Abstract
The objective of this work is to evaluate the potential of oil palm biomass (OPB) in terms of physicochemical properties for producing biofuels via pyrolysis processes. The OPB included oil palm trunk (OPT), oil palm fronds (OPF), oil palm shell (OPS), oil palm roots (OPR), oil palm decanter cake (OPDC), empty fruit bunches (EFB), oil palm fiber (OPFB), and oil palm sewage sludge (OPSS). Their physicochemical properties are considered on several physical, chemical, and thermal aspects. The results showed that particle size distribution and bulk density of ground OPB were different. The proximate analysis results of OPB were consistent with the lignocellulose content and extractives. The carbon and hydrogen content of the OPB were also correlated with the organic components. Some OPB contained high lignin and extractives. The lignin content of OPB strongly influenced to thermal decomposition trend. OPB contained high inorganic elements such as potassium (K), calcium (C), and iron (Fe). The higher heating value and potential use as energy equivalent with fossil fuels of the OPB were relatively low. OPB had low thermal conductivity, and the dielectric constant, loss factor, and tangent loss of the OPB were also low. Thus, these results will be beneficial for the researchers and biofuel producers for choosing the appropriate OPB, as well as the operating conditions and reactor types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The relentless growth of the global population inevitably leads to higher energy consumption [1]. In 2018, British petroleum (BP) reported that global energy demand was 1.6 Gtoe [2]. Most of the currently available energy is obtained from fossil fuels, including crude oil, coal, and natural gas. The growth in global energy demand strongly influences to the energy systems not only in terms of energy security and sustainability but also concerning environmental and social aspects. Global warming and climate change due to greenhouse gases are already severe problems on a global scale. The grave nature of the situation has prompted both developed and developing countries around the world for searching and innovating the clean energy resources in the forms of renewable energy (RE) and alternative energy (AE) resources [1,2,3,4,5]. However, the potential of RE and AE resources such as solar, hydro, wind, geothermal, tidal, wave, and biomass varies significantly between countries and regions. The potential of RE and AE resources of each country and region depends on several factors such as location, weather, and season, as well as the local agricultural and industrial activities. Biomass is one of the high potential RE and AE resources in some regions or countries. Biomass is defined as organic matters which may be obtained from direct plantation (forestry), animal and human waste, residues and wastes from agricultural and industrial processes, and organic municipal solid wastes [6]. In 2018, biomass used as bioenergy accounted for approximately 14% of total global energy consumption [7,8,9,10]. Bioenergy has the potential to decrease carbon footprints and to reduce environmental impacts because the replantation or new growing of plants consumes the carbon dioxide (CO2) that is emitted from the biomass conversion processes into bioenergy [2]. For this reason, the utilization of bioenergy is considered to be carbon dioxide neutral. However, the biomass potential of each country or region depends on the location, weather, plantations, agricultural activity, and industrial processing in that specific location. Biofuels and bioenergy are most commonly produced by countries whose prime industry is agriculture. Most of the Asian countries have a high degree of dependency on the agricultural sector, with Thailand among them.
As an agro-industry country, Thailand has a high potential for biomass applications. Rice husks, cassava roots, corn stalks, rubber wood bark, rubber wood sawdust, oil palm shell, and empty fruit bunches are examples of the biomass available in Thailand [11]. These biomasses are produced during the replanting, harvesting, and processing of agricultural products. For oil palm biomass (OPB), there is particularly high potential in the south of Thailand, which is among the top-ranked producers and exporters of palm oil, after only Malaysia and Indonesia. The total plantation area of the oil palm tree in Thailand increased to 818,500 ha in 2018, which was 4.2% more than in 2017 [12]. The Thai government has also set the target of increasing the plantation area of palm oil trees to 1,632,000 ha in 2036 [12]. The OPB is obtained from harvesting and processing the fresh fruit bunches, as well as during the replantation of oil palm trees. Typically, oil palm fronds and leaves (2–3 fronds) will be produced when harvesting fresh fruit bunches from each oil palm tree [13, 14]. The replantation of oil palm trees also provides a huge source of OPB, including oil palm trunk (OPT), oil palm fronds and leaves (OPF and OPL), and oil palm roots (OPR) [14]. The milling process of fresh fruit bunches also generates various types of biomass and waste such as oil palm fiber (OPFB), empty fruit bunches (EFB), oil palm kernel shell (OPS), oil palm decanter cake (OPDC), and sewage sludge (OPSS) from wastewater treatment [15,16,17,18]. Previous reports have revealed that the yield of crude palm oil obtained from the milling process of fresh fruits was only 20–25% (wt.) [15, 17, 19]. The other parts were turned into biomass in the form of residue or waste [15,16,17,18, 20]. These reports also indicated that milling 100 tons of fresh fruit bunches generated 4–6 tons of OPS, 20–32 tons of EFB, and 12–19 tons of OPFB [14, 19,20,21]. The Ministry of Energy of Thailand reported that some of the OPB, such as OPS, OPFB, and EFB, has been used as solid biofuel in palm oil factories and other industries for the generation of heat and power [12]. However, OPB, including EFB, OPT, OPF, OPL, OPR, and OPDC, remains high potential for biofuel or bioenergy applications.
The conversion of biomass into biofuels or bioenergy can be performed by several processes such as mechanical conversion, thermochemical conversion, biochemical conversion, and combined processes. The criteria for choosing the biomass conversion process among the options depend on many factors such as the type and properties of the biomass being used, the desired fuel forms or energy products, the cost, storage, transportation considerations, and the utilization target. This is because the conversion of biomass by the mentioned processes provides different final forms of biofuels (liquid, gas, or solid) or bioenergy (heat or power). Pyrolysis is one of the thermochemical conversion processes. It involves the thermal decomposition of raw materials under the absence of oxygen or air at elevated temperatures ranging from 300 to 600 °C [22, 23]. This process can be applied for several materials such as plastic wastes, municipal solid wastes, waste cooking oil, used tires, and biomass. The pyrolysis of biomass provides products in the form of bio-oil, biochar, and pyrolysis gas. There are two types of pyrolysis processes – slow pyrolysis and fast pyrolysis – depending on many factors such as temperature, heating rate, and vapor residence time [24, 25]. Fast pyrolysis is more popular than slow pyrolysis for the production of bio-oil or pyrolysis oil. This is because the bio-oil yield obtained from fast pyrolysis is higher than from slow pyrolysis [26,27,28,29]. However, the yield and quality of the bio-oil or liquid product not only depend on pyrolysis types but also are influenced by many other factors such as the reactor type, operating conditions (heating rate, pyrolysis temperature, vapor residence time, etc.), biomass type, biomass property, and biomass composition [30,31,32,33,34,35,36,37,38,39,40]. Biomass properties such as particle size, bulk density, elemental composition, lignocellulosic composition, and chemical composition have a substantial impact on the bio-oil yield and quality [35, 40,41,42,43,44,45,46,47,48,49,50]. The main advantage of bio-oil is that it has a high energy density compared to raw biomass. Bio-oil is also easy to store, handle, and transport. The bio-oil also has many applications. It can be used directly as fuel in a boiler, upgraded for use as fuel in an engine, and turned into high value-added products for food and chemical processes [29, 49, 50]. For the biochar and pyrolysis gas, they can be used as biofuels to generate heat or power for use in pyrolysis or other processes.
