Abstract
Alkaline hydrogen peroxide (AHP) pretreatment is a promising process for enhancing enzymatic digestibility of lignocellulosic biomass in biorefineries. In the present work, the effects of organic bases (NH4OH and tri-ethylamine) and co-solvents (ethanol, isopropanol, tert-butyl alcohol, water) on AHP pretreatment efficiency of rice straw were studied and compared to the typical aqueous reaction with NaOH. It was found that the glucose recovery from enzymatic hydrolysis of the biomass pretreated by AHP at 35 °C for 24 h using NH4OH in aqueous/tert-butyl alcohol (73.6%) was higher than that achieved using ethanol and isopropanol (31.6–48.6%) and water (71.2%) under the same experimental conditions. Increasing H2O2 concentration from 1 to 10% v/v in the aqueous/tert-butyl alcohol with NH4OH led to enhancing sugar yield to from 349 to 623 mg/g pretreated rice straw, equivalent to the highest glucose recovery of 83.0%. Formation of highly porous structures in pretreated rice straw by removals of hemicelluloses and lignin was revealed by Fourier transform infrared spectroscopy and scanning electron microscopy while the increased crystallinity index was shown by X-ray diffraction. This modified low-temperature AHP pretreatment using organic solvent system is advantageous on recyclability potential of the reagents and potent for further implementation in lignocellulosic biorefineries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand of fossil fuels worldwide has been increasing during the last decade with the expected consumption of 101.0 million barrels per day of crude oil in 2020, mostly for the industrial sector and automobiles [1]. This increasing exploitation of fossil resources led to increasing air pollution and became a major cause of CO2 emission contributing to global warming. Lignocellulosic biomass is a renewable carbon-neutral renewable raw material in biorefinery industry which is considered a sustainable platform industry compared to the conventional process relying on fossil feedstock [2]. The structure of biomass mainly comprises three primary constituents: (i) cellulose, a linear highly crystalline homopolymer structure of glucose linked together via β(1,4)-glycosidic bonds; (ii) hemicellulose, a branched heteropolymer of pentose and hexose sugars which forms a network linking the cellulose microfibrils; and (iii) lignin, a complex polymer of phenolic alcohols, which shields plant cell wall from external stresses and microbial attacks [3]. Separation and conversion of these individual biopolymers to a spectrum of fuels and commodity chemicals (e.g., solvents, building blocks, and bioplastic monomers) are the key issues in modern biorefineries [4]. Among various biomass processing routes, conversion of the polysaccharide parts to sugars as intermediates for subsequent conversion to desirable target products via microbial or catalytic processes is a dominated strategy. This leads to a challenge on development of an efficient process for production of cellulosic sugars at a competitive cost.
A pretreatment step by physical, chemical, or biological processes is generally required for increasing enzymatic digestibility of the recalcitrant cellulose fraction, which can be achieved by removals of hindered hemicellulose and lignin, enhancement of porosity structure, reduction of cellulose polymerization degree, and decrystallization of the cellulose structures [3]. However, thermochemical pretreatment processes, for example, steam explosion and liquid hot water, are usually energy intensive and require investment of high-cost equipment due to the need to perform reactions at high temperature and pressure. Basic chemical pretreatment processes using dilute acids and alkalis also have drawbacks on generation of inhibitory compounds (e.g., organic acids and furans derived from carbohydrate degradation and phenolic compounds derived from lignin) which can cause reduction of enzymatic activity in the hydrolysis step and inhibition of cell growth during fermentation [5,6,7].
Alkaline hydrogen peroxide pretreatment (AHP) using hydrogen peroxide (H2O2) as an oxidative agent is usually performed at milder temperature compared to the acid or alkali pretreatment processes due to its exothermic nature with an advantage on low formation of inhibitory by-products, e.g., hydroxymethylfurfural (HMF) and furfural [8]. An alkali, conventionally, NaOH, is required to adjust the pH to 11.5 for maximal generation of the reactive oxygen radicals, i.e., superoxide (O2·) and hydroxyl radical (OH·) derived from the key reactive species, perhydroxyl anion (HOO−), from dissociation of H2O2 [8]. This leads to efficient delignification by cleaving the lignin side chains and breaking down the aliphatic part of lignin into low molecular weight compounds [9] and also results in partial removal of hemicellulose. The AHP process has been studied on several lignocellulose materials such as wheat straw [10], rice husk [9], corn stover [11], sugarcane bagasse [12], and cashew apple bagasse [13] which resulted in marked improvement in cellulose digestibility. Addition of surfactants (e.g., Tween 80), polymers (e.g., polyethylene glycol), and non-catalytic protein (e.g., bovine serum albumin) in the enzymatic hydrolysis step of biomass pretreated by AHP and other alkaline pretreatments has also been reported to enhance the glucose yield by stabilizing and protecting the enzyme activity and reducing enzyme inhibition during the saccharification process [14,15,16]. However, the high cost of H2O2 reagent, including the additional need for recycling of liquid fraction after the process, has limited implementation of AHP pretreatment [8].
Use of organic solvents such as alcohols, ketones, organic acids, ethylene glycol, and esters in organosolv pretreatment operated under high-temperature range (> 150 °C) allows high efficiency in solubilization of the lignin fraction, decrease of viscosity in the reaction mixture, and decreased recondensation of lignin on the biomass surface which resulted in enhancing the enzymatic digestibility of the cellulose-enriched fraction [17,18,19]. The use of organic bases, for example, ammonia, guanidine, and amino-guanidine, has also been demonstrated in alkali pretreatment of different agricultural wastes with advantages on increasing the surface area through alkaline swelling and effective lignin removal, leading to improvement in enzyme accessibility with advantages on possible chemical recycling and less chemical waste generation [20, 21].
