Abstract
This study was designed to isolate microalgal species which can tolerate severe environmental stresses and can be used for wastewater treatment and biofuel generation. Three different microalgal strains (including Chlorella sorokiniana GEEL-01, Parachlorella kessleri GEEL-02, and Parachlorella kessleri GEEL-03) were isolated from a wastewater treatment plant and Waterwheel Park. The highest growth rate (1.61 OD680nm) was observed for C. sorokiniana GEEL-01 with highest removal of total nitrogen (1.5 mg L−1 day−1) and total phosphorus (1.82 mg L−1 day−1). The biocomponents of the isolated species ranged from 19.47–29.64%, 39.39–52.51%, and 15.08–22.75% for carbohydrate, protein, and lipid, respectively, based on the dry cell weight (DCW). The function groups in microalgal biocompounds were also confirmed by Fourier-transform infrared radiation (FT-IR) spectra, indicated their ability for biofuel generation. Ultimate and proximate analyses of C. sorokiniana GEEL-01 showed high carbon (54.24% DCW) and volatile content (92.69% DCW), compare to other strains. Moreover, it exhibited a significant accumulation of lipid with an increase in the fractions of saturated fatty acids (64.5%), and the major fatty acids were palmitic acid (11.17%), stearic acid (33.86%), oleic acid (9.84%), and linoleic acid (11.56%). Biodiesel derived from the major fatty acids had optimum engine efficiency properties including iodine value (77.34 g I2/100 g oil) and cetane number (54.83). Cultivation of C. sorokiniana GEEL-01 can serve as a dual function of biodiesel feedstock generation and nutrients removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustainable biofuels have reduced the world’s dependence on fossil fuels as they have fewer emissions of greenhouse gases (GHG), less impact on the environment, and greater energy security [1]. Biofuels are generated from edible feedstocks such as maize, sugar cane, and edible oils. Although these biomasses are abundant in nature, there are some challenges in the production of biofuels at different stages. The pretreatment of those biomasses is expensive and economically infeasible. To overcome these challenges, alternative and sustainable sources of biofuel are required [2]. Microalgae are an attractive approach for biofuel production due to their higher oil productivity and fast reproduction rate. They could easily be cultivated on a commercial scale to produce sustainable, eco-friendly, and economically feasible biofuels. Various biofuels are generated from microalgal biomass such as biodiesel (lipid-based), bioethanol (carbohydrate-based), and higher alcohols (protein-based) [3]. Fatty acids composition is species-specific, which would be affected by nutritional, environmental factors, cultivation conditions, and growth phases. Fatty acids profiles have a substantial effect on the characteristics of the produced biodiesel. The higher concentration of saturated fatty acids (palmitic acid and steric acid) resulted in high cetane number and lower iodine value of the biodiesel which led to high engine performance [4]. High content of polyunsaturated fatty acids (linoleic acid and linolelaidic acid) improves the liquefaction value of biodiesel such as cold filter plugging point (°C) that is desirable for colder region [5].
Microalgae belong to a diverse group of organisms categorized by different phyla and classes based on their physiology and the growth requirements. It is estimated that about 80,000 microalgal species are present on earth, of which only 40,000 have been studied and analyzed [4]. The environmental factors in different locations can significantly affect the diversity of the microalgae and their growth rate. Therefore, it is essential to screen and isolate microalgae for high biomass production and biocomponents content at specific sites [6]. The appropriate approach for effectively integrating microalgae cultivation with advanced wastewater treatment is to screen and isolate microalgae species from wastewater since those isolates are already well-adapted to the conditions in wastewaters [1]. Potential progress in the area of microalgal cultivation coupled with wastewater treatment has led to a development in microalgal biofuel production [7]. However, significant efforts are still required for the improvements and optimization of the coupled system to prompt the biomass and treat wastewater. Microalgae with high biocomponents content and growth kinetics in its natural habitat need to be investigated for biofuel production [8].
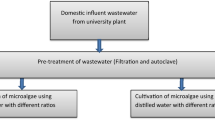
Isolation of microalgae from the natural environment of various habitats in Lanzhou city has not yet been explored. Therefore, this study was designed to isolate microalgal strains which can tolerate severe environmental stresses and could show the potential to be used for many applications including advanced wastewater treatment and biofuel production. Lanzhou city located in the northwest of China and geographical coordinates of this city are 36.057° latitude, 103.840° longitude, and 5,056 ft altitude. It has a semi-arid climate with a very cold winter and hot summers. The identified microalgal species were cultivated in synthetic medium to evaluate their capacity for coupling of biomass production with nutrients removal. The kinetic assessments of microalgal specific growth rate and specific nutrient consumption including total nitrogen (TN) and total phosphorus (TP) were evaluated. The biocomponents content (including lipid, carbohydrate, and protein) was analyzed. This study goes one more step to deeply analyze the biomass elemental composition (CHNS) and functional groups (FTIR). The fatty acids composition was also analyzed along with the estimation of biodiesel quality.
