Abstract
Carbon dioxide (CO2) transfer in the intensive cultivation of microalgae is a crucial process in photobioreactor performance. This study evaluated three operating conditions (bubble size, aeration rate, and CO2 concentration) to improve the growth performance of the microalga Tetradesmus obliquus in a laboratory–scale photobioreactor. Two types of air diffusers were used (glass pipette and a sintered glass diffuser), three aerations rates (0.125, 0.25 and 0.5 vvm), and four CO2–enriched air concentrations (0.04, 0.5, 1.0 and 2.0%) were investigated during the Tetradesmus obliquus cultivations. The results showed that the overall gas-liquid mass transfer coefficient (kLa) CO2 can be raised by increasing the aeration rate and using a sintered glass diffuser; however, CO2 capture efficiency was lower when the highest aeration rates were applied. When the glass diffuser was used at an aeration rate of 0.25 vvm, a kLa CO2 of 11.98 ± 0.6 1/h was provided, in comparison to 4.90 ± 0.19 1/h for the use of pipette at 0.5 vvm (maximum value reached). Similarly, the highest CO2 capture efficiency rate (67.94 ± 3.56%) was found applying an aeration rate of 0.25 vvm. At a CO2 concentration of 1 or 2% the T. obliquus biomass reached approximately 4.3 g/L, values significantly higher (p < 0.05) than the values reported for supplementation of 0.5% (~ 3.9 g/L) and 0.04% (~ 1.5 g/L). In summary, to avoid losses of CO2 to the atmosphere, an addition of 1% CO2 at an aeration rate of 0.25 vvm using a sintered glass diffuser were the optimal conditions to be applied in cylindrical laboratory–scale photobioreactor for T. obliquus growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The continuous increase in the emissions of carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and others greenhouse gases (GHGs) has aggravated climate changes and resulted in several problems in terrestrial and aquatic ecosystems [1]. Particularly, CO2 emissions cause ocean acidification, affecting marine life and fisheries [2]. Several physical, chemical, and biological methods for carbon sequestration have been evaluated, not only in terms of capture efficiency, but also in cost and potential co-products added to the capture. The use of microalgae to capture CO2 is one of the most promising biological ways, because capture is associated with the production of valuable biomass, rich in various metabolites, e.g., lipids, carbohydrates, protein, and other compounds of high commercial value [1, 3]. Nevertheless, CO2 transfer in microalgae cultivation is a key process in photobioreactor performance [4, 5], that can be divided into three distinct stages: (i) CO2 dissolution (transfer from gas to liquid), (ii) CO2 diffusion to microalgae cells and, (iii) CO2 capture by photosynthetic reactions. The combination of all these steps together is defined as the overall gas-liquid mass transfer coefficient (kLa) [6]. This coefficient describes the CO2 mass transfer capacity in a particular system as a function of operating conditions, more precisely the reactor hydrodynamics [7]. In addition, parameters such as aeration and agitation rate, and the type of air diffuser can be adjusted to increase the kLa in photobioreactors [8].
Due to the low partial CO2 pressure in the atmosphere (0.04% v/v), its transfer from gas to liquid usually cannot maintain the demand required for inorganic carbon assimilation during intensive microalgae production, which might limit growth and increase in pH culture medium. The injection of CO2-enriched atmospheric air can prevent this shortage and can boost the CO2 transfer capacity [1]. Despite advances in methods that increase CO2 transport capacity in microalgae cultivation, the direct injection method through CO2-enriched air bubbling is the most widely used strategy, particularly on a laboratory scale. In addition to being a relatively inexpensive way to implement the process in most reactor models, this method is often chosen because it promotes other benefits such as: culture medium mixing – preventing sedimentation and promoting higher cell exposure to the light source; pH control by CO2 dissolution; and O2 excess removal [4, 8].
Furthermore, the critical CO2 concentration to be injected in a microalgae culture should be sufficient to balance the amount of carbon required for optimal growth, limiting CO2 waste, and any additional economic impact [8]. Goldman et al. [10] suggested that when trying to optimize the inorganic carbon supply in photobioreactors, basic gas-liquid mass transport aspects, such as reactor geometry, bubble size, aeration rate and CO2 partial pressure (PCO2) or CO2 concentration, should be assessed as a way to understand the physiological responses to the various combinations of these parameters. Implementing these adjustments can effectively lower the production costs associated with microalgae cultures, thereby enhancing the economic competitiveness and sustainability of the products [11, 12].
Although these parameters are well known, the dynamics of CO2 transfer correlated to microalgal growth and sensibility to shear-forces, are underexplored in intensive microalgae cultures. Much research has focused on investigating the impact of CO2 concentration on microalgae growth. However, comparatively less attention has been given to studying the influence of flow rate and bubble sizes on the rate of mass transfer and CO2 sequestration by microalgae. In view of this, the present study demonstrated the effects of different air flow rates, CO2 concentrations, and bubble size on the growth performance of the green microalga Tetradesmus obliquus cultured in laboratory–scale photobioreactors.
