. The main phenolic components in the bio-oil obtained were (18, 65%) phenol, 2-methoxy-, (17,09%) phenol, 2,6-dimethoxy-, and (13%) phenol. Oxygenated aromatic compounds such as phenols are commonly observed in bio-oil. Phenolic compounds found in bio-oils are composed of both simple phenols consisting chiefly of a single substituted phenolic ring with alcohol, aldehyde, or carboxylic acid groups and oligomeric polyphenols having multiple phenol structural units, each of which is a product of lignin decomposition [48]. A greater number of bio-oil phenolic compounds enhances bio-oil’s HHV [49]. Phenol and its derivatives have applications in various industries, including fine chemicals, medicines, food processing, and resin manufacturing.
Alcohols and esters, although present in smaller quantities, also contribute to the energy value and chemical properties of the bio-oil. Alcohols can be used as solvents and chemical reagents, while esters are valuable for their aromatic properties and potential as biofuels [50].
Alkanes, though in minority, add to the calorific value of the bio-oil and can be used as fuels or lubricants [51].
Conclusion
The aim of this study was to investigate the production of bio-oil through microwave pyrolysis of Triplochiton scleroxylon sawdust. After determining the chemical and thermal characteristics of Triplochiton scleroxylon sawdust, optimization of bio-oil production through microwave pyrolysis using a central composite design was conducted. The three factors studied were microwave power (X1), irradiation time (X2), and amount of wave absorber (X3). Finally, the bio-oil produced under optimal conditions was characterized by GC–MS. The characterization results showed that the biomass studied exhibited good characteristics that could be valorized in pyrolysis for bio-oil production. The bio-oil yield is greatly impacted by the wave absorber as well as the quadratic effect of microwave power and irradiation time. The optimal yield (44.82%) of bio-oil production through microwave pyrolysis was obtained under the following optimal conditions: microwave power of 576 W, irradiation time of 28 min, and wave absorber quantity of 3.81%. The produced bio-oil contained a water content of 25%, an acid content of 47 ± 2 mg KOH/g of bio-oil, and a pH of 4.6 ± 0.7. The molecular composition analysis of the bio-oil by GC–MS revealed that the bio-oil obtained under optimal conditions was a mixture of alkanes, esters, alcohols, ketones, and phenolic compounds. These various compounds are very reactive and can alter the physicochemical properties of the bio-oil. Additional research on the aging of Triplochiton scleroxylon sawdust bio-oil is essential to assess its long-term stability and optimize its industrial application.
Data Availability
Upon reasonable request, the corresponding author will make the datasets used in the current work public.
References
U.S. Energy Information Administration (EIA) (2016) International Energy Outlook 2016 with Projections to 2040
IEA (2022) Africa Energy Outlook 2022. OECD
Demirbaş A, Arin G (2002) An overview of biomass pyrolysis. Energy Sources 24:471–482. https://doi.org/10.1080/00908310252889979
Demirbas A (2010) Fuels from biomass. In: Green energy and technology. Springer London, London
Nanda S, Mohanty P, Pant KK et al (2013) Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res 6:663–677. https://doi.org/10.1007/s12155-012-9281-4
Bhaskar T, Pandey A (2015) Advances in thermochemical conversion of biomass-introduction. Recent advances in thermo-chemical conversion of biomass https://doi.org/10.1016/B978-0-444-63289-0.00001-6
Canabarro N, Soares JF, Anchieta CG et al (2013) Thermochemical processes for biofuels production from biomass. Sustain Chem Process 1:1–10. https://doi.org/10.1186/2043-7129-1-22
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels production through biomass pyrolysis- a technological review. Energies (MDPI) 5:4952–5001. https://doi.org/10.3390/en5124952
Gogoi D, Kumar M, Lakshmi YG (2023) A comprehensive review on “pyrolysis” for energy recovery. BioEnergy Res 16:1417–1437. https://doi.org/10.1007/s12155-023-10568-9
Mutsengerere S, Chihobo CH, Musademba D, Nhapi I (2019) A review of operating parameters affecting bio-oil yield in microwave pyrolysis of lignocellulosic biomass. Renew Sustain Energy Rev 104:328–336. https://doi.org/10.1016/j.rser.2019.01.030