Materials and methods
Collection of wastewater samples and its physicochemical characteristics
Water samples were collected during spring season in 2019 from the Waterwheel Park (WWP) and influent of Yan-Chan wastewater treatment plant (WWTP), Lanzhou, China. The WWP has 108.232° East and 36.063° North geographical position with 5590 m2 of area. While, Yan-Chan WWTP located in Beibhinhe, East Road, Chengguan District. Sterilized plastic bottles (500 mL) were used to collect the water sample and then brought to Green Environmental and Energy Laboratory (GEEL), Lanzhou University. The physicochemical compositions of the wastewater were analyzed (Table 1) after filtering it using a 0.22-μm nylon membrane filter to remove microorganisms and suspended solids. Total dissolved solid (TDS), salinity, and conductivity were measured through PCSTestr 35 multi-parameter (Eutech instruments, USA). Chemical oxygen demand (COD), total nitrogen (TN), and total phosphorus (TP) were analyzed by LH-D100, LH-NT 100, and LH-TP100 kits, respectively, via water-quality auto-analyzer LH-3BN and 5b-3b (V 8) (LianHua Tech-Co., China) [9]. Heavy metals concentration was assessed by inductively coupled plasma optical emission spectroscopy (ICP-OES) (Perkin Elmer, PE Avio 500, America). Sample values were compared to heavy metal mixed standard ICP multi-element standard solution (111355, Merck, USA) [10]. The pH of the wastewater was measured by PHS-3C+ (Table 1). Ten milliliters from collected water of both sites were aseptically added to a 250-mL conical flask that contained 100 mL of autoclaved Bold’s basal medium (BBM) prepared in distilled water [11]. The flasks were placed in shaker incubator MQD S3R (Minquan instruments, Shanghai, China) at 25 °C, 150 rpm under continuous illumination with white fluorescent light (Philips master tl-d eco 51w/54-765) having intensity of 40 μmol m−2 s−1 for 4 weeks [12]. The flasks were examined for microalgal growth using a microscope every 2 days.
Isolation and identification of microalgae
To isolate microalgae, serial dilutions of enriched flask were performed, and 50-μL samples from 1, 2, 4, and 6 dilution fractions were inoculated to a new 500-mL flask containing 250 mL of BBM. A volume of 50 μL from the cultivated samples was sub-cultured on Petri-plates containing BBM solidified with 1.5% (w/v) bacteriological agar. The same volume from different dilutions was transferred to wells of micro-titer plates that had 200 μL BBM and were kept at 25 °C under continuous light for 3 weeks [13]. The culture’s purity was confirmed by repeated culturing and microscopic examinations. The morphological identification was done using botanical approaches [14] and further confirmed by molecular markers.
For DNA extraction, 5–10 mL of fresh microalgal biomass, that was cultured for 10–14 days, was used. Microalgal DNA was extracted using the protocol and instructions of the EZgene™ CP Plant Miniprep Kit (Biomiga, USA). After extraction, the concentration of DNA was measured at 260 nm by a spectrophotometer. The specific coding region (D1-D2) of the rDNA was amplified by a T-Gradient thermocycler (Biometra GmbH, Gottingen, Germany) using the primers, 5′-AGCGGAGGAAAAGAAACTA-′3 as the forward primer and 5′-TACTAGAAGGTTCGATTAGTC-′3 as the reverse primer, using the PCR protocol described [15]. The PCR product on the gel was purified using Tsingke DNA Gel Recovery Kit (Code No. GE0101). Sequencing was analyzed through a Dye Deoxy Terminator Cycle Sequencing Ready Reaction Kit. ABI Prism 377 DNA Sequencer was used for sequencing the fragments. Microalgal strains were identified; the 18S rRNA gene sequences were downloaded to the database, and the genes searched using BLAST [16]. The sequences of gene fragment were analyzed and corrected with Genedoc [17]. The phylogenetic tree was constructed using the neighbor-joining (NJ) Kimura’s two-parameter algorithm, as implemented within the MEGA5 program package [18].
Microalgal growth analyses and nutrients removal
To evaluate the growth kinetics and nutrients removal of identified microalgal species, 150 mL BBM in 250 mL Erlenmeyer flasks was inoculated with the cells (0.1 OD680nm) and incubated at 25 °C with shaking at 150 rpm under continuous white fluorescent lamps (Philips master tl-d eco 51w/54-765) with an intensity of 40 μmol m−2 s−1 for 28 days [19]. The initial inoculum (10% v v−1) used for cultivation of microalgae in this experiment was obtained at the 10th day of active culture (log phase). Microalgal growth was evaluated by measuring the OD at 680nm every 2 days with a spectrophotometer (UV 5500, Metash, China). If the microalgal suspensions had an optical density greater than 1.0, they were diluted to the range of 0.1–1.0. The overall productivity of biomass (Pb) was calculated using the following equation (Eq. 1):
where Pb is the productivity of biomass, x1 is the final biomass concentration at optical density (OD680nm), x0 is the initial biomass concentration at OD680nm, t1 is the final time, t0 is the initial time of cultivation.
The specific growth rate (μ) were calculated using the following equation (Eq. 2):
where x1 and x2 are defined as biomass concentration at optical density (OD680nm) at times t1 and t2, respectively.