Materials and Methods
CO2 Transfer Evaluation in the Reactor
The influence of different aeration rates and bubble sizes on CO2 transfer in the photobioreactor was evaluated. To achieve different bubble sizes, two autoclavable materials were employd: a diffuser constructed of sintered glass with unknown porosity, 1 cm wide and 2.7 cm tall, and a glass pipette with an internal diameter of 0.3 mm. The average bubble sizes and retention time were measured using digital camera images (Sony, Cyber-shot 16.1, Brazil). The obtained images were subsequently analyzed using ImageJ software. Different aeration flows (0.5, 1 and 2 L/min) were applied to each diffuser providing the aeration rates of 0.125, 0.25 and 0.5 vvm.
The overall gas-liquid mass transfer coefficient for oxygen (kLa O2) was determined by the dynamic method. Only culture medium was used in the kLa assays disregarding the effect of the presence of microalgae on kLa values as already demonstrated by Contreras et al. [13] and Langley et al. [8]. Dissolved oxygen (OD) concentration in the culture medium was measured using an oximeter (YSI, YSI Pro ODO, USA). Initially, nitrogen gas was bubbled until the oxygen concentration reached less than 5% (v/v) saturation. Equation (1) was used to estimate kLa values in the proposed conditions.
Where:
dC/dt = O2 transfer velocity (mg O2/L/h).
C\({ }^{\text{*}}\) = Concentration of O2 saturation, in balance with \({p}_{g}\), according to Henry’s law (mg O2/L).
C = O2 concentration in liquid (mg O2/L).
\({p}_{g}\) = O2 partial pressure in gas bubble (atm).
As proposed by Talbot et al. [14], kLa O2 was precisely converted to kLa CO2 as shown on Eq. (2). DCO2 and DO2 are diffusivity values of CO2 and O2 in the culture medium, respectively.
CO2 Capture Efficiency (ECO2)
The CO2 capture efficiency (ECO2) was determined in kLa essays. It was considered, according to Talbot et al. [15] and Ying et al. [16], that ECO2 can be described as the amount of CO2 being transferred to the liquid, divided by the amount of CO2 being injected into the liquid, according to Eq. (3).
For each condition tested, it was considered that the maximum CO2 transfer capacity is equal to the product, kLa C*, since after a few minutes the CO2 concentration approaches saturation. Therefore, to calculate the amount of CO2 transferred to the system, the Eq. (4) was used.
Where, V\({ }_{\text{L}}\) is the liquid volume.
Tetradesmus obliquus Cultivation
Microalgal Strain and Culture Medium
The freshwater microalga T. obliquus strain was isolated and maintained in the Culture Collection of the Laboratory of Algae Cultivation (LCA-UFSC) in an LCA-AD Medium [17], in 2 L photobioreactors with controlled air at 24 ± 1 °C, under constant agitation by bubbling with atmospheric air with an addition of 0.5% of CO2 (v/v) with an aeration flow of 0.5 L/min. The final concentrations (mg/L) of LCA-AD in the nutrient medium were as follows: NaNO3 1,000; CaCl2.2H2O 25; MgSO4.7H2O 75; K2HPO4 25; KH2PO4 58.3; NaCl 25; Na2EDTA.2H2O 50; KOH 31; FeSO4.7H2O 4.98; ZnSO4.7H2O 0.00882; MnCl2.4H2O 0.00144; (NH4)6MoO7O24.H2O 0.00661; CuSO4.5H2O 0.00157; Co(NO3)2.6H2O 0.0004.
Culture Conditions
Cultures were grown in 5 L borosilicate photobioreactors, with a useful volume of 4.5 L, and dimensions of 16.5 cm diameter per 20.5 cm height. The cultures temperature was kept constant at 24 ± 1 °C and monitored daily. For culture illumination, 80 W daylight tubular fluorescent lamps were employed, positioned at a distance of 1 cm on both sides of the reactors, providing an irradiance of 618 µmol photons/m/s.
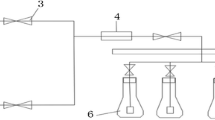
The different CO2 concentrations were achieved by mixing atmospheric air and pure CO2, controlled by rotameters (Aalborg, model P, Canada) (Fig. 1). Before reaching the photobioreactors, the mixture was passed through a mixer to ensure homogeneity of the gases and then through a porosity filter (0.22 μm) to prevent bacterial contamination. The proportions 0.04, 0.5, 1.0 and 2.0% (v/v) were regulated by rotameter control and verified daily with a CO2 measuring instrument (Geotech, G100, UK). To control aeration rate, rotameters were used, with a continuous flow of 1 L/min and by setting an aeration rate of 0.25 vvm. The cultures were acclimated for 7 days to keep the cells in exponential growth phase. They were then concentrated by centrifugation (1.160 x g for 15 min), washed with sterile water and the supernatant discarded to remove nutrient debris. Experimental units (in triplicate) were inoculated with biomass corresponding to the initial concentration of approximately 0.25 g/L.