With OPB, the applications of this biomass for pyrolysis process are still challenging due to many types of OPB that can be applied with pyrolysis processes for producing biofuels in forms of bio-oil, biochar, and pyrolysis gas. Most of the research papers related with pyrolysis have used OPS, OPFB, and EFB [14, 51,52,53,54,55,56]. There are only a few studies that have investigated the pyrolysis of other oil palm biomasses [57,58,59]. Thus, the literature reviews indicated that there are very less studies that revealed the properties of OPB or pyrolysis of OPB in Thailand. Most of the research papers related with OPB and pyrolysis of OPB are published by Malaysian researchers. The aim of this study was, therefore, to investigate the physicochemical properties of OPB for evaluating the potential of biofuels production via pyrolysis processes. The OPB used in this study included OPT, OPF, OPS, OPR, OPDC, EFB, OPFB, and OPSS. The investigated physicochemical properties of these biomasses were considered via proximate analysis, ultimate analysis, higher heating value, and potential use as energy equivalent with fossil fuels, lignocellulose content, thermal decomposition via thermogravimetric analysis (TGA), elemental composition, thermal conductivity, and dielectric property.
2 Materials and methods
2.1 Preparation of biomass samples
The OPT, OPF, and OPR were obtained from oil palm trees which were harvested for 25 years old at Klong Thom District, Krabi Province, Thailand. The OPS, OPDC, EFB, OPFB, and OPSS were residue and waste from crude palm oil production at the Thiando palm oil factory located at Lam Thap District, Krabi Province, Thailand. The fresh OPT (with bark), OPF (without leaves), and OPR were chopped by a chopping machine (MCH-420, Machinery 789, Thailand). All samples were dried inside a solar greenhouse dryer to reduce the moisture content to below 10% (%wt., wet basis). The dried samples were then ground by a grinding machine (Bonny, 2HP, Thailand) equipped with a 2 mm size sieve. The ground samples were kept in a sealed plastic zip bag until ready for further use.
2.2 Determination of physicochemical properties
2.2.1 Particle size distribution
The particle size distribution of the ground OPB was determined by using a vibratory sieve shaker (Analysett 3 pro, Fritsch, Germany) comprising seven sieves with opening sizes of 0.08, 0.11, 0.15, 0.21, 0.30, 0.60, and 1.18 mm and a bottom pan (< 0.08). The determination was performed following the sieve standard ASTM E11 [47]. The result was presented as a weight percentage.
2.2.2 Bulk density
The bulk or apparent density of the ground OPB was determined based on the mass and volume ratio. The cylindrical container with a specific inside diameter and volume (500 mL) was used to determine the bulk density of the biomass samples. The ground biomass samples were poured into a container from a certain height to facilitate the free flow of the samples until the container was full with a specific volume. The net weight of each sample was then recorded, and the bulk density was calculated. To ensure the accuracy of each measurement, it was repeated three times, and the mean value with standard deviation value was presented in the unit of kg/m3 [47].
2.2.3 Proximate and ultimate analysis
The proximate analysis is the determination of the gross components, including moisture content, volatile matter, and fixed carbon content and ash content [48]. These components were determined by using a macro thermogravimetric analyzer (TGA 701, LECO, USA), according to the ASTM D7582 procedure. The basic elemental composition of each sample, including carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sulfur (S), was determined by a CHNS/O Analyzer (FLASH 2000, Thermo Scientific, Italy). The oxygen content was determined based on the analyzer procedure. The C, H, and N were performed according to related EN15104, and the S content was performed according to the ASTM D4239. The mean and standard deviation of values were reported as percentages by weight (wt.%, dry basis).
2.2.4 Higher heating value and potential use as energy equivalent with fossil fuels
The higher heating value (HHV) of the OPB samples was determined by a bomb calorimeter (C5000, IKA® Werke, Germany), according to the EN14918 procedure. The HHV obtained from the bomb calorimeter was also compared to the HHV obtained from the calculation based on the following equations:
where C, H, O, and S are the elemental composition obtained from ultimate analysis (R2 = 0.720) [49].
where FC, VM, and A are the content of fixed carbon, volatile matter, and ash obtained from proximate analysis (R2 = 0.695) [50].
where C, H, O, S, and ash are the compositions of the biomass obtained from ultimate analysis and proximate analysis (R2 = 0.695) [60].
where L is the lignin content of the biomass obtained from lignocellulose content determination (R2 = − 0.875) [61].
The higher heating value (HHV) of the OPB obtained from the bomb calorimeter was then converted to the potential use as energy equivalent with fossil fuels, including coal (bituminous), crude oil, natural gas (NG), and liquefied petroleum gas (LPG). The reference HHV of the coal, crude oil, NG, and LPG were 28.87 MJ/kg, 38.78 MJ/L, 38.95 MJ/m3, and 50.08 MJ/kg, respectively [62, 63].
2.2.5 Lignocellulosic content
The lignocellulosic content of the OPB was determined via the method developed by Georing and Van Soest (1970) [64], Van Soest (1991) [65], Reza et al. (2014) [66], and Kambo and Dutta (2015) [67]. According to this method, the percentage of cellulose, hemicellulose, and lignin was determined in terms of acid detergent fiber (ADF), neutral detergent fiber (NDF), and acid detergent lignin (ADL). The percentage of cellulose, hemicellulose, and lignin was then calculated from the following equations. Extractives are calculated by difference method.
2.2.6 Thermogravimetric analysis (TGA)
Thermal decomposition of the OPB was observed by thermogravimetric analysis (TGA) and differential thermal analysis (DTA) techniques using a thermogravimetric analyzer (Perkin Elmer, USA), according to the ASTM E1131 procedure. The observation was performed at temperatures ranging from 50 to 1000 °C and at a heating rate of 10 °C/min under a nitrogen gas atmosphere.
2.2.7 Major and minor elemental compositions
The major and minor elemental contents, including silicon (Si), iron (Fe), calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), cadmium (Cd), and lead (Pb), of the biomass samples were determined by the Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, AVIO500, Perkin Elmer, USA), according to the EN15290 procedure. The results were reported as mg/kg.
2.2.8 Thermal conductivity
Thermal conductivity of biomass is one of the important factors for designing the conventional heating unit, which the heat transfers from the reaction wall to the biomass. In this study, the thermal conductivity measurement of the ground OPB was performed by the Hot Disk Thermal Constants Analyzer (Hot Disk, TPS 2500S, Sweden). This system determines the thermal conductivity based on a patented transient plane source (TPS) technique, which can be used to study materials with thermal conductivities from 0.005 to 500 W/m•K and cover a temperature range from 30 to 1000 K.
2.2.9 Dielectric properties
Dielectric properties are important factors for designing microwave heating which can be applied for pyrolysis process. Thus, this study determined the dielectric properties, including dielectric constant (ε’), dielectric loss factor (ε”), and tangent loss (tan δ) of the ground OPB by using materials test equipment (E5071C ENA Series Network Analyzer, Keysight Technologies, USA) equipped with a coaxial probe. The ground samples were poured into a cylindrical container, and then the measurement was performed at a temperature of 25 °C and a frequency of 2.45 GHz [68]. The measurement of the dielectric properties for each sample was repeated five times to gain a confidence value, and the mean value of the dielectric properties was presented.
3 Results and discussion
3.1 Particle size distribution of ground samples
Table 1 shows the particle size distribution of the ground OPB. The dried OPB samples were ground with a grinding machine equipped with a 2 mm size sieve. The determination of the particle size distribution showed that most of the ground OPB had particle sizes in the range of 0.30–1.18 mm, except the OPSS, which had a particle size of lower than 0.08 mm. For the pyrolysis process, the particle size of the biomass is one of the main factors that strongly influence product yield and quality. This is because the biomass particles with a small particle size have a high surface area, leading to a high heat transfer rate and also a high heating rate of the biomass inside the pyrolysis reactor. In the case of bio-oil production, the heating rate strongly affects the bio-oil yield and quality. With a fast pyrolysis process, the biomass is usually pyrolyzed by moving the bed pyrolysis reactors with small biomass particles. Pattiya and Suttibak [69] and Sirijanusorn et al. [36] studied bio-oil production from cassava biomass with biomass particle sizes ranging from 0 to 250 and 250–425 to 425–600 μm by using a fluidized bed and counter-rotating twin-screw reactor. They found that the maximum bio-oil yield was obtained with a particle size of 250–425 μm. Pyrolysis of biomass with particles that were too small or large tended to decrease the bio-oil yield. There are two reasons to explain why the biomass particle size of 0–250 μm gave a low bio-oil yield. The first reason is related to the high ash content of the biomass due to the segregation of the feed into a high dirt feed. The second reason is related to the hydrodynamic nature of the fine biomass particles. The tiny biomass particles tend to be blown or entrained out of the reaction zone before completion of the pyrolysis process. For the large biomass particles, the bio-oil yield was found to be low because the larger biomass particles have lower heat transfer. The large particles are usually not complete pyrolyzed, leading to the formation of biochar [36, 69].