Until now, there has been very few reports on AHP pretreatment of lignocellulosic biomass in aqueous organic solvent systems, however with limited demonstration of the types of solvents with no experimental study on its combined use with organic bases [22]. The use of organic solvents with organic bases (TEA and NH4OH) in AHP would allow non-corrosive, non-toxic, and possibility on recovery of chemical reagents by evaporation leading to less generation of chemical waste compared to the conventional AHP pretreatment using NaOH in an aqueous system [17, 20, 23]. In this work, we aimed to study the effects of co-solvents (ethanol, isopropanol, and tert-butyl alcohol) and types of organic bases (NH4OH and tri-ethylamine) on AHP pretreatment of rice straw, one of the world’s most abundant agricultural wastes with the average annual production of 900 million tons/year [24, 25], compared to the use of conventional AHP process. Given the known benefits of co-solvents, organic bases, and the delignification effect of the oxidative H2O2 agent, these combined effects could result in improvement in AHP pretreatment strategy in terms of efficiency, environmental friendliness, and reagent reusability. Physicochemical characteristics and microstructures of the cellulose-enriched solid fraction after alkaline peroxide pretreatment were investigated providing supports for improved processability of the biomass.
Materials and Methods
Materials
Rice straw (RS) was obtained from a paddy field in Pathumthani province, Thailand. Initially, it was physically grinded by a cutting mill (Retsch ZM2000, Haan, Germany) and sieved through a 0.5 mm size screen. The milled rice straw was dried at 70 °C for overnight and stored in plastic bag at room temperature before use. The composition of raw biomass contained 35.5 ± 1.2 wt% of cellulose, 22.5 ± 0.7 wt% of hemicelluloses, 25.0 ± 0.5 wt% of lignin, and 14.2 ± 0.2 wt% of ash determined by the standard NREL analysis [26]. All analytical grade chemicals and organic solvents were provided from major chemical suppliers such as Sigma-Aldrich and Merck.
Alkaline Peroxide Pretreatment
Conventional AHP Process
Based on the conditions from our preliminary study, the dried rice straw (2.5, 5, 7.5, 10, and 12.5%, w/v) was pretreated with 10 mL of hydrogen peroxide solution at different concentrations (1, 2.5, 5, 7.5, and 10% v/v) pre-adjusted to pH 11.5 (approx. 0.4–4.5% w/v of NaOH final concentration depending on concentrations of hydrogen peroxide). The reaction was performed in aqueous media by incubating the samples in different reaction temperatures (25, 30, 35, 40, and 45 °C) for various reaction times (6, 18, 24, 36, and 48 h). After that, the solid residue was collected by vacuum filtration on a paper filter (No. 4) with a pore size of 20–25 μm, washed by distilled water until pH reached to neutral. The residue was dried at 70 °C for overnight and stored at room temperature for further use in enzymatic hydrolysis process.
AHP Process Using Organic Solvents and Organic Bases
AHP pretreatment in aqueous organic solvent system was performed in a solvent mixture comprising different organic solvents (ethanol, isopropanol, or tert-butyl alcohol) and water in the ratio of 70:30. The reaction was performed using an organic bases (NH4OH or tri-ethylamine) for pre-adjusting the reactions to pH 11.5 (approx. 5% v/v final concentration) under the starting condition obtained from the conventional AHP process. The effect of H2O2 concentrations (1, 2.5, 5, 7.5, 10% v/v) at 35 °C for 24 h was then further studied. The collected solid residues were processed for studying the lignocellulosic composition and tested for enzymatic digestibility.
Enzymatic Hydrolysis
Enzymatic hydrolysis reaction consisted of 5% (w/v) pretreated rice straw with cellulase dosage at 20 FPU/g pretreated rice straw using Accellerase® 1500 (Dupont, Rochester, NY) containing 45 FPU/mL of initial activity [27] in 50 mM sodium citrate buffer at pH 4.8 and 0.25% (w/v) of NaN3. The reaction was incubated at 50 °C for 72 h. The sugar product profile was examined using a high-performance liquid chromatograph (SPD-M10A DAD, Shimadzu, Japan) equipped with a refractive index detector and an Aminex HPX-87H column (Bio-rad, Hercules, CA). Sulfuric acid solution (0.005 M) was employed as the mobile phase using a flow rate of 0.5 mL/min with column temperature at 65 °C.
Sugar yield represented the total amount of fermentable sugars (glucose, xylose, and arabinose) obtained from enzymatic hydrolysis of the pretreated biomass calculated based on the weight basis of the substrate used in the hydrolysis reaction (mg/g pretreated RS) according to (Equ. 1). Sugar recovery was defined as the amount of total sugars obtained from the hydrolysis reaction taken weight recovery of the solid fraction from the pretreatment step into an account (mg/g raw RS) according to (Equ. 2). %Glucose recovery was calculated based on the glucose recovered from the hydrolysis reaction compared to the cellulose content (× 1.11) in the raw material on a dried weight basis according to (Equ. 3).
