Abstract
Four microalgal strains, namely, Tetraselmis indica (T. indica), Scenedesmus abundans (S. abundans), Spirulina sp., and Nostoc muscorum (N. muscorum) were cultivated on four different wastewaters in 1000 ml photobioreactors with 750 ml working volume under 94.5 μmol m−2 s−1 light intensity for 14 days for phycoremediation of wastewaters and sustainable biodiesel production. These microalgal strains attained maximum biomass growth in the secondary treated sewage (STS). Maximum biomass yield (0.6533 g L−1) and lipid productivity (25.44 mg L−1 d−1) for T. indica were achieved in STS. T. indica removed (63.6–78.24%) of nitrate, (60.90–65.97%) of phosphate, (61.01–80.01%) of ammonical nitrogen, and (71.16–85.70%) of total organic carbon (TOC) in all four wastewaters. The fatty acid methyl ester (FAME) profile of T. indica shows the presence of myristic acid (1.2%) pentadecylic acid (0.28%), palmitic acid (10.32%), oleic acid (34.59%), linoleic acid (12.38%), and eicosanoic acid (14.88%) in STS. This study demonstrates that T. indica is the most suitable microalgal species among the four microalgal strains selected for phycoremediation of wastewaters and higher biomass production for sustainable biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increased industrialization and urbanization, the demand of renewable energy resources has been increased rapidly (Araujo et al. 2011). The reason could be the limited availability of petroleum-based fuels and their contribution in global warming, which have demanded the development of new renewable energy resources. Currently, renewable energy sources such as biofuel (biodiesel and bioethanol), oils, and biogas have been considered as an alternative energy sources for fossil fuels, which accounts for approximately 10% of the total global energy consumption (Medeiros et al. 2015). Many of the developed and developing countries have adopted the practice of producing sustainable and renewable energy resources such as biodiesel, oil from plants (Jatropha, common hazel, palm, and canola), and crops like corn and sunflower, (Barnwal and Sharma, 2005). Among these, biodiesel production using microalgae have gained tremendous attention over the oil production from plants and crops due to high lipid content, ease of cultivation, easy management, and high cell density.
Microalgae are unicellular and fast growing organism that uses water, carbon dioxide, and nutrients for its growth. Other factors that would affect the growth of microalgae are temperature, light intensity, nutrients, CO2 concentration, location of cultivation, and pH (Chen et al. 2011; Wu et al. 2013; Rai and Gupta, 2016). Algae represent a very diverse and complex group of organisms, which belongs to different phyla and can be distinguished by very different physiological attributes. A direct consequence of this diversity is that the different algae species demanded different environment for their growth in different cultivation medium (Chakraborty et al. 2016). This property of algal species can be used for the screening of microalgae strain in terms of maximal productivity and high lipid content. In a review, considering several marine and freshwater microalgae, Mata et al. (2010) reported that lipid content of marine Dunaliella tertiolecta microalgae (up to 71% on dry weight basis) is much higher than the lipid content of Scenedesmus sp. freshwater microalgae.
Other than biodiesel production, treatment of nitrogen and phosphorus-rich effluents using microalgae has been proved to be beneficial over conventional treatment methods (Gani et al. 2016b). The algal treatment is also a promising approach for the complete removal of nutrients opposite to the conventional tertiary treatment (Ledda et al. 2015). Microalgae can be cultivated in municipal sewage as well as in industrial wastewaters like paper mill effluent, sugar mill effluent, dairy industry wastewater, piggery wastewater, and pharmaceutical industry wastewater (Abou-Shanab et al. 2013; Sirin and Sillanpää, 2015; Gani et al. 2017). These wastewaters are rich in macro nutrients such as nitrogen, phosphorus and micro nutrients, like Mg, Mn, Ca, and Na, which are responsible for activation of different metabolic pathways like photosynthesis, energy storage, and many cellular enzymes (Zhu et al. 2013; Chen et al. 2011). However, optimum concentration of these nutrients is necessary for achieving maximum growth of microalgae. Apart from these inorganic nutrients, carbon is the most essential element as it accounts for about 50% of the total chemical composition of microalgae. Organic carbon present in wastewater is the major source of carbon and is responsible for the microalgae growth (Yang et al. 2016). Several studies were reported in literature which demonstrates that wastewaters are the suitable medium for producing biomass in high amounts (Gupta et al. 2016). Reyimu and Ozçimen, (2017) reported that T. suecia showed highest cell density of 4 × 105 cell/ml on tenth day of cultivation in 50% concentration of municipal wastewater. Sydney et al. (2011) reported a maximum biomass production of 1.88 g L−1 for B. braunii microalgae in secondary effluent of domestic sewage among 20 microalgae strains. No work has been reported in open literature on marine T. india microalgae for phycoremediation of secondary-treated integrated pulp and paper mill effluent and primary municipal-treated wastewater for sustainable biofuel production. Present study focus on the identification of microalgal strains with higher biomass production potential; among four different microalgal strains used in this work for sustainable biodiesel production and simultaneous phycoremediation of wastewaters.