The biomass particle sizes not only influence the bio-oil yield but also affect the quality of the bio-oil or liquid product in terms of water content and solid content. The water content of the bio-oil was increased in line with an increase in biomass particle size. This is because the large particles provided a low heat transfer rate. The high water content in the bio-oil also decreased its heating value [21]. For the effect of biomass particle size on solid content in the bio-oil, pyrolysis of small biomass particles using a moving bed reactor or under a high carrier gases flow rate creates the risk of producing bio-oil with high solid content. This is because the small particles have a low density, which is easily carried out from the reaction zone before the pyrolysis is complete. However, in practice, the desirable biomass particle size for pyrolysis processes may range from millimeters to centimeters, depending on the capacity and type of the pyrolysis reactors [36]. Moreover, the biomass particle size directly affects the preparation cost, transportation, storage, and feeding system [70].
3.2 Bulk density of ground samples
Figure 1 shows the bulk density of the ground OPB, which was in the range of 151.83–895.96 kg/m3, depending on the type of OPB. The OPSS had the highest bulk density (895.96 kg/m3), followed by the OPS (702.61 kg/m3) and the OPDC (592.88 kg/m3), while the OPT had the lowest bulk density of 151.83 kg/m3. When comparing the bulk density of the ground OPB with ground cassava rhizomes (230 kg/m3) and cassava stalk (200 kg/3), the results indicate that the bulk density of the OPT, OPF, OPR, and OPFB is relatively lower than that of the cassava biomass [71]. For the biofuel and bioenergy application of biomass, the bulk density of the biomass particles is one of the most important physical properties. Bulk density of biomass or biomass particles is defined as the mass of the biomass particles divided by the total volume of those particles in the standard container. The total volume includes the volume of the biomass particles, the void volume between the biomass particles, and the volume of the pores inside the biomass particles.
Usually, each biomass has a different bulk density because it has different physical features and structure, as well as different compositions. In the case of OPB particles, such as OPT, OPF, EFB, OPR, and OPPF, they have low bulk density because these biomasses are fibrous and bulky. The chopping or grinding of dried fibrous biomass into powder or small particles helps to improve its bulk density. However, the bulk density of the ground sample is relatively low compared to solid or dense particles such as in coal and sand. For the ground OPS, OPSS, and OPDC samples, they have high bulk density since these biomasses have a solid or dense structure. Grinding dense biomass provides small and dense solid particles, leading to high bulk density. In the pyrolysis process, the bulk density of the biomass particles is one of the key parameters for designing the pyrolysis reactor and feeding system of continuous pyrolysis systems. Beside the reactor size, the bulk density of biomass also directly influences energy density (MJ/m3), storage area requirement and cost, and transportation cost [72,73,74,75].
3.3 Proximate analysis results
Table 2 shows the results of the proximate analysis, including the moisture content (MC), volatile matter (VM), fixed carbon content (FC), and ash content (AC) of the OPB samples. The results show that the MC, VM, FC, and AC of the OPB samples were in the ranges of 8.11–14.42, 51.70–79.98, 11.42–26.14, and 1.35–36.43 (wt.%), respectively. The moisture content of the biomass samples after drying with a solar greenhouse dryer was relatively low, implying that these biomass samples are appropriate for being used as raw biomass to produce biofuels via pyrolysis processes. In pyrolysis processes, particularly in the production of bio-oil through the fast pyrolysis process, the initial moisture content of the biomass strongly influences the water content in the obtained bio-oil or liquid product [21, 76]. The water content of the bio-oil usually varies in the range of 15–40% (wt., wet basis), depending on the moisture content of the biomass and pyrolysis conditions [69, 77, 78]. The pyrolysis of biomass with high moisture content results in bio-oil with high water content. Thus, the moisture content in the biomass should be as low as possible in practice. However, the water content in the bio-oil or liquid product not only depends on the moisture content in the biomass but also is influenced by the pyrolysis conditions, such as the heating rate, reactor type, vapor residence time, and pyrolysis temperature [77, 78]. Aside from these aspects, the pyrolysis of moistened biomass also leads to a loss of energy during dehydration. In practice, the biomass can be dried to the desired moisture content lower than 10% (wt. wet basis). However, drying biomass to the low moisture content affects energy consumption and cost. Thus, the biomass can be dried from the waste heat or the heat obtained from the combustion of the pyrolysis gas.
For volatile matter, the results reveal that the OPT, OPF, EFB, and OPFB had volatile mater in the range of 75–80% (wt.), which is close to the volatile matter results of pinewood (81% wt.) [79], cassava rhizomes (81.5% wt.,), and cassava stalk (81.2% wt.) [69]. The volatile matter of the OPR, OPS, and OPDC was in the range of 68–72% (wt.). The OPSS had the lowest amount of volatile matter (51.70% wt.), which is not much different from the works of [80,81,82]. The OPT, OPF, and EFB had high volatile matter content because they contain high amounts of cellulose and hemicellulose, while the amount of lignin is low (this will be discussed in more detail in the next part.). Volatile matter is an essential component of biomass for producing bio-oil via pyrolysis processes because the yield of bio-oil or liquid product depends on the volatile matter, which is the part of the solid biomass that can be converted into vapors or gases in the form of both condensable and non-condensable vapors. The previous works related to pyrolysis processes have reported that the pyrolysis of biomass with the high volatile matter provided high yields of bio-oil or liquid products [23]. In the part of fixed carbon and ash content, it can be seen in Table 2 that the OPS and OPR had fixed carbon content higher than 20% (wt.), and the OPDC and OPSS had relatively high ash content (14.79, 36.43% wt.). For the pyrolysis process, the fixed carbon content is the part of the biomass that cannot be converted into vapors or gases. This product is called biochar. Thus, for the pyrolysis of biomass to produce a high yield of biochar, biomass with the high fixed carbon content is appropriate. The last part of the proximate analysis is the ash content, which is an inorganic composition in biomass. Biomass with the high ash content indicates that it is not suitable for bio-oil production via pyrolysis processes because it leads to a low yield of bio-oil (liquid product) and high amounts of biochar. However, producing bio-oil by the co-pyrolysis of sewage sludge with woody and non-woody biomass can help to improve the bio-oil yield [47, 80, 81].