Analytical Methods
Chemical Composition
Chemical composition of the pretreated RS solid recovery was determined according to the standard NREL analysis method [26]. The %cellulose recovery, %hemicellulose removal, and %lignin removal were calculated according to Equations 4–6.
where cellulose recovery is the percentage of cellulose remaining after the AHP pretreatment compared with the initial cellulose content; CI is the percentage of initial cellulose content; CF is the percentage of remaining cellulose content in the final solid recovery.
where hemicellulose removal is the percentage of hemicelluloses removed after AHP process compared with the initial hemicellulose content; HI is the percentage of initial hemicellulose content; HF is the percentage of remaining hemicellulose content in the final solid recovery.
where lignin removal is the percentage of lignin removed after AHP process compared with the initial lignin content; LI is the percentage of initial lignin content; LF is the percentage of remaining lignin content in the final solid recovery.
Scanning Electron Microscopy
The microstructure and morphology of the raw and pretreated biomass obtained from AHP pretreatment in aqueous and organic solvent systems was determined by scanning electron microscope (SEM) using a S-3400N Type II SEM (Hitachi, Tokyo, Japan). The samples were dried and coated with gold according to the standard protocol [28]. An electron beam energy of 5 kV was applied for analysis.
X-Ray Diffraction Analysis
The crystallinity of raw and pretreated biomass was analyzed by X-ray diffraction (XRD) using an X’Pert PRO diffractometer (PANalytical, Almelo, The Netherlands). The samples were scanned at a speed of 0.5°/min in a range of 2θ = 10–30° with a step size of 0.02° at 40 kV, 30 mA, and radiation at Cu Kα (λ = 1.54 Å). The average crystallite size was calculated by the Scherrer equation. The crystallinity index (CrI) was calculated according to the (Equ. 7) [29].
where CrI is the crystallinity index (%); I002 is the highest intensity for the crystalline portion at 2ϴ = 22.40 and Iam is the peak for the amorphous portion at 2ϴ = 18.00.
Fourier Transform Infrared Spectroscopy
The functional groups on the lignin samples were analyzed by Fourier-transformed infrared spectroscopy (FT-IR) (Perkin-Elmer System 2000, Waltham, United States) with infrared spectra collected in the wave number range of 600–4000 cm−1.
Results and Discussion
AHP Pretreatment in Aqueous System
Firstly, the conventional AHP pretreatment of rice straw using water as the medium and pH adjusted with NaOH was studied under different reaction conditions with varying concentrations of H2O2, solid loading, temperature, and reaction time to study the optimized conditions for maximizing the glucose recovery (Fig. 1). The effect of H2O2 dosage was initially studied in the range of 1–10% v/v in the reactions containing 5% initial solid loading at 35 °C for 18 h. The result showed that increasing H2O2 dosage from 2.5 to 10% led to decreasing sugar yield from 700 to 664 mg/g pretreated RS with a decrease on solid recovery from 70.0 to 58.0%. This led to the decreasing sugar recovery from 490 to 385 mg/g raw RS, corresponding to the glucose recovery in the range of 82.5 to 64.5% (Fig. 1a). The lower sugar yield obtained from the reactions with higher H2O2 dosages could be due to the excessive dosage of H2O2 which led to lower cellulose recovery together with formation of inhibitory compounds from the delignification reaction [8, 30]. Next, the effect of solid loading was studied in the range of 2.5–12.5% w/v with a fixed reaction volume using the optimum H2O2 concentration (2.5% v/v) at the fixed temperature and time. It was found that the optimal solid loading at 7.5% w/v gave the highest sugar yield and recovery as shown in Fig. 1b. The excessive biomass loading at 10% w/v resulted in reduction of free water which caused reduced efficiency in mass transfer in the reaction and could lead to higher energy consumption for mixing in an up-scaled process [8]. The effect of temperature was subsequently studied at 25–45 °C in the reactions containing 2.5% v/v of H2O2 and 7.5% w/v of solid loading for 18 h. The highest sugar product and glucose recovery were achieved at 35 °C (Fig. 1c). Higher temperature (45 °C) could lead to an excessive generation of radicals in AHP pretreatment which reflected in the decrease in the solid recovery and thus the yield of sugar products. Optimization of reaction time was then studied in the range of 6–48 h based on optimal condition identified. The result demonstrated that increasing reaction time from 6 to 36 h led to an increase in the sugar yield of 720–834 mg/g pretreated RS (Fig. 1d). The reaction time at 24 h under the optimal AHP conditions (7.5% biomass loading, 2.5% H2O2 at 35 °C) was a good candidate for achieving the sugar recovery of 540 mg/g raw RS, equivalent to 93.3% glucose recovery which was 3.1 times compared to that obtained from hydrolysis of untreated RS (105 mg/g raw RS).
Effects of reaction parameters on AHP pretreatment with NaOH in water as the medium. The standard reaction contained 7.5% w/v solid loading with 2.5% v/v H2O2 and incubated 35 °C for 24 h. The total reaction volume was fixed at 10 mL. The reaction parameters were varied for a H2O2 concentration; b solid loading; c temperature; and d reaction time
The glucose recovery obtained using the conventional AHP process in this study was higher than that reported from most previous works on several lignocellulosic feedstocks such as rice husk, sugarcane bagasse, and corn stover where the glucose recovery yields of 54.3–75.0% were obtained under varying AHP pretreatment conditions [31,32,33]. Banerjee et al. (2012) studied scaling-up of AHP pretreatment of corn stover for converting the released sugars to ethanol. The optimal conditions of AHP process were at 0.125 g H2O2/g biomass at 22 °C for 48 h under atmospheric pressure, resulting in a glucose recovery of 75.0% after saccharification [33]. The AHP pretreatment of rice husk using 7.5% H2O2 v/v at 35 °C for 24 h showed a lower glucose yield of 240 mg/g rice husk, equivalent to 60.7% glucose recovery after enzymatic hydrolysis at 45 °C for 120 h [32]. It is noted that a lower glucose yield of 69.4% after enzymatic hydrolysis was also obtained from AHP pretreated sugarcane bagasse using contained 5% H2O2 v/v at 20 °C for 24 h [31]. The results thus suggested high efficiency and selectivity of the conventional AHP process for pretreatment of rice straw under the optimized conditions in this study.