Physical and Chemical Parameters
A radiometer (LI-COR, LI-250 A, USA) was used to measure light intensity outside and inside the photobioreactor studied. To characterize the light regimen during cultivation, an attenuation curve was elaborated considering position A (Center) and position B (Lateral) as shown in Fig. 1.
The dissolved inorganic carbon (DIC) was considered as the sum of CO2/H2CO3, HCO3− and CO32− in the aqueous solution, which can be calculated by Eq. (5) according to Lee et al. [18].
Where:
TA = total alkalinity (mEq/L – measure via titration); [H+] = hydrogen ionic activity (i.e., 10− pH) where the pH of the culture was measured with a digital pHmeter (YSI, pH100, USA); KW, K1 and K2 are dependent of the temperature (T) and are dissociation constants as shown in Eqs. (6), (7) and (8), respectively:
Growth Evaluation
Biomass was estimated indirectly using absorbance readings at 700 nm in a UV-Vis spectrophotometer (Genesis 10 S, Thermo, USA), according to the Eq. (9):
Biomass data were used to determine the maximum achieved biomass (Bmax) and total productivity (Ptotal).
Carbon Fixation Evaluation
To determine the maximum CO2 fixation rate (RCO2 max), maximum yield values were determined by linear regression applied in the exponential growth phase for each evaluated growth curve. The carbon content in biomass (C c) was considered to be 50% for application in Eq. (10), according to Tang et al. [19].
Where:
C\({ }_{C}\) = carbon content in algal biomass (% weight/weight);
P\({ }_{\text{m}\text{a}\text{x}}\) = maximum productivity achieved (g/L/h);
M\({ }_{C{O}_{2}}\) = 44 g/mol;
M\({ }_{C}\) = 12 g/mol
The CO2 fixation efficiency represents the ratio of CO2 fixed by the microalgae culture to the amount of CO2 injected into the system, as described in Eq. (11). The rate of CO2 injected into the culture (VCO2) was calculated using the ideal gas law.
Where:
V\({ }_{C{O}_{2}}\) = CO2 injected rate into the culture (g/L/h).
Statistical Analysis
Data are presented as the mean ± standard deviation (n = 3). Data from kLa CO2, CO2 dissolution efficiency, Bmax, P, and RCO2 max were tested for variance homogeneity (Levene’s test) and normality (Shapiro-Wilk test) using the Statistica 7.0 software. An ANOVA test was applied, followed by Tukey’s post-hoc test, when necessary, using the GraphPad Prism 7.0 software, to evaluate significance difference between the means. For all analysis, a significant level of 5% was adopted.
Results and Discussion
CO2 Transfer Study
The overall gas-liquid mass transfer coefficient (kLa) was determined to compare the use of a sintered glass diffuser (smaller bubbles) with a glass pipette (larger bubbles) at three aeration rates (0.125, 0.25 and 0.5 vvm). As shown in Fig. 2a, a significant increase (p < 0.05) in the aeration rate led to higher values of kLa CO2, when using either the diffuser or pipette. The diffuser promoted higher values of kLa CO2 than the pipette for all aeration rates tested, reaching a maximum value of 16.45 ± 0.36 1/h at the highest applied aeration rate, while the maximum value of kLa CO2 for the pipette was 4.90 ± 0.19 1/h at the aeration rate of 0.5 vvm. According to Langley et al. [8], the values of kLa for photobioreactors using air bubbling stay in the range of 5 to 100 1/h. The short retention time of bubbles in the water column, due to the small column in the photobioreactor, created limitations in the mass transfer of CO2 in the system studied. Thus, to achieve satisfactory gas-liquid mass transfer capability, even with a lower aeration rates, the use of smaller bubbles was more suitable.
kLa CO2 (a) and CO2 dissolution efficiency (b) as a function of aeration rates for pipette (\(\blacksquare\)) and diffuser (\(\bullet\)). All values are presented as the mean (n = 3) ± standard deviation. Different letters indicate significant differences by the Tukey test’s (p < 0.05) between aeration rate, while * indicates significant difference (p < 0.05) between the two types of diffuser
The kLa is composed of the mass transfer coefficient of the liquid phase (kL), which mainly depends on the properties of the liquid (density, viscosity, diffusivity, temperature, etc.) and the interfacial area, which is a function of gas holdup and bubble sizes [20]. Generally, to improve mass transfer capacity, the design and operating conditions of photobioreactors are studied to maximize the interfacial area [21]. Talbot et al. [15] demonstrated that the interfacial area “\(a\)” is strongly dependent on the average diameter of the bubbles produced and the aeration flow applied. In the present study, the comparison of the two dispersers determined that the average diameter of bubbles in the diffuser was 15 times smaller, which expanded the interfacial area and explains the significantly higher values of kLa. The increment in aeration rate under the same bubble size conditions increased the kLa, probably generated by a higher gas holdup, indicating a gain in the volume of the gas fraction in the photobioreactor, a behavior that has been widely observed in bubble column reactors [8, 22]. However, a higher aeration rate also tends to increase the flow velocity of the bubbles, causing greater CO2 losses to the atmosphere, thereby reducing the CO2 capture efficiency [14] and, depending on the species cultivated, may cause cellular damage from intensive shear-forces [23].