Materials and methods
Microorganism media
Three (S. abundans, Spirulina sp., and N. muscorum) freshwater microalgae and one (T. indica) marine microalga were used in this present work. T. indica, a marine microalga, was purchased from National Facility for Marine Cyanobacteria Centre (NFMC), Bharathidasan University Tamil Nadu, India and was maintained in ASN III medium (25 g, NaCl; 3.5 g, MgSO4·7H2O; 2.0 g, MgCl2·6H2O; 0.75 g, NaNO3; 0.75 g, K2HPO4·3H2O; 0.5 g, CaCl2·2H2O; 0.5 g, KCl; 0.02 g, NaCO3; 3.0 mg, Citric acid; 3.0 mg, Ferric ammonium citrate; 0.5 mg, Mg EDTA; 10 μg, Vitamin B12; 1 ml, A-5 trace minerals (2.86 g, H3BO3; 1.81 g, MnCl2·4H2O; 0.222 g, ZnSO4·7H2O; 0.39 g, Na2MoO4·2H2O; 0.079 g, CuSO4·5H2O; 49.0 mg, Co(NO3)2·6H2O; dilute to 1 L distilled water). N. muscorum, Spirulina sp., and S. abundans were purchased from National Chemical Laboratory (NCL) Pune, India. N. muscorum was maintained in BG11 medium (0.075 g, MgSO4·7H2O; 0.04 g, K2HPO4; 1.5 g, NaNO3; 0.036 g, CaCl2·2H2O; 0.006 g, Citric acid; 0.006 g, Ferric ammonium citrate; 0.001 g, EDTA (disodium salt); 0.02 g, Na2CO3; and 1 ml trace metal mix A5 (49.4 mg, Co(NO3)2·6H2O; 0.079 g, CuSO4·5H2O; 0.39 g, Na2MoO4·2H2O; 2.86 g, H3BO3; 1.81 g, MnCl2·4H2O; 0.222 g, ZnSO4·7H2O) diluted in 1 L distilled water). Spirulina sp. and S. abundans were maintained in BBM medium. BBM medium comprising of 25 g, NaNO3; 2.5 g, CaCl2.2H2O; 7.5 g, MgSO4.7H2O; 7.5 g, K2HPO4; 17.5 g, KH2PO4; 2.5 g, NaCl; 50 g, EDTA solution; 4.98 g, acidified iron solution; 11.4 g, H3BO3 and trace metals solution diluted in 1 L of distilled water. All the cultures were maintained in 250 ml of Erlenmeyer flasks and were incubated at 25 ± 2 °C with 94.5 μmol−2 s−1 light intensity.
Wastewater characterization
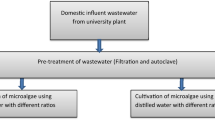
Four different wastewaters such as primary-treated sewage (PTS), secondary-treated sewage (STS) from domestic sewage treatment plant, IIT Roorkee Saharanpur Campus wastewater (CWW) and secondary treated pulp and paper mill wastewater (PWW) from an integrated pulp and paper mill were used for the cultivation of microalgae. All the four wastewater samples were stored at 4 °C. Before cultivation of microalgae in the wastewater, it was filtered using 0.45 μm pore size Whatman filter paper. Further, it was sterilized using autoclave at 121 °C for 15 min to remove the any microbial contamination.