Effects of Base Types and Organic Solvents in AHP Pretreatment
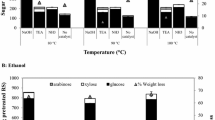
The effects of base (NH4OH and TEA) in the presence of water and various co-solvents (ethanol, isopropanol, and tert-butyl alcohol) were initially studied based on the optimal AHP condition obtained from the conventional process (7.5% w/v solid loading in presence of 2.5% v/v H2O2). The pH was adjusted using different bases to pH 11.5 and incubated at 35 °C for 24 h (Fig. 2). The use of NH4OH for pH adjustment in AHP pretreatment showed a high sugar yield in the range of 178–427 mg/g pretreated RS and sugar recovery of 164–320 mg/g raw RS, equivalent to the glucose recovery of 35.3–73.6% in the aqueous-organic solvent system. A lower sugar yield of 176–208 mg/g pretreated RS and sugar recovery of 165–185 mg/g raw RS, equivalent to 31.6–36.8% glucose recovery, was obtained using TEA. For both bases, higher sugar yield and recovery were obtained using the AHP process in tert-butyl alcohol system compared with those from the water system. The highest glucose recovery of 73.6% was achieved using NH4OH in tert-butyl alcohol as the co-solvent, which was higher than that obtained using the water system under the same experimental conditions (71.2%), suggesting advantages on using this co-solvent in AHP process. The result agreed well with the higher sugar yield obtained from AHP in ethanol system with KOH compared to that from the water system previously demonstrated [22].
Effects of co-solvents and water in AHP pretreatment of rice straw using different organic bases on total sugar product and glucose recovery based on enzymatic digestibility; a NH4OH; b TEA. Reactions contained 7.5% w/v solid loading of rice straw in different aqueous organic systems (70% v/v) in the presence of 2.5% (v/v) H2O2 with 5% (w/v) organic base and incubated at 35 °C for 24 h
Aqueous ammonia or NH4OH has several advantages including high selectivity on delignification of biomass by cleaving the C–O–C linkages in lignin molecule together with breaking the ester and ether bonds in the lignin-carbohydrate complexes with less degradation of cellulose [34]. The use of NH4OH could also allow recycling of the base reagent by evaporation due to the high volatility of ammonia at relatively low temperatures [20]. Tert-butyl alcohol was shown as a superior solvent related to selectivity to cellulose fraction in the pretreatment step and enzymatic hydrolysis compared to the other alcohols (ethanol and isopropanol) and water under the same experimental conditions. The use of tert-butyl alcohol (partition coefficient: log P tert-butyl alcohol = 0.35) has been reported to result in higher solubilization of lignin compared to the use of other alcohols (ethanol, 1-propanol, and 2-propanol; log P = − 0.31, 0.25, and 0.05, respectively), as demonstrated in organosolv pretreatment of sorghum bagasse [35].
Compared to water, the aqueous organic solvent can lead to efficient swelling of the biomass during the pretreatment step due to its larger molecular size. A strong linear correlation between the activation energy on biomass swelling and the molecular weight of solvent was reported [22]. This swelling effect can result in higher accessibility of H2O2 radicals and subsequently by enzymes to the polysaccharide fractions. According to its partition coefficient [35], the use of organic solvent can also allow more efficient solubilization of lignin and lignin-degraded products generated from oxidative cleavages on the ester and ether bonds by the actions of superoxide (O2·) and hydroxyl radical (OH·) species during the pretreatment step [8, 34]. Overall, this can result in increase in pretreatment efficiency and sugar yield from enzymatic hydrolysis of the biomass. The results thus suggested the possibility of using the organic base and co-solvent for the modified low-temperature AHP pretreatment with high efficiency and selectivity.
Effects of Peroxide Loading in the Aqueous Tert-butyl Alcohol System Using NH4OH
The effects of H2O2 concentration in the tert-butyl alcohol system using NH4OH on total sugar product and glucose recovery are shown in Fig. 3. In contrast to the trend observed for the conventional AHP process, it was found that increasing H2O2 concentration from 1 to 10% v/v led to higher sugar yield (349–623 mg/g pretreated RS) and sugar recovery (290–411 mg/g raw RS), equivalent to the maximum glucose recovery of 83.0% at 7.5% v/v of H2O2 concentration. The glucose recovery achieved in this study was higher than those reported in many of the previous works using conventional AHP process on rice straw (44.4–80.0%) [36, 37]. The optimal concentration of H2O2 shown in this study agreed well with the previous reports using conventional AHP processes on different rice wastes. Saha and Cotta (2007) reported that increasing H2O2 concentration from 0 to 7.5% v/v improved release of sugars from rice husk with the highest glucose recovery of 52.2% [32]. The increase in H2O2 concentration (1–4% w/v) also improved enzymatic digestibility of rice husk as reported by Cabrera et al. (2014) [9]. However, excess H2O2 concentration was reported to result in a progressive decrease in sugar product due to non-specific degradation of cellulose along with degradation of released sugars to inhibitory compounds [38].