The CO2 capture efficiency is an important parameter to be evaluated to support efforts to reduce the amount of CO2 lost to the atmosphere as can be observed in the Fig. 2b. The increase in aeration rates led to a reduction in CO2 capture efficiency in both bubble sizes produced. However, decreasing the average bubble size by using the diffuser instead of the pipette significantly increased (p < 0.05) the efficiency values when the same aeration rates were applied.
Both effects are similar to other studies as reported by Ying et al. [16], where the authors emphasize that reducing bubble size is considered more promising than increasing aeration flow because it has a positive effect on both CO2 transfer rate and CO2 capture efficiency. Thus, in addition to improving the growth performance of microalgae, it also leads to lower losses of CO2 to the atmosphere. By using the glass diffuser and applying an aeration rate of 0.25 vvm it was possible to achieve a kLa CO2 value of 11.98 ± 0.6 1/h and a CO2 capture efficiency of 67.94 ± 3.56%. Raising aeration flow decreases CO2 capture efficiency to 46.62 ± 1.02%, while increasing gas-liquid transfer capacity.
CO2 Concentrations Effect on T. obliquus Cultivation
Tetradesmus obliquus was grown for 360 h (15 days) under different CO2 concentrations, as observed in the growth curves (Fig. 3a). After a short acclimation phase in the first 12 h (lag phase), the CO2 supplemented cultures began exponential growth and reached maximum yields in the period from 80 to 100 h. After this point, growth became linear, which might be caused by the reduction of light passage in the photobioreactor, possibly by self-shading from cells in the culture, as can be seen in Fig. 3b. Between the biomass concentration of 0.2 g/L and 1.4 g/L, the cultures of T. obliquus were under a light regimen without shading in the middle of the reactor. Therefore, the availability of CO2 to the system during this period was considered the limiting factor for cell growth, since other nutrients were in excess in the medium. Nitrate (NO3−) and phosphate (PO43−) assimilated in the culture medium, for example, were fully consumed only on day 7 (data not shown). The maximum productivity (P max) data obtained in this period were used to calculate the maximum CO2 fixation rates (RCO2 max).
Biomass accumulation (a), ligh attenuation (b), dissolved inorganic carbon (c). and pH values (d) of Tetradesmus obliquus cultures conducted under different CO2 concentrations: 0.04% (▲); 0.5% (\(\blacklozenge\)); 1% (\(\blacksquare\)); 2% (\(\bullet\)). All values are presented as the mean (n = 3) ± standard deviation
The treatment using only atmospheric air (0.04% CO2) had low growth values and after 350 h an average B max of 1.45 ± 0.10 mg/L and a P total of 3.75 ± 0.31 mg/L/h. Dissolved inorganic carbon (DIC) in the culture medium represents the carbon source for algal growth, being composed of CO2, HCO3− and CO32−. As a result, low concentrations of CO2 in the atmosphere and the lack of efficiency in transfering the CO2 led to inferior concentrations of DIC in the culture medium, as can be seen in Fig. 3c. More photosynthetic activity in the culture accelerates the removal of carbon dioxide and nitrate consumption, which may lead to a high increase of pH in the medium. The combination of these factors may have made CO2 scarce in the liquid, limiting cell growth.
As observed in Fig. 3c, during the first 60 h, on approximately the third day of cultivation (period of maximum yield), higher CO2 concentrations (0.04–2% v/v) raised the DIC concentrations in the culture medium from 13.47 to 91.35 mg/L while the pH decreased from 10.42 to 7.93. At this point of cultivation, 0.5% CO2 promoted significantly lower DIC values (p < 0.05) than 1% and 2% CO2. However, between 150 and 350 h DIC concentrations stabilized, reaching a possible DIC saturation for all treatments where CO2 was supplemented. In this period, a no significant difference (p > 0.05) was observed between the mean values of 143.7 ± 0.97 to 149.65 ± 1.16 at concentrations of 0.5% and 2% respectively. DIC accumulation was therefore faster in the early hours for 1% and 2% CO2 conditions, different from the 0.5% concentration.