Analyses of microalgal biocomponents
The microalgal biomass was harvested after the cultivation time (28 days) to analyze the biocomponents content (including lipid/fatty acid, carbohydrate, and protein) as per protocol reported by Pandey et al. [20]. The lipid were extracted from dried microalgal biomass using the method described by Bligh and Dyer [21]. In detail, 40 mg microalgal biomass, chloroform, and methanol (1:2 v v−1) were added in a glass tube, sonicated for 30 min, and kept in a shaker overnight. The chloroform layer was pipetted, and 2.5 mL chloroform was added, mixed well, and kept in a shaker in for 2 h. It was then centrifuged at 3900 rpm for 10 min; the chloroform recollected and washed with 5 mL NaCl (5%). The samples were evaporated in a dry oven at 50 °C, and the crude lipid content was weighed gravimetrically. Fatty acids (FAs) were analyzed by modifying the method of Lepage and Roy [22]; 1 mL was taken from the crude lipid layer, and 1 mL methanol and 0.3 mL H2SO4 were added. The mixture was vortexed for 3–5 min and incubated at 100 °C for 10 min; 1 mL dH2O was added, centrifuged for 3–5 min, and then centrifuged at 4000 rpm for 10 min. Fatty acids methyl esters (FAMEs) were evaluated with a gas chromatograph equipped with a flame ionization detector GC-(FID) (Foli instruments, China). For FAMEs, the conditions of the inlet and detector were 240 °C and 260 °C, respectively, and the oven was set at 100 °C (2 min), raised by 4 °C min−1 to 180 °C (10 min), and raised by 5 °C min−1 to 235 °C (9 min). Then, 1 μL sample was injected using nitrogen as the carrier gas. The mixture of FAMEs standard, Supelco ® 37-component (Sigma-Aldrich, USA), was used to identify FAs composition present in the extracted microalgal lipid [6]. The properties of the biodiesel produced from microalgae were calculated based on the FAMEs characteristics according to the following equations [23]:
where UD is the unsaturation degree (%), MUFA is monounsaturated fatty acids, PUFA is polyunsaturated fatty acids, N% is the percentage of each fatty acid, M represents the molecular weight of the fatty acid, D is the number of double bonds, SV is saponification value (mg KOH g−1), IV is iodine value (gI2/100 g oil), CN is the cetane number, LCSF is long-chain saturation factor, and C16:0, C18:0, C20:0, C22:0, and C24:0 represent the weight percentage of the corresponding fatty acids.
The carbohydrate content in the microalgal biomass was assessed using the phenol-sulfuric acid method [24]. The carbohydrate concentrations were measured by taking the optical density at 490nm using a spectrophotometer. The concentration was compared to a glucose standard. Microalgal protein content was evaluated by the Lowry method [25]. The freeze-dried algal biomass (1 mg mL−1) was diluted with 0.5 mL Folin ciocalteu reagent (Sigma Co., St. Louis, MO, USA) and 5 mL Lowery reagent. The protein was measured at 750nm using a spectrophotometer. The values were compared to the Bovine serum albumin (BSA) standard.
Comprehensive characteristics of microalgal biomass
The moisture of the samples was assessed by heating the samples at 105 °C for 24 h. The volatile content and ashes were calculated using the Leco TGA 701 instrument according to EN 14776 by heating the sample for 2.5 h at 550 °C under a constant air supply [26]. The elemental analysis determined the percentages of carbon, hydrogen, nitrogen, sulfur, and oxygen of microalgal freeze-dried biomass using the Elementar Vari EL cube (Germany). Freeze-dried samples (3–4 mg dry weight) were placed in tin boats and folded several times to expel any trapped air to reduce background levels of carbon (C) and nitrogen (N) contamination. Sulfanilamide standards were co-analyzed to calibrate the responses for C, H, N, and S [27].
The higher heating value (HHV) of microalgal biomass was calculated from C, H, N, and S using the Dulong equation [28].
Fourier transform infrared (FTIR) spectroscopy predicts the structural and chemical boding nature (targeting functional groups) of different biomolecules in microalgal biomass. The Freeze-dried microalgal biomass (1.5 mg) was analyzed through the MAGNA 550 Nicolet (Madison, USA). The biomass was ground with 100 mg of spectroscopic grade potassium bromide (KBr) powder in an agate mortar. The instrument was equipped with a mercury cadmium telluride (MCT) detector, and the spectra were recorded in the frequency range of 500–4000 cm−1 at a resolution of 4 cm−1 [28].
All the experiments were carried out in triplicate and data are expressed as mean ± standard deviation (SD). SPSS 22.0 (IBM SPSS statistics) was used to perform all statistical analysis in this study. The difference between microalgal strains and variables (including growth kinetics, TN, and TP removal) were analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests (p < 0.05 level of confidence).
Results and discussion
Isolation and identification of microalgae
Isolation is a vital step to obtain pure culture for bioprocess development. It is the first step for the selection of potential microalgae for biofuel production and nutrient removal from wastewater [20]. Microalgal strains, which were isolated from different water sources at Lanzhou City, Gansu, China, were investigated for identification, characterization, growth kinetics, and biochemical content analysis. A total of nine strains were isolated from both sites, and three strains out of them showed abundance due to high growth in original samples and were further cultivated under laboratory conditions. Microscopic examination confirmed the purity of microalgal cultures. Based on morphology and successful culturing, three microalgal isolates (GEEL-01, GEEL-02, and GEEL-03) had spherical cells. A single-celled colony with a diameter of 4–10 μm was selected (Fig. S1). Microalgae species exhibit different morphological and biocomponents variations based on cultivation time and conditions [29]. The identification of microalgae based on morphological differentiation seemed to be very challenging. Therefore, the isolated strains were subjected to molecular identification and were identified as C. sorokiniana GEEL-01, Parachlorella. kessleri GEEL-02, and Parachlorella kessleri GEEL-03.