Beneroso D, Monti T, Kostas ET, Robinson J (2017) Microwave pyrolysis of biomass for bio-oil production: scalable processing concepts. Chem Eng J 316:481–498. https://doi.org/10.1016/j.cej.2017.01.130
Zhang X, Rajagopalan K, Lei H et al (2017) An overview of a novel concept in biomass pyrolysis: microwave irradiation. Sustain Energy Fuels 1:1664–1699. https://doi.org/10.1039/C7SE00254H
Mokhtar NM, Ethaib S, Omar R (2018) Effects of microwave absorbers on the products of microwave pyrolysis of oily sludge. J Eng Sci Technol 13:3313–3330
Fernandez BF (2015) Microwave potential for bioenergy production. Renew Energy Focus 16:156–159. https://doi.org/10.1016/j.ref.2015.10.023
Mushtaq F, Abdullah TAT, Mat R, Ani FN (2015) Optimization and characterization of bio-oil produced by microwave assisted pyrolysis of oil palm shell waste biomass with microwave absorber. Bioresour Technol 190:442–450. https://doi.org/10.1016/j.biortech.2015.02.055
Li J, Dai J, Liu G et al (2016) Biochar from microwave pyrolysis of biomass: a review. Biomass Bioenerg 94:228–244. https://doi.org/10.1016/j.biombioe.2016.09.010
Neves D, Thunman H, Matos A et al (2011) Characterization and prediction of biomass pyrolysis products. Prog Energy Combust Sci 37:611–630. https://doi.org/10.1016/j.pecs.2011.01.001
Guedes RE, Luna AS, Torres AR (2018) Operating parameters for bio-oil production in biomass pyrolysis: a review. J Anal Appl Pyrolysis 129:134–149. https://doi.org/10.1016/j.jaap.2017.11.019
Mari Selvam S, Balasubramanian P (2023) Influence of biomass composition and microwave pyrolysis conditions on biochar yield and its properties: a machine learning approach. BioEnergy Res 16:138–150. https://doi.org/10.1007/s12155-022-10447-9
Lyu G, Wu S, Zhang H (2015) Estimation and comparison of bio-oil components from different pyrolysis conditions. Front Energy Res 3:1–11. https://doi.org/10.3389/fenrg.2015.00028
Chiaramonti D, Bonini M, Fratini E et al (2003) Development of emulsions from biomass pyrolysis liquid and diesel and their use in engines - Part 1: Emulsion production. Biomass Bioenerg 25:85–99. https://doi.org/10.1016/S0961-9534(02)00183-6
Effendi A, Gerhauser H, Bridgwater AV (2008) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sustain Energy Rev 12:2092–2116. https://doi.org/10.1016/j.rser.2007.04.008
Goyal HB, Seal D, Saxena RC (2008) Bio-fuels from thermochemical conversion of renewable resources: a review. Renew Sustain Energy Rev 12:504–517. https://doi.org/10.1016/j.rser.2006.07.014
Atyi REA, Poufoun JN, Awono JPM et al (2016) Economic and social importance of fuelwood in Cameroon. Int For Rev 18:52–65. https://doi.org/10.1505/146554816819683735
Kamkuimo P, Ayouba S, Anicet J (2018) Étude sur la situation de référence de valorisation des rebuts de l’exploitation forestière et de scierie dans la région de l’Est -Cameroun
Ackom EK, Alemagi D, Ackom NB et al (2013) Modern bioenergy from agricultural and forestry residues in Cameroon: potential, challenges and the way forward. Energy Policy 63:101–113. https://doi.org/10.1016/j.enpol.2013.09.006
Nzali BT, Mvogo PO, Ali A, Mouangue R (2019) Effect of particle size on syngas production using sawdust of Cameroonian Triplochiton scleroxylon. Sci African 6:e00182. https://doi.org/10.1016/j.sciaf.2019.e00182
Assabjeu AC, Noubissié E, Desobgo SCZ, Ali A (2020) Optimization of the enzymatic hydrolysis of cellulose of Triplochiton scleroxylon sawdust in view of the production of bioethanol. Sci African 8. https://doi.org/10.1016/j.sciaf.2020.e00438
Tchuidjang TT, Noubissié E, Ali A (2021) Optimization of the pre-treatment of white sawdust (Triplochiton scleroxylon) by the organosolv process for the production of bioethanol. Oil Gas Sci Technol-Rev d’IFP Energies Nouv 76:23. https://doi.org/10.2516/ogst/2021004
Cano-Pleite E, Rubio-Rubio M, Riedel U, Soria-Verdugo A (2021) Evaluation of the number of first-order reactions required to accurately model biomass pyrolysis. Chem Eng J 408:127291. https://doi.org/10.1016/j.cej.2020.127291
Westerhof RJM, Kuipers NJM, Kersten SRA, Van Swaaij WPM (2007) Controlling the water content of biomass fast pyrolysis oil. Ind Eng Chem Res 46:9238–9247. https://doi.org/10.1021/ie070684k
Shrivastava P, Khongphakdi P, Palamanit A et al (2021) Investigation of physicochemical properties of oil palm biomass for evaluating potential of biofuels production via pyrolysis processes. Biomass Convers Biorefin 11:1987–2001. https://doi.org/10.1007/s13399-019-00596-x