3.4 Ultimate analysis results
Table 3 shows the ultimate analysis results, including carbon (C), nitrogen (N), hydrogen (H), sulfur (S), and oxygen (O) content of the OPB samples. The atomic ratio (H/C and O/C) of the biomass is also presented in Fig. 2. The results show that the C, N, H, O, and S content of the OPB samples were in the ranges of 32.39–53.29, 0.19–5.07, 4.83–6.92, 32.08–44.60, and < 0.01–0.77% (wt.). These results indicate that the type of OPB used affected the elemental composition. The carbon and hydrogen of the OPB samples (except OPSS) were relatively high compared to the elemental composition of the lignocellulosic biomass such as cassava rhizomes, cassava stalk, wheat straw, corn stalk, wood sawdust, corncob, and rice husk [69, 82, 83]. The results of the ultimate analysis are consistent with the proximate analysis results. High fixed carbon content in the biomass leads to a high carbon element as clearly observed in the cases of OPS, OPR, and OPFB. Sukiran (2016) [82] reported that there is a good relationship between atomic H/C and O/C ratios in OPB, indicating fairly high energy contained in the C–H and C–O bonds. Chaiwong et al. (2013) [84] explained that a high ratio of H/C indicates a high content of aliphatic hydrocarbon compounds in the biomass. A relatively low O/C ratio in the biomass indicates that it has a small number of polar compounds [84]. For the oxygen content, it was found that the OPT, OPF, EFB, and OPSS had an oxygen content of about 32–33% (wt.), which was lower than the oxygen content in the OPS, OPR, and OPFB (41.54–44.60% wt.).
For bio-oil production via pyrolysis processes, the biomass with high carbon, high hydrogen, and low oxygen content is favorable [85]. This is because carbon and hydrogen can be converted into useful aromatics, bio-oil, or liquid products. On the other hand, the oxygen will bond with the hydrocarbon molecules as phenol, acetic acid, and other oxygenated compounds, which is a disadvantage of biomass for producing bio-oil. Besides, the pyrolysis of biomass with high oxygen content presents the risk of obtaining bio-oil or liquid products with high water content. This is because reaction water can be formed from the reaction between hydrogen and oxygen [85]. The higher water content in bio-oil leads to lower heating values, as well as the instability of liquid products [76, 86]. For nitrogen and sulfur, they have a small bio-oil or liquid composition. These elements are transformed by pyrolysis into gas products. In terms of the nitrogen and sulfur content in the OPB samples, they were found to be low, except in the OPSS which had high contents of both nitrogen and sulfur. The nitrogen and sulfur content levels of the biomass samples obtained from this study are consistent with the previous studies [47, 80, 81]. The nitrogen and sulfur content in the biomass have less influence on the nitrogen and sulfur content in the bio-oil. However, most of the sulfur and nitrogen remained in the biochar [84, 87].
3.5 Minor and major elemental compositions
The minor and major elements in the OPB samples are shown in Table 4. It can be clearly seen that the minor and major elements of the OPB samples are silicon (Si), iron (Fe), calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K), with the content of these elements ranging from 142.07 to 10,907.68 mg/kg for Si, 581.11 to 7959.66 mg/kg for Fe, 841.39 to 21,407.33 mg/kg for Ca, 10.98 to 18,892.55 mg/kg for Mg, 47.87 to 526.18 mg/kg for Na, and 175.63 to 70,791.31 mg/kg for K. The investigated OPB samples had very low levels of cadmium (Cd) and lead (Pb), ranging from 0.10 to 1.55 mg/kg and 0.96 to 10.47 mg/kg, respectively. The OPSS had the highest content of the minor and major elements, which is consistent with the results of the proximate analysis, indicating the highest ash content is in the OPSS. For biomass, some of the minor and major elements function as macro and micronutrients. These results indicate that OPB is a biomaterial which is safe to be used as biofuel and bioenergy because it contains very low levels of heavy metals (Cd and Pb). The obtained results were consistent with the results of Loh, 2016 [82]. However, there are many works which have revealed the alkali content of OPB, such as the amount of Na, Mg, K, and Ca, to be especially important in thermochemical conversion processes. This is because these elements may react with the Si, which then leads to operational problems in the reactors, furnaces, and boilers [82, 88, 89].
For the pyrolysis process, Alvarez et al. (2015) reported that the metals in the biomass resulted in catalytic activity on the pyrolysis products by promoting secondary reactions such as cracking and dehydration [73]. These effects led to obtaining low bio-oil yields and high gas yields. Moreover, Eom et al. (2012) concluded that some inorganic elements such as Ca, Mg, and K would promote the degradation of cellulose and hemicellulose from the biomass, favoring the formation of gas [90]. Additionally, many papers have investigated the effects of catalytic activity of inorganic elements in co-pyrolysis processes [47, 90, 91]. Zhang et al. (2015) found the synergetic effect of the co-pyrolysis of sewage sludge and rice husk [92]. The high inorganic elements in sewage sludge led to a high gas yield of co-pyrolysis. Zuo et al. (2014) also found that the co-pyrolysis of poplar sawdust and sewage sludge in a fixed bed reactor provided the maximum yield of bio-oil when they mixed 80% sludge and 20% sawdust [93]. Thus, the co-pyrolysis of OPB such as OPT, OPR, OPS, EFB, and OPFB with OPSS is still interesting.
3.6 Higher heating value and potential use as energy equivalent with fossil fuels
The higher heating value (HHV) or gross calorific value (GCV) of the OPB samples obtained from both determinations by bomb calorimeter and estimation by correlations is shown in Table 5. It can be seen that the HHV of the OPB obtained from the bomb calorimeter was in the range of 11.19–18.72 MJ/kg. The HHV (HHV1-HHV4) can be estimated by correlations, but the accuracy of the results depends on the correlation used. Based on the values of the estimated HHVs, they indicated that the HHV estimated by using the results from the proximate analysis provided the best estimation values for all biomass samples. The obtained HHV of the OPB from this study was consistent with the results obtained from Sukiran, 2016 [82]. The heating value (both LHV and HHV) of the biomass depends on many factors such the type, composition, age, plantation region, and weather conditions. The composition of the biomass is one of the most important factors that influence its heating value. Normally, biomass consists of moisture, organic components, and inorganic components.
For energy applications, the biomass should contain the lowest moisture content possible. This is because biomass with the high moisture content will release low useful thermal energy (net heating value or lower heating value). The heating value of the biomass depends on the gross components, including the volatile matter (VM), fixed carbon content (FC), and ash content (AC). Biomass with a high VM and FC, but with low AC, will provide high heating values such as in the cases of the OPS, EFB, and OPFB for OPB samples. The heating value of the biomass can also be described by ultimate analysis results based on the relative content of the main elements, including C, H, and O. This is due to the fact that the overall values of these elements are directly related to the biochemical components of the cell wall, such as the lignocellulose content [3]. In the case of OPB, it can be seen that the biomass samples with high cellulose and lignin tended to have high HHV. The previous works reported that lignin has higher levels of HHV than cellulose and starch [63]. The OPB samples have high calorific values conforming the high carbon and hydrogen content of the biomass as well as its low moisture content, as shown in Table 3.
For the pyrolysis process, the heating value of the biomass did not affect the heating value of the bio-oil. The biomass with high HHV provided bio-oil with a low HHV [69]. This is because different types of biomass with the same heating value may have different compositions (volatile matter, fixed carbon content, lignocellulose content). These components can be converted into condensable vapors under different conditions during the pyrolysis process [92]. Table 6 shows the potential use of the OPB samples as energy equivalent with fossil fuels. These HHVs were calculated to the equivalent quantity of fossil fuels, including crude oil, coal, LPG, and NG. The potential uses of the OPB samples as energy equivalent with crude oil, coal, LPG, and NG were 0.29–0.52 L, 0.39–0.70 kg, 0.22–0.40 kg, and 0.29–0.52 m3, respectively. These results indicated that the utilization of OPB as bioenergy could help to reduce the reliance on fossil fuels and subsequently lessen environmental impacts, particularly the net carbon dioxide emission.