The amplified region LSU rDNA (D1-D2) with a size of 741–772 bp was noted for all strains. The amplified region LSU rDNA has more variation than the SSU rDNA region, and thus provides more genotypic variation among closely related species using short diagnostic sequences [15]. It was found that the identified isolates C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03, were similar to Chlorella and Perchlorella based on 98 and 99% sequence similarities, respectively. The genome sequences of the isolates were published in the NCBI databases.
The fragment size of the amplified LSU rDNA (D1-D2) regions of the isolates and their accession numbers and the nearest identified match present in the GenBank nucleotide database are shown in Table 2. The identified isolates were also confirmed by results obtained from the phylogenetic analysis of the LSU rDNA D1-D2 sequence. C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 were similar to the microalgal strains Chlorella sorokiniana KU361155, Parachlorella kessleri FR865655, and Parachlorella kessleri KJ676116 (Fig. 1 and Table 2). The isolated strains were further investigated for growth kinetics in BBM under laboratory conditions.
Growth rates of the microalgal strains
Microalgal strains were cultured in BBM to analyze biomass production. The growth was calculated after every 2 days by taking OD680nm through spectrophotometer. C. sorokiniana had a higher growth rate than the other strains (Fig. 2a). The average growth rate of C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 was 1.61, 1.39, and 1.23 OD680nm respectively, after 28 days. Microalgal growth was statistically significant (p < 0.05) (Fig. 2a, b). The highest specific growth rate for each strain was observed during the lag phase of the cultivation time (Fig. 2b), due to the ideal environmental conditions (such as active inoculum and less competition among the cells) and proper availability of nutrients which accelerate the microalgal growth [30]. While, the average specific growth rate values of 28 days for C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 were 0.149, 0.156, and 0.133 day−1, respectively (Table 3). The maximum growth of chlorella was 0.4 OD540nm after 10 day, as reported by Asuthkar et al. [34]. The growth rate of the isolates varied under similar environmental conditions. Microalgal growth is related to the nutrients availability, light source, pH of the growth medium, temperature, and addition of initial inoculum volume [35]. An increase in inoculum resulted in increased cell proliferation, microalgal biomass, and efficient nutrient recovery [36]. The pH of the cultivation medium for all strains increased with time. The initial pH of the medium was kept constant at 6.8 [37], during the lag phase a slight increase was noted, while, during the log phase there was a differential increase in the pH due to the rise in microalgal photosynthetic activity and the last day the pH of the medium was 9.4–10.5 (Fig. 2c). The increased changes in the pH, from 6.6 to 10.1, of the culture medium with an increase in time did not inhibit the growth of Chlorella sp. IMMTCC-2 [31]. The productivity of biomass for C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 were 0.056, 0.049, and 0.043 OD 680nm day−1. This finding suggests that C. sorokiniana GEEL-01 is the most suitable for biomass production.
Growth curve of isolated microalgae in BBM. a Daily specific growth rate of isolates. b pH variation in cultivation media of the isolated microalgae. c Statistical differences between the microalgal strains in growth kinetics; specific growth rate were identified by one-way (ANOVA) followed by Duncan’s multiple comparisons (p < 0.05). Statistically differences are marked by various alphabets
It is necessary to investigate potential microalgae for high nutrient removal as well as high biomass production [38]. Three microalgal strains could effectively remove TN and TP from the culturing medium. C. sorokiniana GEEL-01 showed improved removal efficiency when compared to the other stains (Fig. 3). The initial TN and TP concentration in BBM was 43.003 mg L−1 and 51.2 mg L−1, respectively, and the C. sorokiniana GEEL-01 reduced the final TN concentration to 0.046 mg L−1 and TP concentrations to 0.354 mg L−1 after 28 days (Fig. 3a, b). Nutrient (such as TN and TP) removal ability of the microalgal strains was statistically significant (p < 0.05) (Fig. 3a, b). Nitrogen is needed for the growth of microalgae and ranges from 1 to 10% of the cell weight. To overcome growth limitation, the N to P ratio in the medium should be adequate to the stoichiometric ratio (Redfield ratio i.e.,16:1) of microalgal biomass. Coupling wastewater treatment with microalgae cultivation ensures the availability of N and P for microalgal growth making this coupled technology cost-effective for microalgal biomass production for biofuel generation and nutrients recycling [1]. The concentration of N determined the biocomponents percentage in the microalgae, and the lower concentration in the medium might result in a high accumulation of lipid. In contrast, a high concentration leads to high protein content [39]. P is also important for cell functions, DNA, and energy transfer. The primary mechanism involved for TN and TP elimination might be microalgal biomass uptake [40]. The different microalgal species uptake TN and TP at different rates during the cultivation period [41].