The chemical compositions of the solids obtained under the optimal pretreatment conditions and enzymatic hydrolysis using NH4OH in aqueous tert-butyl alcohol and NaOH in water were studied compared with the raw RS (Fig. 4). The solid recovery after pretreatment was in the range of 65–66%. According to Fig. 4a, the hemicellulose removal and lignin removal after AHP pretreatment were 10.7% and 58.0% for the NaOH/water process and 9.4% and 52.5% for the NH4OH/tert-butyl alcohol process, respectively, with more than 90% cellulose recovery. Enzymatic hydrolysis led to conversion of the cellulose fraction to glucose with further removal of the hemicellulose and lignin fractions (Fig. 4b). This led to a further increase in accumulated hemicellulose to 73.8% and 39.1% for the NaOH/water and NH4OH/tert-butyl alcohol processes, respectively, while the lignin fraction was mainly not degraded and enriched in the solid residues. Interaction efficiency between hemicellulose and lignin removals by both AHP processes under the optimal conditions and glucose recovery after enzymatic hydrolysis was significant with high correlation based on a statistical analysis of the regression model (R2 = 0.92 and 0.96 and P value < 0.05). This agrees with strong correlation of xylan and lignin removals to glucose yield from AHP pretreatment of sugarcane bagasse [14]. Mass balance of the pretreatment processes with the focus on the cellulose fraction is shown in supplementary Fig. S1, showing balance of starting cellulose, the released glucose, and the residual non-digested cellulose in the solid residues. The results thus showed effective delignification of the biomass with slight hemicellulose removal while the majority of the cellulose fraction was obtained with high enzymatic digestibility. The results on degrees of hemicellulose and lignin removals were correlated to several previous works using water system with NaOH [13, 39,40,41]. Xing et al. (2013) reported that AHP pretreatment of bamboo using 30% w/w H2O2 concentration at 80 °C for 1 h led to reduction of the lignin fraction from 26 to 12.7%, with no significant decrease of glucan and xylan [40]. The AHP process of corn stover containing 1% (w/w) of H2O2 with 4% of solid loading at 37 °C for 3 h resulted in hemicellulose and lignin removals of 6.25% and 41.1%, respectively, together with 87.3% cellulose recovery [39].
Biomass composition on AHP pretreatment (a) and enzymatic hydrolysis (b) using modified and conventional AHP process based on analysis of hemicellulose removal, lignin removal, and glucose recovery. Rice straw was pretreated using NH4OH in 70% v/v aqueous tert-butyl alcohol (7.5% solid loading, 7.5% v/v H2O2, 35 °C, 24 h) and NaOH in water (7.5% solid loading, 2.5% v/v H2O2, 35 °C, 24 h) under the optimal condition compared to raw rice straw
Physicochemical Characterization of Pretreated Rice Straw in Organic Solvent System
The structural alteration of RS samples pretreated by the AHP process in the presence of NaOH in the aqueous medium and NH4OH in the aqueous organic of tert-butyl alcohol system was analyzed by SEM compared with the native RS (Fig. 5). It can be seen that the native RS showed intact physical structure with no cracks and cavities and covered with an intact wax-coated surface. Overall, AHP pretreatment in the presence of different bases both in water and solvent systems resulted in structural changes in the biomass surface due to efficient removals of the cuticle wax and silica layers. The use of NaOH/aqueous and NH4OH/tert-butyl alcohol systems led to formation of higher porous structure and increased surface area of the biomass. Their modified structure allowed increasing accessibility of the enzymes to the inner cellulose microfibers in the substrate. This result was corresponded to the previous work reported by Qi et al. (2009) [42], which showed that the AHP pretreatment of wheat straw in the aqueous medium using NaOH led to rugged, rough, and broken surface as well as appearance of porous structure in the pretreated solid fraction compared to the native biomass which showed an even and smooth flat surface.
SEM analysis of AHP-pretreated rice straw using modified and conventional AHP process under the optimal condition compared to raw rice straw; a, b pretreated rice straw in water with NaOH (7.5% solid loading, 2.5% v/v H2O2, 35 °C, 24 h) at 200×, 500×; c, d pretreated rice straw in 70% v/v tert-butyl alcohol with NH4OH (7.5% solid loading, 7.5% v/v H2O2, 35 °C, 24 h) at 200×, 500×; and e, f native rice straw at 200×, 500×
The crystallinity index of AHP-pretreated samples pretreated under the optimal condition was analyzed by XRD. It was found that the use of NH4OH in aqueous tert-butyl alcohol systems resulted in an increase in crystallinity index (56.1%) compared with the native RS (45.2%) but lower than that obtained using NaOH in aqueous media (59.1%). This could be due to the effect of AHP process which led to more solubilization of amorphous lignin and hemicellulose fractions. The effect of AHP pretreatment on crystallinity index of different lignocellulosic biomass has been reported by several researches. Reis et al. (2016) reported the increase of crystallinity index to 47.6 and 47.5% after AHP pretreatment of sugarcane bagasse and sweet sorghum bagasse, respectively, compared to 34.0% and 33.8% of these biomasses in their native forms, respectively [43]. Moreover, the effect of aqueous ammonia addition could also result in removal of the amorphous lignin and hemicellulose, leading to increasing crystallinity index after biomass pretreatment and enhancing the enzymatic digestibility as reported in the ammonia soaking pretreatment process of corn stover [44].