3.7 Lignocellulose content
Table 7 shows the chemical composition of the OPB samples in terms of cellulose, hemicellulose, lignin, and extractives. The cellulose, hemicellulose, lignin, and extractives of the OPB were in the range of 2.59–54.35, 8.25–25.97, 6.64–38.15, and 2.55–58.69% (wt.), respectively. The type of OPB had a strong effect on lignocellulosic compositions. The OPT, OPF, EFB, and OPFB had high cellulose and hemicellulose content. The OPS, OPR, and OPFB had high cellulose content, but their lignin content was also high. The OPDC and OPSS had high extractives, which are part of the inorganic elements and ash. The ratios of hemicellulose/lignin and cellulose/lignin are depicted in Fig. 3. It can be seen that the OPT, OPF, EFB, and OPFB had relatively high ratios, indicating high cellulose and hemicellulose content in the biomass samples. For the pyrolysis process, biomass with the high cellulose and hemicellulose contents usually produces higher yields of bio-oil or liquid than biomass with high lignin content. This is because the thermal decomposition of lignin is more difficult than that of cellulose and hemicellulose. The pyrolysis of biomass with a high lignin content usually leads to a high biochar yield [94]. The effects of the cellulose, hemicellulose, and lignin content of the biomass on the yield of pyrolysis products have been studied by many authors. Quan et al. (2016) studied the pyrolysis behavior of cellulose, hemicellulose, and lignin at 500 °C [95]. They found that the pyrolysis of these components provided liquid yields of 18.67, 30.83, and 0.5% (wt.), respectively. The mentioned effects were also investigated by Kim et al. (2013), who found a higher bio-oil yield when using biomass with higher cellulose and hemicellulose content [43]. Qu et al. (2011) explained that the pyrolysis of cellulose provided a high bio-oil yield because cellulose is more volatile than hemicellulose [96].
In addition to the liquid yield, variations in the composition of the cellulose, hemicellulose, and lignin in the biomass also influence the chemical compounds of the bio-oil or liquid products. For biomass with high levels of cellulose, the liquid fraction usually contains acids, alcohols, aldehydes, ketones, esters, heterocyclic derivatives, and phenolic compounds [97]. Stefanidis et al. (2014) studied the pyrolysis of cellulose, hemicellulose, and lignin separately [98]. They found that the liquid products obtained from cellulose pyrolysis were composed of sugars, simple phenols, ketones, aldehydes, and alcohols. The main compounds in bio-oil produced from hemicellulose pyrolysis were ketones, phenols, acids, and aldehydes. The bio-oil produced from lignin is composed of complex phenols with high molecular weights [98, 99].
3.8 Thermal decomposition behavior
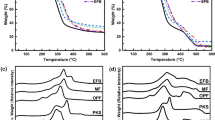
The thermal decomposition behavior of the OPB via TGA and DTA is shown in Fig. 4a, b, respectively. The observation results indicate that there are four main stages (as shown in the DTA curves) in the thermal decomposition of the OPB samples. The thermal decomposition of the OPB samples also occurred at different trends due to the different components of the lignocellulosic biomass, namely, cellulose, hemicelluloses, and lignin. The first stage occurred at temperatures of 50–100 °C. At this stage, the weight of the biomass decreased slightly due to the evaporation of the moisture content. In the second stage at temperatures of 100–250 °C, the weight of the OPB was relatively constant due to the lower amount of evaporation of the light volatile compounds. Most of the thermal energy supplied to the biomass was used to increase its temperature during this stage. The next stage is the main thermal decomposition of the OPB, which was occurred at temperatures of 250–400 °C. For the last stage, the thermal degradation of the biomass samples was prolonged due to the decomposition of the lignin. The weight of the biomass samples decreased mainly in the third stage due to the decomposition of the cellulose and hemicellulose into both condensable and non-condensable vapors.
A previous study found that the decomposition of hemicellulose, generally represented by xylan, mainly takes place within the temperature range of 250–350 °C, followed by cellulose decomposition, which primarily occurs at the temperature range of 325–400 °C with levoglucosan as the main pyrolysis product. Recent investigations have reported that lignin was the most stable component, decomposing in the higher temperature range of 300–900 °C [100]. Figure 4a also showed the less thermal decomposition of the OPSS. This is because the main composition of the OPSS was extractives, which are mainly composed of inorganic elements and ash. The thermal decomposition trends of the OPB shown in Fig. 4a were consistent with the trends for the lignocellulosic content of the OPB samples shown in Table 7. The lignin is covalently linked to hemicellulose and cross-linked to the polysaccharide, and thus, it is highly rigid and hard. Besides, lignin consists mostly of aromatic rings; thus, the bio-oil produced from lignin biomass is rich in aromatic compounds [98].
3.9 Thermal conductivity and dielectric properties
Table 8 shows the thermal conductivity and dielectric property of the OPB samples. The results reveal that the thermal conductivity of the OPB samples was in the range of 0.08–0.17 W/m•K. The OPS, OPDC, OPFB, and OPSS had thermal conductivity higher than 0.10 W/m•K, while the OPSS had the highest thermal conductivity (0.17 W/m•K). For the dielectric property of the OPB samples, it was reported in terms of dielectric constant (ε’), dielectric loss factor (ε”), and tangent loss (tan δ). The dielectric constant (ε’), dielectric loss factor (ε”), and tangent loss (tan δ) of the OPB samples were in the ranges of 0.95–2.03, 0.00–0.10, and 0.00–0.05, respectively. The dielectric property of the OPS, OPDC, OPFB, and OPSS was relatively high, which is consistent with the values of thermal conductivity. Comparing the dielectric property and thermal conductivity of the biomass samples with their inorganic elements indicated that the OPB with high Fe, K, and Ca content also provided high values of dielectric property and thermal conductivity. These results indicate that the type of OPB affected the thermal conductivity and dielectric property. The solid biomass has low thermal conductivity because it is an anisotropic material [101].
Many previous studies have determined the thermal conductivity of biomass. For example, Mason et al. (2016) reported that the thermal conductivities of dried wood pellets, miscanthus, and willow were between 0.10 and 0.12 W/m•K, while agricultural residues such as wheat and rape straws had a relatively low thermal conductivity value of 0.05 W/m•K [102]. Gupta et al. (2003) found that the thermal conductivities of softwood, softwood bark, and softwood char were in the range of 0.095–0.205 W/m•K [103]. For the pyrolysis process, the thermal conductivity of biomass strongly affects the heating systems and reactor design, heating rate, and kinetics of pyrolysis [51]. The significance of the heat transfer properties of small biomass particles in evaluating the chemical kinetics of pyrolysis and char combustion has been described by Hayhurst [104]. Differences in thermal conductivity affect the internal temperatures and heating rates in the particle, which, in turn, affect the reaction kinetics [105].
For the dielectric property, it is the key property of the materials or biomass for microwave heating. This is because the microwave absorbability and conversion to the heat of any materials depends on the dielectric property. The ε’ represents the ability of molecules to become polarized under the electric field. The ε” indicates the ability of materials to convert electromagnetic energy into heat. The ability or potential of materials that can be heated by microwave can be evaluated by the loss tangent, which is the ratio of the dielectric loss factor to the dielectric constant of the materials [52, 106]. In general, materials can be classified into three types according to their loss tangent, namely, high (> 0.5), medium (0.1–0.5), and low (< 0.1) microwave absorbing materials [107]. In the case of OPB, the loss tangent of oil palm fiber, oil palm shell, and empty fruit bunches was 0.08, 0.12, and 0.3, respectively [53, 106]. The loss tangent of biomass is low when compared to the loss tangent of carbon materials such as biochar and activated carbon (0.1–0.8). Consequently, carbon materials are good microwave absorbers with a high capacity to absorb and convert microwave energy into heat [108, 109]. Thus, the biomass pyrolysis heated by microwave irradiation could be a more promising heating technique because of the many advantages it offers over conventional heating methods [52, 106, 110].