The different treatments had distinct pH responses in the culture medium as observed in Fig. 3d. During the first 40 h, the pH increased rapidly in all treatments due to the exponential growth period, where the CO2 fixation rate by the culture was probably higher than the gas dissolution rate. After approximately 84 h the pH values remained constant for the treatments using CO2-enriched air, ranging from 7.8 to 8.7.
pH is recognized to be a controlling factor in the reactions that regulates the appearance of different forms of DIC in the culture, influencing the CO2 mass transfer in the system [24]. For T. obliquus, besides absorbing CO2 passively, there is strong evidence that HCO3− is directly absorbed by algae through a bicarbonate pump that is activated by the consumption of ATP [25, 26]. For this reason, this microalga is likely to be capable of maintaining photosynthetic activity at high pH, between 8 and 9, where HCO 3− is still available in water under standard temperature and salinity conditions.
However, some studies suggest that Scenedesmus sp. chooses to absorb CO2 rather than bicarbonate in a more saturated CO2 medium, probably because it is a lower energy expenditure option for cells. Yang and Gao [27] demonstrated that by decreasing culture pH, thereby increasing CO2 in the medium (3, 21 and 186 µM CO2) and at a constant DIC concentration (1.68 mM), it was possible to boost the specific growth rate of T. obliquus. Azov [28] also cultivated T. obliquus adapted to high CO2 concentrations by providing DIC at a controlled concentration with pH between 8.1 and 9.3 (0.2 increase between treatments) and obtained a constant specific CO2 fixation rate for all treatments applied at this pH range. The results obtained in this study for pH are in agreement with the data demonstrated by these experiments, so the physiological effect of pH probably had no effect on T. obliquus growth in treatments with CO2-enriched air.
The increase in CO2 concentrations led to better conditions for microalgae growth in terms of biomass gain, reaching a maximum biomass (Bmax) with mean values ranging from 3.93 to 4.35 g/L and total productivity (Ptotal) with mean values of 10.99 to 12.39 mg/L/h at concentrations of 0.5% and 2%, respectively (Table 1). Although growth curves followed similar trends in biomass gain among the treatments where the cultures received CO2-enriched air, applying 1 and 2% CO2 promoted the highest values (p < 0.05) for both B max and P total in comparison to 0.5%, while no significant difference (p > 0.05) between 1 and 2% treatments. Typically, P total values for T. obliquus have been reported in the literature between a range of 5.83 to 35.17 mg/L/h over high CO2 concentrations and applying irradiance with values from 35 to 420 µmol photons/m2/s [29,30,31,32]. Overall, the focus of these studies had been to demonstrate the ability of T. obliquus to tolerate high CO2 concentrations (5–50%) on the premise of using gaseous effluents from industrial processes. Tang et al. [19] for example, cultivated T. obliquus in a 1 L photobioreactor at an aeration rate of 0.25 vvm using different concentrations (0.03, 5, 10, 20, 30 and 50%) and obtained better results using 10% of CO2 with mean values for Bmax of 1.84 ± 0.01 g/L and Pmax of 6.58 ± 0.17 mg/L/h. Ho et al. [33] also obtained better results by cultivating T. obliquus with 10% CO2 reaching B max values of 3.51 g/L and P total of 12.17 mg/L/h in a 1 L photobioreactor. Likewise, the results obtained by these authors demonstrate that concentrations above 10% negatively affect microalgae growth. An excess of CO2 in the culture may cause lower pH values and consequently intracellular acidification leading to an inhibition of the carbon anhydrase enzyme [34]. Chiu et al. [35] reported complete growth inhibition of Chlorella sp. when using 5%, 10% and 15% of CO2, and better results were achieved by applying 2% of CO2, which allowed reaching B max of 1.2 g/L. However, tolerance to high CO2 concentrations may vary from species to species [36].
Values expressed as mean (n = 3) ± standard deviation. Different letters on the same column indicate statistical differences (p < 0.05). The values obtained for P max were calculated by linear regression of growth curves during the exponential growth phase.
It should be noted that the CO2 concentrations applied are not related to the performance of the cultivation system in these cases, but are rather an indication of the species’ tolerance to pH and the applied CO2 values. However, when air is enriched with CO2 and injected into a microalgae culture, the total flow of transferred CO2 is related to aeration rate, concentration or PCO2 and CO2 transfer capacity. Märkl [37], studying the effect of light intensity as a function of CO2 concentration applied to microalgae cultivation, reported that the critical value of CO2 injected into photobioreactor is dependent on the specific light regime and CO2 transfer coefficient of each system. Thus, since there are no established photobioreactor standards, it is not possible to determine a critical concentration of CO2 to be applied for a particular species.
The CO2 fixation rate and efficiency were evaluated on the effect of increasing CO2 concentrations on T. obliquus cultivation in a 4.5 L photobioreactor applying an aeration rate of 0.25 vvm, which provided a kLa CO2 of 11.89 ± 0.4 1/h. Higher fixation rates were obtained with increased CO2 concentration, as opposed to lower fixation efficiency as more CO2 was supplied. This inverse relationship between CO2 fixation rate and efficiency can be seen in Fig. 3. Zhang et al. [38] evaluated increasing aeration rates using 5% and 10% of CO2 on Synechocystis aquatilis algae growth, and defined two stages where increasing the aeration rate had different effects on culture productivity and CO2 fixation efficiency. By doubling the aeration rate (from 0.0025 to 0.005 vvm) the algae had a 70% productivity gain. But, from this point forward, when the aeration flow was enhanced 20 times (from 0.005 to 0.1 vvm) the productivity gain was only 50%. The authors affirmed that a point of intersection between these two situations should be determined to increase culture productivity and the efficiency of carbon fixation.