Wastewater samples were characterized for ammonical nitrogen (NH3-N), nitrate nitrogen (NO3-N) and phosphate phosphorus (PO4-P), TOC, and TDS. Nitrate nitrogen (NO3-N) and phosphate phosphorus (PO4-P) were characterized using Ion exchange chromatography (850 professional IC, Metrohm). Inductively coupled plasma mass spectrometry (ICP-MS) (Teledyne Leeman Labs, Prodigy SPEC) was used for the heavy metals detection in the wastewater. Total organic carbon was detected by total organic carbon analyzer (CPH, SHIMAZADU). Ammonical nitrogen was determined as described by standard procedure (APHA, 1992). TDS, EC, and pH were estimated using Hanna digital meter (HI 3512, Hanna Instruments).
Experimental
All the experiments were performed in the 1000 ml of glass reagent bottles used as photobioreactors with working volume of 750 ml. Air pumps (pressure 0.02 Mpa, output 3.5 L/min) were used to provide ambient air as a source of carbon dioxide in the photobioreactors. An aliquot of 15 ml from each culture was used as inoculums for the cultivation of microalgae in the four different wastewaters. A dark and light cycle of 16:08 h with light intensity of 94.5 μmol m−2 s−1 was maintained using white LED. Temperature was maintained at 25 ± 2 °C for 14 days of cultivation period. All the experiments were performed in triplicates (Kim et al. 2016; Bhatnagar et al. 2011).
Growth pattern
Samples were collected from all the photobioreactors at an interval of every 24 h till all the four microalgal strains reached saturation level after 14 days of cultivation period. After centrifugation at 6200 rpm for 5 min samples were allowed to dry overnight in vacuum oven at 104 °C. The dry biomass was estimated gravimetrically. Biomass productivity (mg L−1 d−1) was calculated using the following equation.
Biomass productivity = (final dry biomass–initial dry biomass)/cultivation period.
The specific growth rate (μ) and doubling time (td) were calculated using equations 1 and 2 (Gani et al. 2016a).
Lipid extraction
The modified Bligh and Dyer method (Bligh and Dyer, 1959) was used for total lipid extraction from the dried microalgal biomass. 50 ml of algal broth culture was centrifuged for 5 min at 3000 rpm, and the supernatant was removed. The residue obtained after the centrifugation was washed two times with double distilled water and allowed to dry overnight in vacuum oven at 104 °C. The residual algal biomass was sonicated for 5 min at 20 kHz and after addition of 10 ml of chloroform: methanol (2:1 v/v) it was allowed to stir for 30 min. Extract obtained was filtered using sintered glass funnel containing 5 ml of 0.034% MgCl2 and then centrifuged at 3000 rpm for 5 min. The aqueous upper layer was allowed to aspirate and the residual organic phase was washed with 1 ml of 2 N KCl: methanol (4:1 v/v) and later on addition of 5 ml of artificial upper phase (chloroform:methanol:water; 3:48:47,v/v/v) until the phase boundary becomes clear. For internal standard nonadecanoic acid (0.1 μg/ml) was used. The chloroform layer at bottom was transferred to test tube, and the lipid yield was determined gravimetrically. Fatty acid methyl ester was analyzed with GC-MS (Agilent, Santa Clara, CA, USA) using DB-5MS capillary column (30 m × 0.25 m × 0.25 μm). Sample injection was done by splitless injection mode (1 μL at 250 °C) using helium as a carrier gas.
Biodiesel properties
Biodiesel quality properties such as iodine value (IV), oxidative stability (OS), long-chain saturation factor (LCSF), cetane number (CN), high heating value (HHV) saponification value (SV), and density were determined based on FAME profile obtained from the GC-MS analysis for each microalgal strain. Following empirical equations were used to calculate these values (Arora et al. 2016).
Where DB = no of double bond, M = molecular mass of each fatty acid, and FC = % of each fatty acid component.