4 Conclusions
This study determined the physicochemical properties of oil palm biomass (OPB), including oil palm trunk (OPT), oil palm fronds (OPF), oil palm shell (OPS), oil palm roots (OPR), oil palm decanter cake (OPDC), empty fruit bunches (EFB), oil palm fiber (OPFB), and oil palm sewage sludge (OPSS) to evaluate the potential use for producing biofuels via pyrolysis processes. The physicochemical properties of the OPB were considered from particle size distribution, bulk density, proximate and ultimate analysis, higher heating value and potential use as energy equivalent with fossil fuels, elemental composition, lignocellulose content, thermogravimetric analysis, thermal conductivity, and dielectric properties. The results indicate that:
-
Most of the ground OPB had low bulk density, except for the OPS, OPDC, and OPSS which had relatively high bulk density.
-
The OPT, OPF, OPFB, and EFB contained high amounts of volatile matter (75.43–79.98%). The OPS and OPR had high amounts of fixed carbon, while the OPDC and OPSS contained high ash content. The main elemental compositions of OPB were consistent with the proximate analysis results.
-
The higher heating value (HHV) of OPB was in the range of 11.19–18.72 MJ/kg, which was relatively low as compared to fossil fuels.
-
The OPF and OPR had the high cellulose content, while the OPT and OPFB had the high hemicellulose content. The OPS, OPR, and OPSS had high lignin. The extractives were high in OPDC and OPSS. The thermal decomposition trend of the OPB depended on the lignocellulosic components, particularly the lignin.
-
The content of inorganic elements such as potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) in the OPB depended on the OPB types. The inorganic elements in the OPB may act as a catalyst for the pyrolysis processes, which influence the product yield and quality.
-
The thermal conductivity and dielectric properties of OPB were low. The need for an appropriate design of heating unit both conventional and microwave heating. The microwave heating of the OPB may be applied with carbon materials for improving the heat generation and heating rate inside the reactor.
Therefore, based on these results, it can be concluded that some OPB has the potential to produce biofuels via pyrolysis processes, while the co-pyrolysis of the OPB may help to improve the utilization of OPB, waste, and residues. The results from this study will help the researchers and biomass industries to identify the most appropriate way to convert OPB into biofuels via pyrolysis processes.
References
Abuelnuor AAA, Wahid MA, Hosseini SE et al (2014) Characteristics of biomass in flameless combustion: a review. Renew Sust Energ Rev 33:363–370. https://doi.org/10.1016/j.rser.2014.01.079
Demirbaş A (2001) Relationships between lignin contents and heating values of biomass. Energy Convers Manag 42:183–188
Tanger P, Field JL, Jahn CE et al (2013) Biomass for thermochemical conversion: targets and challenges. Front Plant Sci 4:218. https://doi.org/10.3389/fpls.2013.00218
Yelmen B, Tarık Çakir M (2016) Biomass potential of Turkey and energy production applications. Energy Sources, Part B Econ Planning, Policy 11:428–435. https://doi.org/10.1080/15567249.2011.613443
British petroleum (2017) BP statistical review of world energy. BP p.l.c. 2017. https://euagenda.eu/upload/publications/untitled-96772-ea/. Accessed 26 June 2019
Mishra RK, Mohanty K (2019) Pyrolysis characteristics, fuel properties, and compositional study of Madhuca longifolia seeds over metal oxide catalysts. Biomass Convers Biorefinery:1–17. https://doi.org/10.1007/s13399-019-00469-3
Abbasi T, Abbasi SA (2010) Biomass energy and the environmental impacts associated with its production and utilization. Renew Sust Energ Rev 14:919–937. https://doi.org/10.1016/j.rser.2009.11.006
Laser M, Lynd LR (2014) Comparative efficiency and driving range of light- and heavy-duty vehicles powered with biomass energy stored in liquid fuels or batteries. Proc Natl Acad Sci 111:3360–3364. https://doi.org/10.1073/pnas.1314039111
Khosravanipour Mostafazadeh A, Solomatnikova O, Drogui P, Tyagi RD (2018) A review of recent research and developments in fast pyrolysis and bio-oil upgrading. Biomass Convers Biorefinery 8:739–773. https://doi.org/10.1007/s13399-018-0320-z
Fernando AL, Duarte MP, Almeida J, Boléo S, Mendes B (2010) Environmental impact assessment of energy crops cultivation in Europe. Biofuels Bioprod Biorefin 4:594–604. https://doi.org/10.1002/bbb.249
Jagustyn B, Patyna I, Skawińska A (2013) Evaluation of physicochemical properties of palm kernel shell as agro biomass used in the energy industry. Chemik 67:552–559
Preechajarn S, Prasertsri P (2018) Thailand biofuels annual. USDA foreign agricultural service. https://www.biofuelsdigest.com/bdigest/tag/thailand/thaibiofuelsannual2018. Accessed 2 July 2019
Hosseini SE, Wahid MA, Ganjehkaviri A (2015) An overview of renewable hydrogen production from thermochemical process of oil palm solid waste in Malaysia. Energy Convers Manag 94:415–429. https://doi.org/10.1016/j.enconman.2015.02.012
Asadullah M, Ab Rasid NS, Kadir SASA, Azdarpour A (2013) Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy 59:316–324. https://doi.org/10.1016/j.biombioe.2013.08.037
Prasertsan S, Presertsan P (1996) Biomass residues from palm oil mills in Thailand: an overview on quantity and potential usage. Biomass Bioenergy 11:387–395. https://doi.org/10.1016/S0961-9534(96)00034-7
Kurnia JC, Jangam SV, Akhtar S, Sasmito AP, Mujumdar AS (2016) Prolysis advances in biofuel from oil palm and palm oil processing waste: a review. Biofuel res. J. 9:332–346. https://doi.org/10.18331/BRJ2016.3.1.3
Loh SK (2017) The potential of the Malaysian OPB as a renewable energy source. Energy Convers Manag 141:285–298
Iskandar MJ, Baharum A, Anuar FH, Othaman R (2018) Palm oil industry in South East Asia and the effluent treatment technology—a review. Environ Technol Innov 9:169–185. https://doi.org/10.1016/j.eti.2017.11.003
Areerat K (2006) Appropriate technology evaluation for oil palm by-products utilization in Krabi province. Dissertation, Mahidol University
Papong S, Yuvaniyama C, Lohsomboon P, Malakul P (2004) Overview of biomass utilization in Thailand. In: ASEAN Biomass Meeting, Bangkok, pp 1–10
IRENA (2015) A background paper to renewable energy in manufacturing. https://www.irena.org/publications/2014/Jun/Renewable-Energy-in-Manufacturing. Accessed 2 July 2019
Shuit SH, Tan KT, Lee KT, Kamaruddin AH (2009) OPB as a sustainable energy source: a Malaysian case study. Energy 34:1225–1235. https://doi.org/10.1016/j.energy.2009.05.008
Mckendry P (2002) Energy production from biomass (part 2): conversion technologies. Bioresour Technol 83:47–54. https://doi.org/10.1016/S0960-8524(01)00119-5
Velghe I, Carleer R, Yperman J, Schreurs S (2011) Study of the pyrolysis of municipal solid waste for the production of valuable products. J Anal Appl Pyrolysis 92:366–375. https://doi.org/10.1016/j.jaap.2011.07.011
Bridgwater AV, Peacocke GVC (2000) Fast pyrolysis processes for biomass. Renew Sust Energ Rev 4:1–73
Yaman S (2004) Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manag 45:651–671. https://doi.org/10.1016/S0196-8904(03)00177-8