Raveendran K, Ganesh A, Khilar KC (1996) Pyrolysis characteristics of biomass and biomass components. Fuel 75:987–998. https://doi.org/10.1016/0016-2361(96)00030-0
Hu M, Chen Z, Wang S et al (2016) Thermogravimetric kinetics of lignocellulosic biomass slow pyrolysis using distributed activation energy model, Fraser-Suzuki deconvolution, and iso-conversional method. Energy Convers Manag 118:1–11. https://doi.org/10.1016/j.enconman.2016.03.058
Wang S, Liu Q, Luo Z et al (2007) Mechanism study on cellulose pyrolysis using thermogravimetric analysis coupled with infrared spectroscopy. Front Energy Power Eng China 1:413–419. https://doi.org/10.1007/s11708-007-0060-8
Cagnon B, Py X, Guillot A et al (2009) Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour Technol 100:292–298. https://doi.org/10.1016/j.biortech.2008.06.009
Demirbas MF, Balat M (2007) Biomass pyrolysis for liquid fuels and chemicals: a review. J Sci Ind Res (India) 66:797–804
Zhou R, Lei H, Julson J (2013) The effects of pyrolytic conditions on microwave pyrolysis of prairie cordgrass and kinetics. J Anal Appl Pyrolysis 101:172–176. https://doi.org/10.1016/j.jaap.2013.01.013
Shang H, Lu RR, Shang L, Zhang WH (2015) Effect of additives on the microwave-assisted pyrolysis of sawdust. Fuel Process Technol 131:167–174. https://doi.org/10.1016/j.fuproc.2014.11.025
Mohan D, Pittman CU, Philip S (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuels 62:848–889
Uemura Y, Omar WN, Razlan S et al (2012) Mass and energy yields of bio-oil obtained by microwave-induced pyrolysis of oil palm kernel shell. J Japan Inst Energy 91(10):954–9
Sinag A, Uskan B, Gülbay S (2011) Detailed characterization of the pyrolytic liquids obtained by pyrolysis of sawdust. J Anal Appl Pyrolysis 90:48–52. https://doi.org/10.1016/j.jaap.2010.10.003
Steele PH, Yu F, Gajjela S (2009) Past, present, and future production of bio-oil. Woody Biomass Util 2009 Conf Proc 17–22
Kumar R, Strezov V, Weldekidan H et al (2020) Lignocellulose biomass pyrolysis for bio-oil production: a review of biomass pre-treatment methods for production of drop-in fuels. Renew Sustain Energy Rev 123:109763. https://doi.org/10.1016/j.rser.2020.109763
Rezaei PS, Shafaghat H, Daud WMAW (2014) Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: a review. Appl Catal A Gen 469:490–511. https://doi.org/10.1016/j.apcata.2013.09.036
Milne T, Agblevor F, Davis M, et al (1997) A review of the chemical composition of fast-pyrolysis oils from biomass. Dev Thermochem Biomass Convers 409–424. https://doi.org/10.1007/978-94-009-1559-6_32
Oasmaa A, Peacocke C (2010) Properties and fuel use of biomass-derived fast pyrolysis liquids. A guide
Kim JS (2015) Production, separation and applications of phenolic-rich bio-oil - a review. Bioresour Technol 178:90–98. https://doi.org/10.1016/j.biortech.2014.08.121
Nanda S, Mohanty P, Kozinski JA, Dalai AK (2014) Physico-chemical properties of bio-oils from pyrolysis of lignocellulosic biomass with high and slow heating rate. Energy Environ Res 4:21–32. https://doi.org/10.5539/eer.v4n3p21
Oppong Francis, Cangsu Xu, Xiaolu Li ZL (2022) Esters as a potential renewable fuel: a review of the combustion characteristics. Fuel Process Technol 229:107185
Lu Q, Zhang ZB, Liao HT et al (2012) Lubrication properties of bio-oil and its emulsions with diesel oil. Energies 5:741–751. https://doi.org/10.3390/en5030741
Acknowledgements
The authors would like to thank the Supervisor of the Industrial Chemistry and Bioresources Laboratory of the National Advanced School of Agro-Industrial Sciences at Ngaoundere University (Cameroon) for permitting the execution of the experiments.
Author information
Authors and Affiliations
Contributions
The methodology and writing of the manuscript were performed by Badza Kodami and Kom Regonne Raïssa. Tsatsop Tsague Roli Karole, Ze Bilo’o Philemon, and Ngassoum Martin Benoit analyzed the data. After reading this draft of the manuscript, all the authors provided their approval.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badza, K., Raïssa, K.R., Karole, T.T.R. et al. Optimal Bio-Oil Production Using Triplochiton scleroxylon Sawdust Through Microwave-Assisted Pyrolysis. Bioenerg. Res. (2024). https://doi.org/10.1007/s12155-024-10788-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12155-024-10788-7