Nutrient removal by isolated microalgae during cultivation. Total nitrogen removal (a). Total phosphorus removal (b). Statistical differences between the microalgal strains and nutrient removal were identified by one-way (ANOVA) followed by Duncan’s multiple comparisons at p < 0.05. Statistically differences are marked by different alphabets and were represented as (a > b > c)
Biomass composition
Microalgal biocomponents composition
All microalgal strains showed a high accumulation of protein content (39.39–52.51%) followed by carbohydrate content (19.47–29.64%), and then lipid content (15.08–22.75%) comprising 84–92% of the total cell biomass (Table 3). The microalgal cell biocomposition were compared to others strains which carry same range of the biocomponents concentration (Table 3). Gao et al. [33] observed an increase in lipid accumulation of P. Kessleri up to 33.2% under nitrogen stress. The limitation in N, P resulted in lipid accumulation in microalgal cells [42]. In the present study, C. sorokiniana GEEL-01 showed high lipid content which might be due to the reduction in N, P at the end of the cultivation (Table 3 and Fig. 3). Moreover, the proximate analysis showed that C. sorokiniana GEEL-01 has highest volatile content, 92.69% of the total dry cell weight (DCW) when compared to the other strains (Table 4). Ash content of 7.31%, 6.15%, and 7.02% of the DCW were noted for C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 strains, respectively. The high ash content in microalgal biomass could result in low energy production if the biomass is directly subjected to high heat. It has been reported that the ash content of Chlorella sp. was heated to 1700 °C but did not evaporate [26].
CHNS and FTIR analysis
The main elements and their estimated composition of the dried microalgal biomass are presented in Table 4. Microalgal isolates C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 had a carbon content of 44.56–54.24%, an oxygen content of 31.63–40.74%, a hydrogen content of 6.77–7.96%, a sulfur content of 0.28–0.398%, and a nitrogen content of 5.72–7.56%. P. kessleri GEEL 02, and P. kessleri GEEL-03, had a high C/N ratio and increased protein content. The high protein content discriminates microalgal biomass from lignocellulosic feedstocks, which has usually less than < 1% N [26]. The microalgal isolates had higher carbon content and hydrogen content, which makes them improved potential candidates for biofuel production rather than lignocellulosic biomass (rice straw) and fossil fuels (coal) (Table 4). A high sulfur and nitrogen content in microalgal biomass leads to increased emissions of nitrogen oxides (NO×) and sulfur oxides (SO×) [45]. As the S content is negligible in the isolated microalgae, the emission of SO× is not expected. The N content was similar to (6.64–7.72%) that reported by Pandey et al. [20], and the increased nitrogen content may be due to availability of high TN in the culture medium. The lower O content in C. sorokiniana GEEL-01 resulted in a high HHV 24.04664 MJ Kg−1 DCW, which is higher than previous studies [46]. These findings can predict that C. sorokiniana GEEL-01 and P. kessleri GEEL-02, which have high carbon content and HHV and low N and S, can be used as feedstock for biodiesel production.
FT-IR spectra of the microalgal biomass were recorded, and the bands were compared with those from reference literature. The FT-IR transmittance of the isolated microalgal species C. sorokiniana GEEL-01, P. kessleri GEEL-02, and P. kessleri GEEL-03 represented the alcoholic group (OH), carboxylic group (COOH), amino (NH2), and organic compound groups (Fig. 4). The region 3550–3200 cm−1 in the spectra represents the presence of lipids in the biomass and is due to the symmetrical and asymmetrical stretching vibration of CH2 [47]. This functional group comprised the backbone of the lipid and stretches at 2830–2695 cm−1 [48]. Alkane groups are seen at 3100–2800 cm−1, aldehyde groups at 2830–2695 cm−1, ester group at 1800–1700 cm−1, and carboxylic group at 1420–1330 cm−1. The alkane and alkene groups show the presence of saturated and unsaturated hydrocarbons. The aldehyde, carboxylic, and ester groups display an abundance of carbohydrates, proteins, and lipids. Distinct bands were observed in the regions of 1750–1720 cm−1, 1163–1210, and 1–4 cm−1, which shows the presence of esters in the isolated microalgal biomass. The increases in the peaks at 1700–1800 cm−1 (carbonyl stretch) confirm higher concentrations of fatty acids [49]. This indicates that microalgal biomass gives a distinct peak in the FT-IR spectra for triglycerides (lipids) and fatty acids. FT-IR spectroscopy performed a rapid screening of microalgal biomass and thus appears to be a viable analytical approach to evaluate microalgae as potential candidates for biofuel production.
Fatty acids profile and biodiesel properties
The fatty acids profiles of the three microalgal strains are summarized in Table 5. The predominant FAs composition of C. sorokiniana GEEL-01 was palmitic acid (C16:0), stearic acid (C18:0), palmitoleic acid (C16:1), oleic acid (C18:1), and linoleic acid (C18:2). The saturated fatty acids (SFAs) content was 31 to 64.5%, the monounsaturated fatty acids (MUFAs) content was 15.23–33.14%, and the polyunsaturated fatty acids (PUFAs) content was 20.40 to 35.34% for all observed microalgae species (Fig. 5). The properties of biodiesel are dependent on the composition of fatty acids [50]. The biodiesel characteristics derived from the cultured biomass are shown in comparison with other microalgal species and internationally recognized standards of biodiesel, such as the European standard EN 14214 and the American standard ASTM 6751−03 in Table 6. The important values of biodiesel derived from isolated microalgae such as unsaturation degree (UD), saponification value (SV), cetane number (CN), iodine value (IV), cold filter plugging point (CFPP), and long-chain saturated factor (LSCF), were calculated based on the fatty acids profile and were acceptable according to the recommend international standards.