FT-IR spectra of the rice straw pretreated under the optimal conditions for NaOH/water and NH4OH/tert-butanol system compared to the native biomass are shown in Fig. 6. The functional groups of AHP-pretreated samples were corresponded to the assignment of FT-IR spectra of pretreated oak wood, sugarcane, and sweet sorghum bagasse reported earlier [43, 45]. The raw rice straw showed the peak of C=C stretching of aromatic ring of lignin at 1604 cm−1, C=C aromatic skeletal vibration stretching of the benzene ring in lignin at 1513 cm−1, and C–O–C stretching of primary alcohol in cellulose and hemicelluloses at 1053 cm−1. All functional groups in the raw rice straw were identified in the pretreated rice straw samples according to the FT-IR spectra. However, the lower intensity of these peaks was observed after pretreatment by the AHP process, suggesting solubilization of the lignin and partial hemicellulose fractions. In addition, the higher intensity of C–H deformation of glucose ring in cellulose and hemicellulose at 889 cm−1 of the AHP-pretreated samples compared to that of the raw material also suggested the change in cellulose structure after the pretreatment step.
Comparison of total sugar product and glucose recovery achieved in our study and previous works on pretreatment of rice straw by different methods is summarized in Table 1. The glucose recovery achieved in this study using the modified tert-butyl alcohol/NH4OH-based AHP process or the optimized conventional AHP process was higher or at least comparable compared to that previously reported using different chemical (e.g., acid and ionic liquid) and hydrothermal (e.g., liquid hot water and steam explosion) methods or their combinations which led to a varying glucose recovery in the range of 57.0–83.0% [5,6,7, 46,47,48]. This can reflect the synergized effect of high lignin solubility in tert-butyl alcohol and efficient biomass delignification caused by H2O2. Compared to previous studies on pretreatment of rice straw using different chemical and hydrothermal methods, the developed AHP processes in this study were performed under markedly milder conditions and hence could result in less energy requirement and lower equipment cost. The developed AHP pretreatment by NH4OH/tert-butyl alcohol system is also advantageous compared to the conventional AHP pretreatment using NaOH/aqueous system in terms of reusability of the organic solvent and organic base which can lead to generation of less waste water and chemical waste. Recycling of tert-butyl alcohol using distillation method was demonstrated [49] while NH4OH can be recovered by simple evaporation technique [20]. However, further study on the solvent and base recycling step is needed. The results thus demonstrated the potential of the modified AHP pretreatment developed in this work for biomass utilization in biorefineries.
Conclusion
AHP pretreatment using NH4OH in the aqueous tert-butyl alcohol system was reported in this study. The developed process led to marked improvement on enzymatic digestibility of rice straw, resulting in high glucose recovery with high selectivity on separation of the cellulose. This was associated efficient delignification with partial hemicellulose removal, and hence increase in crystallinity of the cellulose-enriched fraction. The developed AHP pretreatment using the organic base and co-solvent allowed high pretreatment efficiency under mild reaction conditions leading to advantages on low energy consumption and lower formation of toxic compound during the pretreatment step with potential on solvent and base recycling. This work presented a promising modified AHP pretreatment for rice straw and other agricultural wastes for further application in biomass conversion industry.

Highlights
-
A modified AHP process was reported for pretreatment of rice straw.
-
The effects of bases and solvents on efficiency of AHP pretreatment were studied.
-
The modified AHP using NH4OH and tert-butanol led to 83.0% glucose recovery.
-
Physical characteristics of cellulose fraction were analyzed by SEM, XRD, and FTIR.
References
Daily global crude oil demand 2006–2020 (2020) https://www.statista.com/statistics/271823/daily-global-crude-oil-demand-since-2006/. Accessed 5 May 2020.