Results and discussion
Growth of microalgae
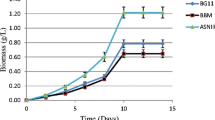
The growth profile of the microalgae depends upon the nutrients present in the cultivation media and culture conditions. In this study, algal growth was determined in different wastewaters with varying nutrients concentration, under identical culture conditions. (Table 1). A relevant criteria of the selection microalgal species is high specific growth rate and less doubling time. Among all four strains marine microalga T. indica showed the significant growth in all the cultivation media (Fig. 1). In case of T. indica, the cell growth started from the first day of cultivation in STS with a lag phase of 4 days and followed by an exponential growth phase persisted up to seventh day. Stationary phase was attained after the eleventh day of the cultivation period (Fig. 1d). T. indica showed highest biomass yield of 0.65 g L−1 in STS followed by 0.37, 0.35, and 0.32 g L−1 in PTS, CWW, and PWW, respectively. Previously, T. indica show highest biomass yield 0.88 g L−1 in secondary-treated domestic sewage at 135 μmol m−2 s−1 light intensity (Amit et al. 2017). T. indica has achieved a lag phase of 4 days before entering into exponential growth phase, when cultivated in PWW. In addition, T. indica showed highest biomass yield of 0.32 g L−1 in PWW among the all four algal strains. In case of CWW, T.indica started growing from the first day of cultivation with a lag phase of 3 days and followed by the exponential growth phase thus the 0.0261 g L−1 d−1 maximum biomass productivity had been achieved. Michels et al. (2014) reported a maximum of 0.5 g L−1 biomass concentration with T. suecia in fish farm wastewater. Figure 1 shows that maximum biomass yield for all the strains was obtained in STS. Thus, among all the four growth media, STS was found the most suitable for the growth of all four microalgal strains (Table 2). Comparison of biomass yield for all the four strains in STS is presented in Fig. 2. PTS found to be less effective than the STS, it is due to the presence of unwanted toxic compounds hindering the metabolic activities of microalgal cells resulting in low growth of microalgal biomass. Among all the four microalgal strains, T. indica achieved maximum biomass productivity of 0.0566 g L−1 d−1 Table 2. Reyimu and Ozçimen (2017) reported 0.5430 and 0.4778 d−1 maximum specific growth rate for N. oculata and T. suecica, when cultivated in 75 and 25% wastewater, respectively. Among the freshwater microalgae, S. abundans showed the maximum biomass yield of 0.55 g L−1 in STS, on twelfth day of cultivation period (Table 2). As shown in Fig. 1b. S. abundans has a lag phase of 4 days before entering into the exponential phase. Stationary phase was attained on the twelfth day of cultivation period. Sharma et al. (2014) reported that maximum dry biomass obtained was 0.79 g L−1 with S. abundans, when cultivated in municipal wastewater. The lowest biomass growth for all the four microalgal strains was observed in PWW media as shown in Fig. 1. It may be due to the presence of mercury and chlorophenolic compounds, which are toxic in nature and prevent microalgal growth. As compared to the STS medium, lower growth was found in PTS. This may be due to higher concentration of macro nutrients present in PTS medium.
The specific growth rate of T. indica is consistently higher than all other three microalgae strains in all the four growth media with short of doubling time Table 2. Sirin and Sillanpää (2015) reported specific growth rate of 0.19 d−1 at 25 °C for growth of T. suecica on secondary domestic-treated sewage under constant photon flux density. T. indica showed highest specific growth rate of 0.30 d−1 in STS followed by 0.244, 0.296, and 0.315 d−1 in PTS, CWW, and PWW, respectively.
Reduction in pollution load
For its growth, microalgae consume nutrients which helps in phycoremediation of pollution load in wastewater. Thus, this process well supports the purpose of coupling microalgal remediation of wastewater and biodiesel production.