In this study, C. sorokiniana GEEL-01 had high total SFAs contents (64.5% DCW) with IV of 77.34 g I2/100 g oil and CN of 54.83. The biodiesel characteristics from C. sorokiniana GEEL-01 biomass were in the limit of the international biodiesel standard (EN 14214) and are desirable for oxidative stability [5]. While, high concentration of PUFAs (35% DCW) in P. kessleri GEEL-02 biomass resulted in a lower CFPP (− 4.44 °C) of biodiesel which are recommended for colder region without blocking engine filter (Table 6). The properties for biodiesel assessment are important to understand the performance and quality of biodiesel in engines. Emission of toxic gases, such as nitrogen oxide, carbon monoxide, and low hydrocarbons, is due to high UD values [54]. The viscosity and CN are dependent on the LCSF values, and it could improve engine efficiency and ignition properties of biodiesel. CFPP depends on the local region, and higher CFPP values of biodiesel resulted in problems in filter plugging and flow of fuels [50]. The biodiesel derived from isolated microalgae could be an alternative to fossil fuels and could reduce energy scarcity and environmental pollution.
Conclusions
Microalgal strains were found to be promising for biofuel production and nutrient removal. C. sorokiniana GEEL-01 attained the highest growth rate when compared to the other strains. The biochemical content analysis showed that C. sorokiniana GEEL-01 had an increased lipid content at 22.75%. C. sorokiniana GEEL-01 could remove TN and TP at 1.5 mg L−1 day−1 and 1.82 mg L−1 day−1, respectively. High C content and low N content differentiated the isolated microalgae from lignocellulosic biomass (rice straw) and fossil fuels (coal) as a sustainable source of biofuel production. The high concentration of C16 and C18 fatty acids in the lipid content improved the biodiesel properties. The high nutrient removal rate and lipid content of C. sorokiniana GEEL-01 makes it a viable option for using as feedstock for biofuel production and wastewater treatment.
References
Salama E-S, Kurade MB, Abou-Shanab RAI, El-Dalatony MM, Yang I-S, Min B, Jeon B-H (2017) Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew Sust Energ Rev 79:1189–1211. https://doi.org/10.1016/j.rser.2017.05.091
Aditiya H, Chong W, Mahlia T, Sebayang A, Berawi M, Nur H (2016) Second generation bioethanol potential from selected Malaysia’s biodiversity biomasses: a review. Waste Manag 47:46–61. https://doi.org/10.1016/j.wasman.2015.07.031
Staples MD, Malina R, Barrett SR (2017) The limits of bioenergy for mitigating global life-cycle greenhouse gas emissions from fossil fuels. Nat Energy 2:16202. https://doi.org/10.1038/nenergy.2016.202
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36. https://doi.org/10.1186/s12934-018-0879-x
Abomohra AE-F, El-Naggar AH, Amajuba B (2018) Potential of macroalgae for biodiesel production: screening and evaluation studies. J Biosci Bioeng 125:231–237. https://doi.org/10.1016/j.jbiosc.2017.08.020
Shuba ES, Kifle D (2018) Microalgae to biofuels:‘Promising’alternative and renewable energy, review. Renew Sust Energ Rev 81:743–755. https://doi.org/10.1016/j.rser.2017.08.042
Ferro L, Miranda F, Gentili F, Funk C (2020) Photosynthesis at high latitudes: adaptation of photosynthetic microorganisms to Nordic climates. In: Lee NM (ed) biotechnological applications of extremophilic microorganisms. Walter de Gruyter
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
Ndayisenga F, Yu Z, Yu Y, Lay C-H, Zhou DJB (2018) Bioelectricity generation using microalgal biomass as electron donor in a bio-anode microbial fuel cell. Bioresour Technol 270:286–293. https://doi.org/10.1016/j.biortech.2018.09.052
Chen G, Shan R, Li S, Shi J (2015) A biomimetic silicification approach to synthesize CaO-SiO2 catalyst for the transesterification of palm oil into biodiesel. Fuel 153:48–55. https://doi.org/10.1016/j.fuel.2015.02.109
Kanz T, Bold H (1969) Physiological studies, morphological and taxonomical investigation of Nostoc and Anabaena in culture. University of Texas, Austin Publication No 6924
Fistarol GO, Hargreaves PI, Walter JM, Viana TV, Gomes PD, Lourenço CB, Rezende CE, Gregoracci G, Rua C, Thompson CC (2018) Rapid isolation of culturable microalgae from a tropical shallow lake system. J Appl Phycol 30(3):1807–1819. https://doi.org/10.1007/s10811-018-1404-7
Cobos M, Castro JC, Paredes JD, Pérez S, Maddox JD, Estela SL, Rodríguez HN, Tresierra AB, Saldaña JR, Marapara JL (2019) Isolation, characterization, and biotechnological potential of native microalgae from the Peruvian Amazon. In: Milada Vítová microalgae-from physiology to application. IntechOpen. https://doi.org/10.5772/intechopen.89515