In this study, increasing CO2 concentrations from 0.04 to 1% led to a productivity gain of 72.3%. When higher CO2 concentrations were applied (between 1% and 2%), the increase in culture yield was only 2.3%, while CO2 fixation efficiency decreased from 10.21 to 5.28%. When CO2 was used at concentrations below 1%, the mass transfer of this gas was a limiting factor for culture growth in this study and lower values for this parameter were obtained. The probable limitation on CO2 transfer also matches the data presented for DIC in the medium as shown previously. The stabilization of the CO2 fixation rate (above 1%) in this case may have occurred due to DIC saturation in the culture. From this point on, the boost in CO2 concentration had little effect on the fixation rate, and only led to increased CO2 losses to the atmosphere, which might cause an increase in biomass production costs.
Considering both the highest yield and the highest CO2 fixation efficiency, 1% supplementation was considered the most effective concentration, defined as the critical CO2 concentration to be applied to the T. obliquus cultures cultivated in this study. According to Langley et al. [8], the critical CO2 partial pressure (PCO2 CRIT) is the lowest partial pressure value of CO2 in the inlet gas that facilitates the gas-liquid CO2 transfer rate, which is equal to the maximum rate that CO2 can be used through photosynthesis. Thus, increasing the CO2 concentration in the inlet gas above the critical concentration results in little or no gain in culture yield and, consequently decreases the CO2 fixation efficiency. By cultivating Chlorella vulgaris in a 3.8 L airlift photobioreactor, Langley et al. [8] were able to determine PCO2 CRIT at 0.0012 atm (0.12%), obtaining maximum yields (13 mg/L/h) and achieving 26% of CO2 removal. The difference between the concentrations applied in both studies may mainly be related to the difference in kLa CO2 of 33.84 1/h, because of the light regimen employed and the physiological difference between species.
Due to the low gas-liquid mass transfer rate in the system, despite obtaining the desired increase in productivity from the higher CO2 concentration, there was a negative effect on CO2 fixation efficiency. The same pattern was observed in other studies where CO2 removal in low CO2 concentrations was evaluated [4]. Both CO2 fixation and removal efficiency determine the ability of the culture to absorb the injected carbon. However, the removal of CO2 represents the total amount absorbed by the system, considering other forms of carbon absorption, such as DIC accumulation, and not only the portion fixed by the algae. From an engineering standpoint, the performance of most carbon photobioreactors used for carbon sequestration is unsatisfactory in terms of CO2 fixation or removal efficiency [4]. Some advances have been reported regarding increased CO2 removal efficiency as reported by Chiu et al. [39] and de Godos et al. [9], but, generally when high concentrations are applied, little of what is injected into a culture is absorbed by the system or fixed by microalgae.
Laboratory scale microalgae cultivation in batches is usually done with simple and easily sterilized materials such as glass flasks or carboys [39]. Photobioreactors used in this condition are generally characterized by low gas-liquid mass transfer capacity and consequently low efficiency in CO2 fixation. Some strategies to increase CO2 recovery have been presented in the literature and can be implemented in these reactor models. Conducting a comprehensive investigation into the interplay between CO2 concentration and aeration rates within photobioreactors utilized for intensive microalgae cultivation contributes to optimizing the performance of CO2 sequestration reactors [40, 41]. This advancement paves the way for the development of zero-carbon industries, as microalgae cultures can be seamlessly integrated into various production processes, utilizing solid, liquid, and gaseous waste as inputs [42, 43].
Conclusions
In this study, the use of the sintered glass diffuser caused a gain in gas-liquid mass transfer capacity by decreasing the mean bubble diameter and increasing the interfacial area from kLa CO2. Raising the aeration flow in the reactor led to an increase in kLa CO2 values and a parallel increase in CO2 losses to the atmosphere. Thus, to obtain optimum growth in terms of biomass production and optimize the use of CO2, it is concluded that the use of a 1% CO2 concentration is the best conditions to be applied in the studied system.
Data Availability
Data will be made available on request.