Chandel AK, Garlapati VK, Singh AK, Antunes FAF, da Silva SS (2018) The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Bioresour Technol 264:370–381. https://doi.org/10.1016/j.biortech.2018.06.004
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318. https://doi.org/10.1016/j.biortech.2018.04.099
Choi S, Song CW, Shin JH, Lee SY (2015) Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng 28:223–239. https://doi.org/10.1016/j.ymben.2014.12.007
Kim I, Lee B, Park J-Y, Choi S-A, Han J-I (2014) Effect of nitric acid on pretreatment and fermentation for enhancing ethanol production of rice straw. Carbohydr Polym 99:563–567. https://doi.org/10.1016/j.carbpol.2013.08.092
Lee C, Zheng Y, VanderGheynst JS (2015) Effects of pretreatment conditions and post–pretreatment washing on ethanol production from dilute acid pretreated rice straw. Biosyst Eng 137:36–42. https://doi.org/10.1016/j.biosystemseng.2015.07.001
Banoth C, Sunkar B, Tondamanati PR, Bhukya B (2017) Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 7(5):334. https://doi.org/10.1007/s13205-017-0980-6
Dutra ED, Santos FA, Alencar BRA, Reis ALS, de Souza RFR, Aquino KAS, Morais MA Jr, Menezes RSC (2018) Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: status and perspectives. Biomass Convers Biorefin 8(1):225–234. https://doi.org/10.1007/s13399-017-0277-3
Cabrera E, Muñoz MJ, Martín R, Caro I, Curbelo C, Díaz AB (2014) Alkaline and alkaline peroxide pretreatments at mild temperature to enhance enzymatic hydrolysis of rice hulls and straw. Bioresour Technol 167:1–7. https://doi.org/10.1016/j.biortech.2014.05.103
Yuan Z, Wen Y, Li G (2018) Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour Technol 259:228–236. https://doi.org/10.1016/j.biortech.2018.03.044
Alencar BRA, Reis ALS, de Souza RFR, Morais MA, Menezes RSC, Dutra ED (2017) Recycling the liquid fraction of alkaline hydrogen peroxide in the pretreatment of corn stover. Bioresour Technol 241:928–935. https://doi.org/10.1016/j.biortech.2017.06.022
Rabelo SC, Andrade RR, Maciel Filho R, Costa AC (2014) Alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis and fermentation of sugarcane bagasse to ethanol. Fuel 136:349–357. https://doi.org/10.1016/j.fuel.2014.07.033
Correia JAC, Júnior JEM, Gonçalves LRB, Rocha MVP (2013) Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: study of parameters. Bioresour Technol 139:249–256. https://doi.org/10.1016/j.biortech.2013.03.153
Zhang H, Huang S, Wei W, Zhang J, Xie J (2019) Investigation of alkaline hydrogen peroxide pretreatment and tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol Biofuels 12(1):107. https://doi.org/10.1186/s13068-019-1454-3
Lu J, Liu H, Xia F, Zhang Z, Huang X, Cheng Y, Wang H (2020) The hydrothermal-alkaline/oxygen two-step pretreatment combined with the addition of surfactants reduced the amount of cellulase for enzymatic hydrolysis of reed. Bioresour Technol 308:123324. https://doi.org/10.1016/j.biortech.2020.123324
Ding D, Li P, Zhang X, Ramaswamy S, Xu F (2019) Synergy of hemicelluloses removal and bovine serum albumin blocking of lignin for enhanced enzymatic hydrolysis. Bioresour Technol 273:231–236. https://doi.org/10.1016/j.biortech.2018.11.024
Zhang K, Pei Z, Wang D (2016) Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour Technol 199:21–33. https://doi.org/10.1016/j.biortech.2015.08.102
Inkrod C, Raita M, Champreda V, Laosiripojana N (2018) Characteristics of lignin extracted from different lignocellulosic materials via organosolv fractionation 11. https://doi.org/10.1007/s12155-018-9895-2
Raita M, Denchokepraguy N, Champreda V, Laosiripojana N (2017) Effects of alkaline catalysts on acetone-based organosolv pretreatment of rice straw. 3 Biotech 7(5):340. https://doi.org/10.1007/s13205-017-0969-1
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Li W, Wang W, Xu P, Xu P, Zhao X, Wang Y (2015) Pretreatment of Miscanthus stalk with organic alkali guanidine and amino-guanidine. Bioresour Technol 179:606–610. https://doi.org/10.1016/j.biortech.2014.11.110
Arpan JAINTHW (2013) Pretreatment composition for biomass conversion process. US Patent WO2013151927A1 (2013-10-10)
Teramura H, Sasaki K, Oshima T, Matsuda F, Okamoto M, Shirai T, Kawaguchi H, Ogino C, Hirano K, Sazuka T, Kitano H, Kikuchi J, Kondo A (2016) Organosolv pretreatment of sorghum bagasse using a low concentration of hydrophobic solvents such as 1-butanol or 1-pentanol. Biotechnol Biofuels 9(1):27. https://doi.org/10.1186/s13068-016-0427-z
Chen W-H, Hsu M-H, Wu A-Y, Hwang W-S (2017) Efficient extraction and recovery of xylan and lignin from rice straw using a flow-through hydrothermal system. J Taiwan Inst Chem Eng 79:103–109. https://doi.org/10.1016/j.jtice.2017.04.021
Sen B, Chou Y-P, Wu S-Y, Liu C-M (2016) Pretreatment conditions of rice straw for simultaneous hydrogen and ethanol fermentation by mixed culture. Int J Hydrog Energy 41(7):4421–4428. https://doi.org/10.1016/j.ijhydene.2015.10.147
Sluiter A, Hames B, Ruiz R, Scralata C, Sluiter J, Templeton D (2011) Determination of structural carbohydrates and lignin in biomass, NREL/TP-51042618. Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory
Ghose TK (1987) Measurement of cellulase activities 59 (2):257. https://doi.org/10.1351/pac198759020257
Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW (2012) Scanning electron microscopy Curr Protoc Microbiol Chapter 2:Unit2B.2-2B.2. https://doi.org/10.1002/9780471729259.mc02b02s25