Sime I (2004) The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae, edited by David M. John, Brian A. Whitton and Alan J. Brook. Cambridge University Press, Cambridge, 2002, 702 pp. ISBN 0-521-77051-3. Aquat Conserv Mar Freshwat Ecosyst 14:105–105. https://doi.org/10.1002/aqc.579
Sonnenberg R, Nolte AW, Tautz D (2007) An evaluation of LSU rDNA D1-D2 sequences for their use in species identification. Front Zool 4:6. https://doi.org/10.1186/1742-9994-4-6
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Nicholas K, Nicholas H (1997) GeneDoc. A tool for editing and annotating multiple sequence alignments: multiple sequence alignment editor and shading utility version 2.6.002. http://www.psc.edu/biomed/genedoc
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. https://doi.org/10.1093/molbev/msm092
Abou-Shanab RA, Matter IA, Kim S-N, Oh Y-K, Choi J, Jeon B-H (2011) Characterization and identification of lipid-producing microalgae species isolated from a freshwater lake. Biomass Bioenergy 35:3079–3085. https://doi.org/10.1016/j.biombioe.2011.04.021
Pandey A, Srivastava S, Kumar S (2019) Isolation, screening and comprehensive characterization of candidate microalgae for biofuel feedstock production and dairy effluent treatment: a sustainable approach. Bioresour Technol 293:121998. https://doi.org/10.1016/j.biortech.2019.121998
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/y59-099
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Francisco ÉC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403. https://doi.org/10.1002/jctb.2338
Dubois M, Gilles K, Hamilton J, Rebers P, Smith FJAC (1956) Phenol sulphuric acid method for total carbohydrate. Anal Chem 26:350
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Rizzo AM, Prussi M, Bettucci L, Libelli IM, Chiaramonti D (2013) Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior. Appl Energy 102:24–31. https://doi.org/10.1016/j.apenergy.2012.08.039
Quigg A, Finkel ZV, Irwin AJ, Rosenthal Y, Ho T-Y, Reinfelder JR, Schofield O, Morel FM, Falkowski PG (2003) The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nat 425:291–294. https://doi.org/10.1038/nature01953
Sudhakar K, Premalatha M (2015) Characterization of micro algal biomass through FTIR/TGA/CHN analysis: application to Scenedesmus sp. Energy Source A Recov Util Environ Eff 37:2330–2337. https://doi.org/10.1080/15567036.2013.825661
Suparmaniam U, Lam MK, Uemura Y, Lim JW, Lee KT, Shuit SH (2019) Insights into the microalgae cultivation technology and harvesting process for biofuel production: a review. Renew Sust Energ Rev 115:109361. https://doi.org/10.1016/j.rser.2019.109361
Chia MA, Lombardi AT, Melao MDGG (2013) Growth and biochemical composition of Chlorella vulgaris in different growth media. An Acad Bras Cienc 85:1427–1438. https://doi.org/10.1590/0001-3765201393312
Nayak M, Jena J, Bhakta S, Rath SS, Sarika C, Rao BVSK, Pradhan N, Thirunavoukkarasu M, Mishra SK, Panda PK (2011) Screening of fresh water microalgae from eastern region of India for sustainable biodiesel production. Int J Green Energy 8:669–683. https://doi.org/10.1080/15435075.2011.588764
Kumar K, Dasgupta CN, Das D (2014) Cell growth kinetics of Chlorella sorokiniana and nutritional values of its biomass. Bioresour Technol 167:358–366. https://doi.org/10.1016/j.biortech.2014.05.118
Gao Y, Feng J, Lv J, Liu Q, Nan F, Liu X, Xie S (2019) Physiological changes of Parachlorella Kessleri TY02 in lipid accumulation under nitrogen stress. Int J Environ Res Public Health 16:1188. https://doi.org/10.3390/ijerph16071188
Asuthkar M, Gunti Y, Rao R, Rao C, Yadavalli R (2016) Effect of different wavelengths of light on the growth of Chlorella pyrenoidosa. Int J Pharm Sci Res 7:847–851. https://doi.org/10.13040/IJPSR.0975-8232.7(2).847-51
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628. https://doi.org/10.1016/j.biortech.2009.10.062
Lau P, Tam N, Wong Y (1995) Effect of algal density on nutrient removal from primary settled wastewater. Environ Pollut 89:59–66. https://doi.org/10.1016/0269-7491(94)00044-E
Barsanti L, Gualtieri P (2014) Algae: anatomy, biochemistry, and biotechnology. CRC press, Boca Raton. https://doi.org/10.1201/b16544
Chiu S-Y, Kao C-Y, Chen T-Y, Chang Y-B, Kuo C-M, Lin C-S (2015) Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour Technol 184:179–189. https://doi.org/10.1016/j.biortech.2014.11.080