References
Prasad R, Gupta SK, Shabnam N, Oliveira CYB, Nema AK, Ansari FA, Bux F (2021) Role of microalgae in global CO2 sequestration: physiological mechanism, recent development, Challenges, and future prospective. Sustainability 13(23):13061. https://doi.org/10.3390/su132313061
Tai TC, Calosi P, Gurney-Smith HJ, Cheung WW (2021) Modelling ocean acidification effects with life stage-specific responses alters spatiotemporal patterns of catch and revenues of american lobster, Homarus americanus. Sci Rep 11(1):23330. https://doi.org/10.1038/s41598-021-02253-8
Oliveira CYB, Jacob A, Nader C, Oliveira CDL, Matos ÂP, Araújo ES, Shabnam N, Ashok B, Gálvez AO (2022) An overview on microalgae as renewable resources for meeting sustainable development goals. J Environ Manag 320:115897. https://doi.org/10.1016/j.jenvman.2022.115897
Cheng L, Zhang L, Chen H, Gao C (2006) Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep Purif Technol 50:324–329. https://doi.org/10.1016/j.seppur.2005.12.006
Verma R, Srivastava A (2018) Carbon dioxide sequestration and its enhanced utilization by photoautotroph microalgae. Environ Develop 27:95–106. https://doi.org/10.1016/j.envdev.2018.07.004
Ojah A, Sabri LS, Aldahhan MH (2021) Local volumetric mass transfer coefficient estimation for Scenedesmus microalgae culture in a cylindrical airlift photobioreactor. J Chem Technol Biotechnol 96(3):764–774. https://doi.org/10.1002/jctb.6590
Tebbani S, Filali R, Lopes F, Dumur D, Pareau D (2014) CO2 biofixation by microalgae: modeling, estimation and control. John Wiley & Sons, Oxford
Langley N, Harrison S, van Hille R (2012) A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem Eng 68:70–75. https://doi.org/10.1016/j.bej.2012.07.013
de Godos I, Mendoza J, Acién F, Molina E, Banks C, Heaven S, Rogalla F (2014) Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour Technol 153:307–314. https://doi.org/10.1016/j.biortech.2013.11.087
Goldman JC, Dennett MR, Riley CB (1981) Inorganic carbon sources and biomass regulation in intensive microalgal cultures. Biotechnol Bioeng 23:995–1014. https://doi.org/10.1002/bit.260230508
Chia SR, Nomanbhay SBHM, Chew KW, Munawaroh HSH, Show PL (2022) Algae as potential feedstock for various bioenergy production. Chemosphere 287:131944. https://doi.org/10.1016/j.chemosphere.2021.131944
ElFar OA, Chang CK, Leong HY, Peter AP, Chew KW, Show PL (2021) Prospects of industry 5.0 in algae: customization of production and new advance technology for clean bioenergy generation. Energy Convers Manag X 10:100048. https://doi.org/10.1016/j.ecmx.2020.100048
Contreras A, Garcia F, Molina E, Merchuk JC (1998) Interaction between CO2-mass transfer, light availability, and hydrodynamic stress in the growth of Phaeodactylum tricornutum in a concentric tube airlift photobioreactor. Biotechnol Bioeng 60:317–325. https://doi.org/10.1002/(SICI)1097-0290(19981105)60:3<317::AID-BIT7>3.0.CO;2-K
Talbot P, Gortares MP, Lencki RW, de la Noüe J (1991) Absorption of CO2 in algal mass culture systems: a different characterization approach. Biotechnol Bioeng 37:834–842. https://doi.org/10.1002/bit.260370907
Talbot P, Lencki RW, de la Noüe J (1990) Carbon dioxide absorption characterization of a bioreactor for biomass production of Phormidium bohneri: comparative study of three types of diffuser. J Appl Phycol 2:341–350. https://doi.org/10.1007/BF02180924
Ying K, Al-Mashhadani MKH, Hanotu JO, Gilmour DJ, Zimmerman WB (2013) Enhanced mass transfer in microbubble driven airlift bioreactor for microalgal culture. Engineering 5:735–743. https://doi.org/10.4236/eng.2013.59088
Oliveira CYB, Viegas TL, da Silva MFO, Fracalossi DM, Lopes RG, Derner RB (2020) Effect of trace metals on growth performance and accumulation of lipids, proteins, and carbohydrates on the green microalga Scenedesmus obliquus. Aquac Int 28(4):1435–1444. https://doi.org/10.1007/s10499-020-00533-0
Lee JY, Hong ME, Chang WS, Sim SJ (2015) Enhanced carbon dioxide fixation of Haematococcus pluvialis using sequential operating system in tubular photobioreactors. Process Biochem 50:1091–1096. https://doi.org/10.1016/j.procbio.2015.03.021
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. https://doi.org/10.1016/j.biortech.2010.10.047
Chisti Y, Jauregui-Haza UJ (2002) Oxygen transfer and mixing in mechanically agitated airlift bioreactors. Biochem Eng J 10(2):143–153. https://doi.org/10.1016/S1369-703X(01)00174-7
Fan LH, Zhang YT, Zhang L, Chen HL (2008) Evaluation of a membrane-sparged helical tubular photobioreactor for carbon dioxide biofixation by Chlorella vulgaris. J Membrane Sci 325:336–345. https://doi.org/10.1016/j.memsci.2008.07.044