Şensöz S, Can M (2002) Pyrolysis of pine ( Pinus Brutia ten.) chips: 1. effect of pyrolysis temperature and heating rate on the product yields. Energy Sources 24:347–355. https://doi.org/10.1080/00908310252888727
Shastri Y, Hansen A, Rodríguez L, Ting KC (2014) Engineering and science of biomass feedstock production and provision. Springer Science + Business media, New York
Lozano-García B, Parras-Alcántara L (2013) Short-term effects of olive mill by-products on soil organic carbon, total N, C: N ratio and stratification ratios in a Mediterranean olive grove. Agric Ecosyst Environ 165:68–73
Bridgwater AV (1999) Principles and practice of biomass fast pyrolysis processes for liquids. J Anal Appl Pyrolysis 51:3–22
Meier D, Faix, O (1999) State of the art of applied fast pyrolysis of lignocellulosic materials-a review. Bioresour Technol 68, 71–77. https://doi.org/10.1016/S0960-8524(98)00086-8
Bridgwater AV (2012) Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 38:68–94. https://doi.org/10.1016/j.biombioe.2011.01.048
Isahak WNRW, Hisham MWM, Yarmo MA, Yun Hin T (2012) A review on bio-oil production from biomass by using pyrolysis method. Renew Sust Energ Rev 16:5910–5923. https://doi.org/10.1016/j.rser.2012.05.039
Fonts I, Gea G, Azuara M, Ábrego J, Arauzo J (2012) Sewage sludge pyrolysis for liquid production: a review. Renew Sust Energ Rev 16:2781–2805. https://doi.org/10.1016/j.rser.2012.02.070
Sirijanusorn S, Sriprateep K, Pattiya A (2013) Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour Technol 139:343–348
Alvarez J, Amutio M, Lopez G, Bilbao J, Olazar M (2015) Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 159:810–818
Huang X, Cao J-P, Zhao X-Y et al (2016) Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel 169:93–98. https://doi.org/10.1016/j.fuel.2015.12.011
Wang Y, Zeng Z, Tian X, Dai L, Jiang L, Zhang S, Wu Q, Wen P, Fu G, Liu Y, Ruan R (2018) Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour Technol 269:162–168
Leng L, Huang H (2018) An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour Technol 270:627–642
Chen X, Li S, Liu Z, Chen Y, Yang H, Wang X, Che Q, Chen W, Chen H (2019) Pyrolysis characteristics of lignocellulosic biomass components in the presence of CaO. Bioresour Technol 287:21493
Pattiya A (2011) Bio-oil production via fast pyrolysis of biomass residues from cassava plants in a fluidised-bed reactor. Bioresour Technol 102:1959–1967
Pattiya A, Suttibak S (2012a) Influence of a glass wool hot vapour filter on yields and properties of bio-oil derived from rapid pyrolysis of paddy residues. Bioresour Technol 116:107–113
Kim SS, Ly HV, Kim J, Choi JH, Woo HC (2013) Thermogravimetric characteristics and pyrolysis kinetics of alga Sagarssum sp. biomass. Bioresour Technol 139:242–248
Kelkar S, Saffron CM, Chai L et al (2015) Pyrolysis of spent coffee grounds using a screw-conveyor reactor. Fuel process Technol 137:170–178. https://doi.org/10.1016/j.fuproc.2015.04.006
Biswas B, Pandey N, Bisht Y, Singh R, Kumar J, Bhaskar T (2017) Pyrolysis of agricultural biomass residues: comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour Technol 237:57–63
Fiore S, Berruti F, Briens C (2018) Investigation of innovative and conventional pyrolysis of ligneous and herbaceous biomasses for biochar production. Biomass Bioenergy 119:381–391. https://doi.org/10.1016/j.biombioe.2018.10.010
Ani FN, Zailani R (1997) Characteristics of pyrolysis oil and char from oil Palm shells. Dev. Thermochem. Biomass convers. Springer Netherlands, Dordrecht, pp 425–432. https://doi.org/10.1007/978-94-009-1559-6_33
Okoroigwe E, Li Z, Stuecken T, Saffron C, Onyegegbu S (2012) Pyrolysis of Gmelina arborea wood for bio-oil/bio-char production: physical and chemical characterisation of products. J Appl Sci. https://doi.org/10.3923/jas.2012.369.374
Saidur R, Abdelaziz EA, Demirbas A, Hossain MS, Mekhilef S (2011) A review on biomass as a fuel for boilers. Renew. Sustain. Energy rev. 15:2262–2289. https://doi.org/10.1016/j.rser.2011.02.015
Acda MN (2016) Physical and chemical properties of fuel pellets from agricultural residues. Former Philipp Agric 99:283–287
Abdullah NA, Mohamed R, Mahmood WMFW, Saad MHM (2017) Black smoke elimination via PID controlled co-firing technique at palm oil mill. Int J Appl Eng Res 12:8050–8056
Salema AA, Ani FN (2011) Microwave induced pyrolysis of OPB. Bioresour Technol 102:3388–3395
Omar R, Idris A, Yunus R, Khalid K, Isma MIA (2011) Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 90:1536–1544
Ruengvilairat P, Tanatavikorn H, Vitidsant T (2012) Bio-oil production by pyrolysis of oil palm empty fruit bunch in nitrogen and steam atmospheres. J Sustain Bioenergy Syst 2:75
Kabir G, Mohd Din AT, Hameed BH (2017) Pyrolysis of oil palm mesocarp fiber and palm frond in a slow-heating fixed-bed reactor: a comparative study. Bioresour Technol 241:563–572
Sukiran MA, Loh SK, Bakar NA (2016) Production of bio-oil from fast pyrolysis of oil palm biomass using fluidised bed reactor. J Energy Technol Policy 6:52–62
Yakub MI, Abdalla AY, Feroz KK, Suzana Y, Ibraheem A, Chin SA (2015) Pyrolysis of oil palm residues in a fixed bed tubular reactor. Journal of Power and Energy Engineering 3:185–193
Sareekam N, Kamarudin SK, Kasmuri NH (2016) Optimization of bio oil from palm oil fronds via fast pyrolysis. Indian J Sci Technol 9:1–13
Bensidhom G, Hassen-Trabelsia AB, Alper K, Sghairoun M, Zaafouri K, Trabelsi I (2018) Pyrolysis of date palm waste in a fixed-bed reactor: characterization of pyrolytic products. Bioresour Technol 247:363–369
Annamalai K, Sweeten JM, Ramalingam SC (1987) Technical notes: estimation of gross heating values of biomass fuels. Trans ASAE 30:1205–1208
Parikh J, Channiwala S, Ghosal G (2005) A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 84:487–494. https://doi.org/10.1016/j.fuel.2004.10.010
Sheng C, Azevedo JLT (2005) Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 28:499–507
Demirbaş A (2001) Relationships between lignin contents and heating values of biomass. Energy Convers Manag 42:183–188
Goering HK, Van Soest PJ (1970) Forage fiber analysis, apparatus, reagents, procedures, and some applications. In: Agriculture handbook, vol. 379. ARS-USDA, Washington, DC
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Reza MT, Uddin MH, Lynam JG, Hoekman SK, Coronella CJ (2014) Hydrothermal carbonization of loblolly pine: reaction chemistry and water balance. Biomass Convers Biorefinery 4:311–321. https://doi.org/10.1007/s13399-014-0115-9
Kambo HS, Dutta A (2015) Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers Manag 105:746–755. https://doi.org/10.1016/j.enconman.2015.08.031
Salema AA, Yeow YK, Ishaque K et al (2013) Dielectric properties and microwave heating of oil palm biomass and biochar. Ind Crop Prod 50:366–374. https://doi.org/10.1016/j.indcrop.2013.08.007
Pattiya A, Suttibak S (2012) Production of bio-oil via fast pyrolysis of agricultural residues from cassava plantations in a fluidised-bed reactor with a hot vapour filtration unit. J Anal Appl Pyrolysis 95:227–235
Duong TL, Nguyen DT, Nguyen HHM, Phan BMQ, Nguyen HL, Huynh TM (2019) Fast pyrolysis of Vietnamese waste biomass: relationship between biomass composition, reaction conditions, and pyrolysis products, and a strategy to use a biomass mixture as feedstock for bio-oil production. J Mater Cycles Waste Manag 21:624–632