Sun FF, Wang L, Hong J, Ren J, Du F, Hu J, Zhang Z, Zhou B (2015) The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw Bioresour Technol 187:354-361. https://doi.org/10.1016/j.biortech.2015.03.051
Govindarajan R, Muthukumar K (2015) Influence of dual salt on the pretreatment of sugarcane bagasse with hydrogen peroxide for bioethanol production. Chem Eng J 260:178–187. https://doi.org/10.1016/j.cej.2014.08.006
Rabelo SC, Filho RM, Costa AC (2008) A comparison between lime and alkaline hydrogen peroxide pretreatments of sugarcane bagasse for ethanol production. Appl Biochem Biotechnol 144(1):87–100. https://doi.org/10.1007/s12010-008-8200-9
Saha BC, Cotta MA (2007) Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzym Microb Technol 41(4):528–532. https://doi.org/10.1016/j.enzmictec.2007.04.006
Banerjee G, Car S, Liu T, Williams DL, Meza SL, Walton JD, Hodge DB (2012) Scale-up and integration of alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis, and ethanolic fermentation. Biotechnol Bioeng 109(4):922–931. https://doi.org/10.1002/bit.24385
Elumalai S, Espinosa AR, Markley JL, Runge TM (2014) Combined sodium hydroxide and ammonium hydroxide pretreatment of post-biogas digestion dairy manure fiber for cost effective cellulosic bioethanol production. Sustain Chem Process 2(1):12. https://doi.org/10.1186/2043-7129-2-12
McKarns SC, Hansch C, Caldwell WS, Morgan WT, Moore SK, Doolittle DJ (1997) Correlation between hydrophobicity of short-chain aliphatic alcohols and their ability to alter plasma membrane integrity. Fundam Appl Toxicol 36(1):62–70
Hideno A (2017) Short-time alkaline peroxide pretreatment for rapid pulping and efficient enzymatic hydrolysis of rice straw. Bioresour Technol 230:140–142. https://doi.org/10.1016/j.biortech.2017.01.058
Morone A, Chakrabarti T, Pandey RA (2017) Assessment of alkaline peroxide-assisted wet air oxidation pretreatment for rice straw and its effect on enzymatic hydrolysis. Cellulose 24(11):4885–4898. https://doi.org/10.1007/s10570-017-1451-2
Martin J, Lorenzo Hernando A, Muñoz R, Blanco S, Bolado S (2016) Saccharification of microalgae biomass obtained from wastewater treatment by enzymatic hydrolysis. Effect of alkaline-peroxide pretreatment. Bioresour Technol 218:265–271. https://doi.org/10.1016/j.biortech.2016.06.087
Selig MJ, Vinzant TB, Himmel ME, Decker SR (2009) The effect of lignin removal by alkaline peroxide pretreatment on the susceptibility of corn stover to purified cellulolytic and xylanolytic enzymes. Appl Biochem Biotechnol 155(1–3):397–406. https://doi.org/10.1007/s12010-008-8511-x
Xing Y, Yu H, Zhu L, Jiang J (2013) Efficient enzymatic hydrolysis of bamboo by pretreatment with steam explosion and alkaline peroxide BioResources 8. https://doi.org/10.15376/biores.8.4.5392-5408
Yu H, You Y, Lei F, Liu Z, Zhang W, Jiang J (2015) Comparative study of alkaline hydrogen peroxide and organosolv pretreatments of sugarcane bagasse to improve the overall sugar yield. Bioresour Technol 187:161–166. https://doi.org/10.1016/j.biortech.2015.03.123
Qi B, Xiangrong C, Yi S, Fei S, Yinhua W (2009) Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Ind Eng Chem Res 48:7346–7353. https://doi.org/10.1021/ie8016863
Reis ALS, Damilano ED, Menezes RSC, de Morais Jr MA (2016) Second-generation ethanol from sugarcane and sweet sorghum bagasses using the yeast Dekkera bruxellensis. Ind Crop Prod 92:255–262. https://doi.org/10.1016/j.indcrop.2016.08.007
Kim TH, Lee YY (2005) Pretreatment of corn stover by soaking in aqueous ammonia. Appl Biochem Biotechnol 121-124:1119–1131
Kubovský I, Kačíková D, Kačík F (2020) Structural changes of oak wood main components caused by thermal modification. https://doi.org/10.13140/RG.2.2.33028.88969
Imman S, Arnthong J, Burapatana V, Champreda V, Laosiripojana N (2015) Influence of alkaline catalyst addition on compressed liquid hot water pretreatment of rice straw. Chem Eng J 278:85–91. https://doi.org/10.1016/j.cej.2014.12.032
Phi Trinh LT, Lee J-W, Lee H-J (2016) Acidified glycerol pretreatment for enhanced ethanol production from rice straw. Biomass Bioenergy 94:39–45. https://doi.org/10.1016/j.biombioe.2016.08.017
Gao J, Xin S, Wang L, Lei Y, Ji H, Liu S (2019) Effect of ionic liquid/inorganic salt/water pretreatment on the composition, structure and enzymatic hydrolysis of rice straw. Bioresour Technol Reports 5:355–358. https://doi.org/10.1016/j.biteb.2018.05.006
Lo K-M, Chien IL (2017) Efficient separation method for tert-butanol dehydration via extractive distillation. J Taiwan Inst Chem Eng 73:27–36. https://doi.org/10.1016/j.jtice.2016.07.040
Acknowledgments
The authors sincerely thank Integrative biorefinery laboratory and National Center for Genetic Engineering and Biotechnology (BIOTEC) for laboratory facility. This project was supported by the Thailand Research Fund (TRG6180013) and the National Science and Technology Development Agency
Funding
The project was supported by the Thailand Research Fund (TRG6180013) and the National Science and Technology Development Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Damaurai, J., Preechakun, T., Raita, M. et al. Investigation of Alkaline Hydrogen Peroxide in Aqueous Organic Solvent to Enhance Enzymatic Hydrolysis of Rice Straw. Bioenerg. Res. 14, 122–134 (2021). https://doi.org/10.1007/s12155-020-10152-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10152-5