Merchuk JC, Gluz M, Mukmenev I (2000) Comparison of photobioreactors for cultivation of the red microalga Porphyridium sp. J Chem Technol Biotechnol 75:1119–1126. https://doi.org/10.1002/1097-4660(200012)75:12<1119::aid-jctb329>3.0.co;2-g
Wang C, Lan CQ (2018) Effects of shear stress on microalgae–a review. Biotechnol Adv 36(4):986–1002. https://doi.org/10.1016/j.biotechadv.2018.03.001
Wetzel RG, Likens GE (2010) Limnological analyses. Springer, New York
Thielmann J, Tolbert NE, Goyal A, Senger H (1990) Two systems for concentrating CO2 and bicarbonate during photosynthesis by Scenedesmus. Plant Physiol 92:622–629. https://doi.org/10.1104/pp.92.3.622
Moroney JV (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119:9–16. https://doi.org/10.1104/pp.119.1.9
Yang Y, Gao K (2003) Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J Appl Phycol 15:379–389. https://doi.org/10.1023/a:1026021021774
Azov Y (1982) Effect of pH on inorganic carbon uptake in algal cultures. Appl Environ Microbiol 43:1300–1306
de Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445. https://doi.org/10.1016/j.jbiotec.2007.01.009
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101:71–74. https://doi.org/10.1016/j.biortech.2009.03.030
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalgae Scenedesmus obliquus CNW-n. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Kaewkannetra P, Enmak P, Chiu T (2012) The effect of CO2 and salinity on the cultivation of Scenedesmus obliquus for biodiesel production. Biotechnol Bioproc E 17:591–597. https://doi.org/10.1007/s12257-011-0533-5
Ho SH, Chen WM, Chang JS (2010) Scenedesmus obliquus CNW-n as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730. https://doi.org/10.1016/j.biortech.2010.06.112
Satoh A, Kurano N, Miyachi S (2001) Inhibition of photosynthesis by intracellular carbonic anhydrase in microalgae under excess concentrations of CO2. Photosynth Res 68:215–224. https://doi.org/10.1023/a:1012980223847
Chiu SY, Kao CY, Chen CH, Kuan TC, Ong SC, Lin CS (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396. https://doi.org/10.1016/j.biortech.2007.08.013
Singh S, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sust Energ 38:172–179. https://doi.org/10.1016/j.rser.2014.05.043
Märkl H (1977) CO2 transport and photosynthetic productivity of a continuous culture of algae. Biotechnol Bioeng 19:1851–1862. https://doi.org/10.1002/bit.260191209
Zhang K, Kurano N, Miyachi S (2002) Optimized aeration by carbon dioxide gas for microalgal production and mass transfer characterization in a vertical flat-plate photobioreactor. Bioproc Biosyst Eng 25:97–101. https://doi.org/10.1007/s00449-002-0284-y
Chiu SY, Tsai MT, Kao CY, Ong SC, Lin CS (2009) The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng Life Sci 9:254–260. https://doi.org/10.1002/elsc.200800113
Martínez-Jerónimo F, Espinosa-Chávez F (1994) A laboratory-scale system for mass culture of freshwater microalgae in polyethylene bags. J Appl Phycol 6:423–425. https://doi.org/10.1007/bf02182159
Leong YK, Chew KW, Chen WH, Chang JS, Show PL (2021) Reuniting the biogeochemistry of algae for a low-carbon circular bioeconomy. Trends Plant Sci 26(7):729–740. https://doi.org/10.1016/j.tplants.2020.12.010
Oliveira CYB, Oliveira CDL, Prasad R, Ong HC, Araujo ES, Shabnam N, Gálvez AO (2021) A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquac 13(3):1594–1618. https://doi.org/10.1111/raq.12536
Fu J, Huang Y, Xia A, Zhu X, Zhu X, Chang JS, Liao Q (2022) How the sulfur dioxide in the flue gas influence microalgal carbon dioxide fixation: from gas dissolution to cells growth. Renew Energy 198:114–122. https://doi.org/10.1016/j.renene.2022.08.057
Funding
This study received financial support from the Brazilian Ministry of Science, Technology, Innovation, and Communications–MCTIC/CGTS/SETEC provided by the Funding Authority for Studies and Projects (FINEP) (Agreement No. 01.10.0457.00) and the National Council for Scientific and Technological Development (CNPq) (Case No. 407513/2013-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CRediT Authorship Contribution Statement
FRFP, RGL, HC, CN and RGC developed the experimental design and conducted the data acquisition and analysis. FRFP, HC, CN and CYBO wrote the manuscript, and all authors conducted the data interpretation and revised the manuscript.
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Fonseca Pchara, F.R., Cella, H., Nader, C. et al. Investigating Carbon Dioxide Transfer for Intensive Cultures of the Microalga Tetradesmus obliquus. Bioenerg. Res. 17, 547–556 (2024). https://doi.org/10.1007/s12155-023-10622-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10622-6