All the four microalgal strains showed good removal of the nutrients from the wastewaters. Among the four-strains used in this study, T. indica was found the most suitable for the removal of nutrients from the wastewaters. The maximum TDS removal of 97.2, 96.2, 95.47, and 93.1%, was achieved in STS, when treated with T. indica, S. abundans, Spirulina sp., and N. muscorum, respectively. Electrical conductivity of PTS was reduced from 11.95.33 ± 1.52 to 0.45 ± 2.08, 0.37 ± 2.51, 0.35 ± 4.72, and 0.23 ± 4.58 with N. muscorum, Spirulina sp., S. abundans, and T.indica, respectively. Nitrogen is an important factor which is responsible for regulating lipid content ranging from 1 to 10% (Abou-Shanab et al. 2014). T. indica removed about 63.6–78.24% of nitrate and 60.90–65.97% of phosphate in all the four wastewaters. However, maximum removal of nutrients (nitrate, phosphate, and ammonium) was found in STS with T. indica. Sirakov and Velichkova, (2014) reported maximum reduction of 78% nitrate and 79% phosphate in aquaculture wastewater with T. chuii. However, for T. indica, highest removal of nitrate of 78.24% occurred in case of CWW having lowest concentration of nitrate. The highest removal of ammonical nitrogen was found to be 80.01, 78.81, 73.42, and 72.12% with T. indica, N. muscorum, Spirulina sp., and S. abundans, respectively in STS. The maximum reduction in TOC was found to be 85.70, 74.12, 77.38, and 74.62% in STS with T. indica, N. muscorum, Spirulina sp., and S. abundans, respectively. Michels et al. (2014) reported that removal efficiency of N and P was 49.4 and 99.0%, respectively, with T. suecica when cultivated in fish farm wastewater. The maximum removal of 61.79% nitrate in domestic sewage with marine microalga Chlorella marina (Kumar et al. 2015). S. abundans removed 63.39–70.54% of nitrate and 54.92–60.63% of phosphate. Significant reduction of nitrate (80%) has been reported, when 10% of inoculums were used in the treatment of sewage with S. abundans (Lekshmi et al. 2015). It can be seen from the Fig. 3 that the S. abundans is very effective in STS for the removal of TOC and ammonical nitrogen. Spirulina sp. removed about 62.61–64.63% of nitrate and 52.74–59.01% of phosphate. Nostoc muscorum removed 52.73–63.91% of phosphate and 56.15–67.9% of nitrate. From Fig. 3, it can be seen that T. indica is the most effective species over the other three strains in order to achieve higher biomass and simultaneous remediation of the wastewaters used in this study.
Reduction in pollutant in all four wastewaters. a Reduction in pollutant in all four wastewaters using Spirulina sp. b Reduction in pollutant in all four wastewaters using N. muscorum. c Reduction in pollutant in all four wastewaters using T. indica. d Reduction in pollutant in all four wastewaters using S. abundans
FAME analysis
The fatty acid composition of harvested algal biomass was estimated by GC-MS. C16-C18 are the most common fatty acids which are suitable for biodiesel production (Knothe 2009). The maximum lipid productivity of 25.44, 8.63, 7.77, and 7.69 mg L−1 d−1 was T. indica, S. abundans, N. muscorum, and Spirulina sp., respectively, in STS (Table 2). Previous study indicates T. suecica achieved the lipid productivity of 27 mg L−1 d−1 in artificial seawater (Montero et al. 2011). The FAME profile for all four microalgae in all four wastewater as shown in Fig. 4. The quality of biodiesel depends on the content of oleic and palmitic acids. Liu et al. (2011) reported that high oleic acid content in algal oil provides reasonable balance of fuel properties including combustion heat, oxidative stability, lubricity, viscosity, cold filter plugging point (CFPP), and ignition quality, while palmitic acid imparts a higher oxidative stability and cetane number as well as lower NOX emissions (Yang et al. 2016). All the physical properties were within the permissible limits of ASTM D6751 fuel standards Table 3; therefore, marine microalga T.indica derived biodiesel can be used in internal combustion diesel engines.
Conclusions
This study clarified that among the four microalgal strains, T. indica is the most suitable for integrated biodiesel production and phycoremediation of wastewater. The highest biomass productivity of 0.05663 g L−1 d−1 was achieved in STS with T. indica, and it was also found very effective in PWW. FAME profile of T. indica shows the presence of myristic acid (1.2%) pentadecylic acid (0.28%), palmitic acid (10.32%), oleic acid (34.59%), linoleic acid (12.38%), and eicosanoic acid (14.88%). Physical properties of biodiesel produced with T. indica in STS media showed the suitability of the derived biodiesel in diesel engines. Thus, production of biomass from wastewater using T. indica could reduce the use of fresh water and expensive nutrients for biodiesel production.