Sirijanusorn S, Sriprateep K, Pattiya A (2013) Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour Technol 139:343–348. https://doi.org/10.1016/j.biortech.2013.04.024
Scott DS, Piskorz J (1984) The continuous flash pyrolysis of biomass. Can J Chem Eng 62:404–412. https://doi.org/10.1002/cjce.5450620319
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Brar JS, Singh K, Wang J, Kumar S (2012) Cogasification of coal and biomass: a review. Int J For Res 2012:1–10. https://doi.org/10.1155/2012/363058
Elliott DC, Hart TR, Neuenschwander GG, Rotness LJ, Olarte MV, Zacher AH (2012) Catalytic hydroprocessing of fast pyrolysis bio-oil from pine sawdust. Energy and Fuels 26:3891–3896. https://doi.org/10.1021/ef3004587
Okoroigwe EC, Saffron CM (2012) Determination of bio-energy potential of palm kernel shell by physicochemical characterization. Niger J Technol 31:329–335
Kang B-S, Lee KH, Park HJ, Park Y-K, Kim J-S (2006) Fast pyrolysis of radiata pine in a bench scale plant with a fluidized bed: influence of a char separation system and reaction conditions on the production of bio-oil. J Anal Appl Pyrolysis 76:32–37
Lu Q, Li W-Z, Zhu X-F (2009) Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers Manag 50:1376–1383
Amutio M, Lopez G, Alvarez J, Olazar M, Bilbao J (2015) Fast pyrolysis of eucalyptus waste in a conical spouted bed reactor. Bioresour Technol 194:225–232
Inguanzo M, Domınguez A, Menéndez JA, Blanco CG, Pis JJ (2002) On the pyrolysis of sewage sludge: the influence of pyrolysis conditions on solid, liquid and gas fractions. J Anal Appl Pyrolysis 63:209–222
Domínguez A, Menéndez JA, Inguanzo M, Pis JJ (2006) Production of bio-fuels by high temperature pyrolysis of sewage sludge using conventional and microwave heating. Bioresour Technol 97:1185–1193
Sukiran MA, Loh SK, Bakar NA (2016) Production of bio-oil from fast pyrolysis of OPB using fluidised bed reactor. J Energy Technol Policy 6:52–62
Maddi B, Viamajala S, Varanasi S (2011) Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour Technol 102:11018–11026
Chaiwong K, Kiatsiriroat T, Vorayos N, Thararax C (2013) Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy 56:600–606
Pimenidou P, Dupont V (2012) Characterisation of palm empty fruit bunch (PEFB) and pinewood bio-oils and kinetics of their thermal degradation. Bioresour Technol 109:198–205
De Bari I, Barisano D, Cardinale M, Matera D, Nanna F, Viggiano D (2000) Air gasification of biomass in a downdraft fixed bed: a comparative study of the inorganic and organic products distribution. Energy Fuel 14:889–898. https://doi.org/10.1021/ef990243g
Berndes G, Börjesson P, Ostwald M, Palm M (2008) Multifunctional biomass production systems–an overview with presentation of specific applications in India and Sweden. Biofuels, Bioprod Biorefining Innov a Sustain Econ 2:16–25
Wei X, Schnell U, Hein K (2005) Behaviour of gaseous chlorine and alkali metals during biomass thermal utilisation. Fuel 84:841–848. https://doi.org/10.1016/j.fuel.2004.11.022
Ninduangdee P, Kuprianov VI (2014) Combustion of palm kernel shell in a fluidized bed: optimization of biomass particle size and operating conditions. Energy Convers Manag 85:800–808
Eom I-Y, Kim J-Y, Kim T-S, Lee S-M, Choi D, Choi I-G (2012) Effect of essential inorganic metals on primary thermal degradation of lignocellulosic biomass. Bioresour Technol 104:687–694
Zabeti M, Nguyen TS, Lefferts L, Heeres HJ, Seshan K (2012) In situ catalytic pyrolysis of lignocellulose using alkali-modified amorphous silica alumina. Bioresour Technol 118:374–381
Zhang W, Yuan C, Xu J, Yang X (2015) Beneficial synergetic effect on gas production during co-pyrolysis of sewage sludge and biomass in a vacuum reactor. Bioresour Technol 183:255–258
Zuo W, Jin B, Huang Y, Sun Y (2014) Characterization of top phase oil obtained from co-pyrolysis of sewage sludge and poplar sawdust. Environ Sci Pollut Res 21:9717–9726
Akhtar J, Amin NS (2012) A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew Sust Energ Rev 16:5101–5109
Quan C, Gao N, Song Q (2016) Pyrolysis of biomass components in a TGA and a fixed-bed reactor: thermochemical behaviors, kinetics, and product characterization. J Anal Appl Pyrolysis 121:84–92
Qu X, Liang P, Wang Z, Zhang R, Sun D, Gong X (2011) Pilot development of polygeneration process of circulating fluidized bed combustion combined with coal pyrolysis. Chem Eng Technol 34:61–68
Klass DL (1988) The US biofuels industry. Institute of Gas Technology, Chicago
Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Lappas AA (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150
Chang G, Miao P, Yan X, Wang G, Guo Q (2018) Phenol preparation from catalytic pyrolysis of palm kernel shell at low temperatures. Bioresour Technol 253:214–219
Dhyani V, Bhaskar T (2018) Chapter 2: kinetic analysis of biomass pyrolysis. In: Waste biorefinery. Elsevier, London
Cai J, He Y, Yu X, Banks SW, Yang Y, Zhang X (2017) Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sust Energ Rev 76:309–322
Mason PE, Darvell LI, Jones JM, Williams A (2016) Comparative study of the thermal conductivity of solid biomass fuels. Energy Fuel 30:2158–2163
Gupta M, Yang J, Roy C (2003) Specific heat and thermal conductivity of softwood bark and softwood char particles☆. Fuel 82:919–927
Hayhurst AN (2013) The kinetics of the pyrolysis or devolatilisation of sewage sludge and other solid fuels. Combust Flame 160:138–144
Jones JM, Saddawi A, Dooley B, Mitchell EJS, Werner J, Waldron DJ (2015) Low temperature ignition of biomass. Fuel Process Technol 134:372–377
Huang Y-F, Chiueh P-T, Lo S-L (2016) A review on microwave pyrolysis of lignocellulosic biomass. Sustain Environ Res 26:103–109
Yin C (2012) Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour Technol 120:273–284
Lam SS, Chase HA (2012) A review on waste to energy processes using microwave pyrolysis. Energies 5:4209–4232
Menéndez JA, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo EG (2010) Microwave heating processes involving carbon materials. Fuel Process Technol 91:1–8. https://doi.org/10.1016/j.fuproc.2009.08.021
Robinson J, Dodds C, Stavrinides A, Kingman S, Katrib J, Wu Z (2015) Microwave pyrolysis of biomass: control of process parameters for high pyrolysis oil yields and enhanced oil quality. Energy Fuel 29:1701–1709
Acknowledgments
The authors express their sincere appreciation to Energy Conservation Promotion Fund, Energy Policy and Planning Office (EPPO), Ministry of Energy of Thailand.
Funding
This study was also supported by Thailand Research Fund (Contract number: MRG6280084), Prince of Songkla University (Contract number: RDO610037S), Graduate School, Prince of Songkla University, and Interdisciplinary Graduate School of Energy systems, Prince of Songkla University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrivastava, P., Khongphakdi, P., Palamanit, A. et al. Investigation of physicochemical properties of oil palm biomass for evaluating potential of biofuels production via pyrolysis processes. Biomass Conv. Bioref. 11, 1987–2001 (2021). https://doi.org/10.1007/s13399-019-00596-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00596-x