Negi S, Barry AN, Friedland N, Sudasinghe N, Subramanian S, Pieris S, Holguin FO, Dungan B, Schaub T, Sayre R (2016) Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. J Appl Phycol 28:803–812. https://doi.org/10.1007/s10811-015-0652-z
Khalid AAH, Yaakob Z, Abdullah SRS, Takriff MS (2019) Analysis of the elemental composition and uptake mechanism of Chlorella sorokiniana for nutrient removal in agricultural wastewater under optimized response surface methodology (RSM) conditions. J Clean Prod 210:673–686. https://doi.org/10.1016/j.jclepro.2018.11.095
Znad H, Al Ketife AM, Judd S, AlMomani F, Vuthaluru HB (2018) Bioremediation and nutrient removal from wastewater by Chlorella vulgaris. Ecol Eng 110:1–7. https://doi.org/10.1016/j.ecoleng.2017.10.008
Ghafari M, Rashidi B, Haznedaroglu BZ (2018) Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 9:147–156. https://doi.org/10.1080/17597269.2016.1221644
Coimbra RN, Paniagua S, Escapa C, Calvo LF, Otero MJRE (2015) Combustion of primary and secondary pulp mill sludge and their respective blends with coal: a thermogravimetric assessment. Renew Energy 83:1050–1058. https://doi.org/10.1016/j.renene.2015.05.046
Paniagua S, Calvo LF, Escapa C, Coimbra RN, Otero M, Garcia AI (2018) Chlorella sorokiniana thermogravimetric analysis and combustion characteristic indexes estimation. J Therm Anal Calorim 131:3139–3149. https://doi.org/10.1007/s10973-017-6734-1
Miranda AF, Biswas B, Ramkumar N, Singh R, Kumar J, James A, Roddick F, Lal B, Subudhi S, Bhaskar T (2016) Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol Biofuels 9:221. https://doi.org/10.1186/s13068-016-0628-5
Gautam R, Varma AK, Vinu R (2017) Apparent kinetics of fast pyrolysis of four different microalgae and product analyses using pyrolysis-FTIR and pyrolysis-GC/MS. Energy Fuel 31:12339–12349. https://doi.org/10.1021/acs.energyfuels.7b02520
Miranda M, Sepúlveda F, Arranz J, Montero I, Rojas C (2018) Physical-energy characterization of microalgae Scenedesmus and experimental pellets. Fuel 226:121–126. https://doi.org/10.1016/j.fuel.2018.03.097
Forfang K, Zimmermann B, Kosa G, Kohler A, Shapaval V (2017) FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for oleaginous fungi. PLoS One 12:e0170611. https://doi.org/10.1371/journal.pone.0170611
Kothari R, Pathak VV, Kumar V, Singh D (2012) Experimental study for growth potential of unicellular alga Chlorella pyrenoidosa on dairy waste water: an integrated approach for treatment and biofuel production. Bioresour Technol 116:466–470. https://doi.org/10.1016/j.biortech.2012.03.121
Patel A, Arora N, Mehtani J, Pruthi V, Pruthi PA (2017) Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew Sust Energ Rev 77:604–616. https://doi.org/10.1016/j.rser.2017.04.016
Bharti RK, Dhar DW, Prasanna R, Saxena AK (2018) Assessment of biomass and lipid productivity and biodiesel quality of an indigenous microalga Chlorella sorokiniana MIC-G5. Int J Green Energy 15:45–52. https://doi.org/10.1080/15435075.2017.1351368
Osundeko O, Davies H, Pittman JK (2013) Oxidative stress-tolerant microalgae strains are highly efficient for biofuel feedstock production on wastewater. Biomass Bioenergy 56:284–294. https://doi.org/10.1016/j.biombioe.2013.05.027
Ashour M, Elshobary ME, El-Shenody R, Kamil A-W, Abomohra AE-F (2019) Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenergy 120:439–447. https://doi.org/10.1016/j.biombioe.2018.12.009
Altun Ş (2014) Effect of the degree of unsaturation of biodiesel fuels on the exhaust emissions of a diesel power generator. Fuel 117:450–457. https://doi.org/10.1016/j.fuel.2013.09.028
Funding
This work was supported by a grant (PCSED-001-18) from Ministry of Higher Education, Kingdom of Saudi Arabia, under the Promising Center for Sensors and Electronic Devices (PCSED) at Najran University, Kingdom of Saudi Arabia and the startup fund for the construction of the double first-class project (No. 561119201), Lanzhou University, China
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• FTIR and CHNS analysis confirmed presences major components in microalgal strains.
• C. sorokiniana GEEL-01 showed growth curve (1.61 OD680nm) and lipid content (22.75%).
• C. sorokiniana GEEL-01 uptake TN (1.82 mg L−1 day−1) and TP (1.5 mg L−1 day−1).
• The protein content in all strain was ≥ 35% while P. kessleri GEEL-03 52.51% protein.
Electronic supplementary material
ESM 1
(DOCX 917 kb)
Rights and permissions
About this article
Cite this article
Arif, M., Wang, L., Salama, ES. et al. Microalgae Isolation for Nutrient Removal Assessment and Biodiesel Production. Bioenerg. Res. 13, 1247–1259 (2020). https://doi.org/10.1007/s12155-020-10136-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10136-5