References
Abou-Shanab RAI, Ji MK, Kim HC, Paeng KJ, Jeon BH (2013) Microalgal species growing on piggery wastewater as a valuable candidate for nutrient removal and biodiesel production. J Environ Manag 115:257–264. https://doi.org/10.1016/j.jenvman.2012.11.022
Abou-Shanab RAI, El-Dalatony MM, El-Sheekh MM et al (2014) Cultivation of a new microalga, Micractinium reisseri, in municipal wastewater for nutrient removal, biomass, lipid, and fatty acid production. Biotechnol Bioprocess Eng 19:510518. https://doi.org/10.1007/s12257-013-0485-z
Amit, Chandra R, Ghosh UK, Nayak JK (2017) Phycoremediation potential of marine microalga Tetraselmis indica on secondary treated domestic sewage for nutrient removal and biodiesel production. Environ Sci Pollut Res 24:20868–20875. https://doi.org/10.1007/s11356-017-9734-6
APHA (1992) Standard methods for the examination of water and wastewater. Water Environ Fed 18th:9–45
Araujo GS, Matos LJBL, Gonçalves LRB, Fernandes FAN, Farias WRL (2011) Bioresource technology bioprospecting for oil producing microalgal strains : evaluation of oil and biomass production for ten microalgal strains. Bioresour Technol 102:5248–5250. https://doi.org/10.1016/j.biortech.2011.01.089
Arora N, Patel A, Sartaj K, Pruthi PA, Pruthi V (2016) Bioremediation of domestic and industrial wastewaters integrated with enhanced biodiesel production using novel oleaginous microalgae. Environ Sci Pollut Res 23:1–11. https://doi.org/10.1007/s11356-016-7320-y
Barnwal BK, Sharma MP (2005) Prospects of biodiesel production from vegetable oils in India. Renew Sustain Energy Rev 9:363–378. https://doi.org/10.1016/j.rser.2004.05.007
Bhatnagar A, Chinnasamy S, Singh M, Das KC (2011) Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl Energy 88:3425–3431. https://doi.org/10.1016/j.apenergy.2010.12.064
E. G. Bligh and W. J. Dyer ARMOTLEAP (1959) Canadian Journal of Biochemistry and Physiology. 37:
Chakraborty S, Mohanty D, Ghosh S, Das D (2016) Improvement of lipid content of Chlorella minutissima MCC 5 for biodiesel production. J Biosci Bioeng 122:294–300. https://doi.org/10.1016/j.jbiosc.2016.01.015
Chen M, Tang H, Ma H, Holland TC, Ng KYS, Salley SO (2011) Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour Technol 102:1649–1655. https://doi.org/10.1016/j.biortech.2010.09.062
Gani P, Sunar NM, Matias-Peralta H et al (2016a) Influence of initial cell concentrations on the growth rate and biomass productivity of microalgae in domestic wastewater. Appl Ecol Environ Res 14:399–409. https://doi.org/10.15666/aeer/1402_399409
Gani P, Sunar NM, Matias-Peralta HM, Latiff AAA, Parjo UK, Embong Z, Khalid A, Tajudin SAA (2016b) The potential of biodiesel production from Botryococcus sp. biomass after phycoremediation of domestic and industrial wastewater. IOP Conf Ser Mater Sci Eng 160:0–9. https://doi.org/10.1088/1757-899X/160/1/012048
Gani P, Sunar NM, Matias-Peralta H, Mohamed RMSR, Latiff AAA, Parjo UK (2017) Extraction of hydrocarbons from freshwater green microalgae (sp.) biomass after phycoremediation of domestic wastewater. Int J Phytorem 19(7):679–685
Gupta PL, Choi HJ, Pawar RR, Jung SP, Lee SM (2016) Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J Environ Manag 184:585–595. https://doi.org/10.1016/j.jenvman.2016.10.018
Kim G, Bae J, Lee K (2016) Nitrate repletion strategy for enhancing lipid production from marine microalga Tetraselmis sp. Bioresour Technol 205:274–279. https://doi.org/10.1016/j.biortech.2016.01.045
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition †. 759–766. doi: https://doi.org/10.1039/b903941d
Kumar D, Santhanam P, Jayalakshmi T, et al (2015) Excessive nutrients and heavy metals removal from diverse wastewaters using marine microalga Chlorella marina ( Butcher ) 44:
Ledda C, Idà A, Allemand D, Mariani P, Adani F (2015) Production of wild Chlorella sp. cultivated in digested and membrane-pretreated swine manure derived from a full-scale operation plant. Algal Res 12:68–73. https://doi.org/10.1016/j.algal.2015.08.010
Lekshmi B, Joseph RS, Jose A, Abinandan S, Shanthakumar S (2015) Studies on reduction of inorganic pollutants from wastewater by Chlorella pyrenoidosa and Scenedesmus abundans. Alexandria Eng J 54:1291–1296. https://doi.org/10.1016/j.aej.2015.09.013
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102:106–110. https://doi.org/10.1016/j.biortech.2010.06.017
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232. https://doi.org/10.1016/j.rser.2009.07.020
Medeiros DL, Sales EA, Kiperstok A (2015) Energy production from microalgae biomass: carbon footprint and energy balance. J Clean Prod 96:493–500. https://doi.org/10.1016/j.jclepro.2014.07.038
Michels MHA, Vaskoska M, Vermuë MH, Wijffels RH (2014) Growth of Tetraselmis suecica in a tubular photobioreactor on wastewater from a fish farm. Water Res 65:290–296. https://doi.org/10.1016/j.watres.2014.07.017
Montero MF, Manuela A, Guillermo GR (2011) Isolation of high-lipid content strains of the marine microalga Tetraselmis suecica for biodiesel production by flow cytometry and single-cell sorting. J Appl Phycol 23(6):1053–1057
Rai MP, Gupta S (2016) Effect of media composition and light supply on biomass, lipid content and FAME profile for quality biofuel production from Scenedesmus abundans. Energy Convers Manag 141:85–92. https://doi.org/10.1016/j.enconman.2016.05.018
Reyimu Z, Ozçimen D€ (2017) Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J Clean Prod 150:40–46. https://doi.org/10.1016/j.jclepro.2017.02.189
Sharma GK, Khan S, Ahmad F, Gupta N (2014) Nutrient sequestration and phycoremediation of sewage waste water by selective microalgae. Green Farming 5:1–4
Sirin S, Sillanpää M (2015) Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour Technol 191:79–87. https://doi.org/10.1016/j.biortech.2015.04.094
Sirakov IN, Velichkova KN (2014) Bioremediation of wastewater originate from aquaculture and biomass production from microalgae species-Nannochloropsis oculata and Tetraselmis chuii. Bulg. J. Agric. Sci 20:66–72
Sydney EB, da Silva TE, Tokarski A, Novak AC, de Carvalho JC, Woiciecohwski AL, Larroche C, Soccol CR (2011) Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl Energy 88:3291–3294. https://doi.org/10.1016/j.apenergy.2010.11.024
Wu LF, Chen PC, Lee CM (2013) The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. Int Biodeterior Biodegradation 85:506–510. https://doi.org/10.1016/j.ibiod.2013.05.016
Yang IS, Salama ES, Kim JO, Govindwar SP, Kurade MB, Lee M, Roh HS, Jeon BH (2016) Cultivation and harvesting of microalgae in photobioreactor for biodiesel production and simultaneous nutrient removal. Energy Convers Manag 117:54–62. https://doi.org/10.1016/j.enconman.2016.03.017
Zhu L, Wang Z, Shu Q, Takala J, Hiltunen E, Feng P, Yuan Z (2013) Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res 47:4294–4302. https://doi.org/10.1016/j.watres.2013.05.004
Acknowledgements
The authors are thankful to Ministry of Human Resource and Development, Government of India, for providing research grant for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• The higher biomass productivity was showed in STS by T. indica.
• Screening of most suitable microalgae in four different wastewaters for higher biomass production.
• T indica was found to be most promising species for nutrients removal and biodiesel production.
• T. indica biodiesel profile is within the specified ASTM D6751 standards.
Rights and permissions
About this article
Cite this article
Amit, Ghosh, U.K. An approach for phycoremediation of different wastewaters and biodiesel production using microalgae. Environ Sci Pollut Res 25, 18673–18681 (2018). https://doi.org/10.1007/s11356-018-1967-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1967-5