Abstract
The potential economic benefits of surfactants addition on enzymatic hydrolysis of steam-exploded lodgepole pine (SELP) and ethanol-pretreated lodgepole pine (EPLP) were investigated in this study. Free cellulase readsorption on fresh substrate was used to recover and recycle cellulase enzymes during the hydrolysis of SELP and EPLP substrate. Supplementing Tween 80 during the hydrolysis could facilitate enzyme recycling for EPLP substrate. A logarithmic correlation was established between surfactant concentration and free cellulase content after lignocellulosic hydrolysis, which was used to compute enzyme cost savings over various Tween 80 concentrations. A simple economic analysis of enzyme cost savings versus the cost of surfactant was undertaken. The results indicated that the addition of Tween 80 (priced at US $0.25/kg) during the hydrolysis of the EPLP substrate could save 60% of the total enzyme cost at concentrations in the 0.025% to 0.2% range. The addition of Tween for the hydrolysis of the SELP substrate significantly reduced the material cost by 24% per 1 gal of ethanol produced, and the ethanol production cost could be reduced by 8.6% with the addition of Tween and enzymes recycle for the hydrolysis of SELP substrate. A schematic concept of recycling enzyme and surfactant was also presented with a recirculation of process streams during hydrolysis. Further analysis indicated a 66% reduction in total enzyme cost could potentially be achieved under the concept.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest biomass provides one of the most promising renewable feedstocks for biofuels production [1]. However, the recalcitrant nature of cellulose, hemicellulose, and lignin in the forest biomass presents a substantial challenge to enzymatic hydrolysis [2–6]. A number of pretreatment processes, including organosolv, steam explosion, dilute acid, and ammonia fiber explosion, have been investigated to improve the accessibility of lignocellulosic biomass to cellulase enzymes and minimize the adverse effect of lignin during enzymatic hydrolysis [7–9]. Among them, steam explosion is recognized as a viable pretreatment method for hardwood and agricultural residues, with the benefits of lower cost and less water usage and energy consumption [4, 10]. Organosolv pretreatment, which is derived from Alcell pulping process developed initially as a chemical pulping method, has also been investigated for biomass bioconversion [7, 11]. Organosolv-pretreated substrate has a relatively low lignin content (between 6.4% and 27.4%) compared to steam-exploded substrate which is typically approximately 45.6% [12, 13]. However, the cost of the biomass fractionation and cellulase enzymes are still major bottlenecks in the potential commercialization of lignocellulose bioconversion to ethanol [14–16]. Therefore, enzyme cost reduction is very critical and should be addressed for biofuels development [17, 18].
Three strategies have been suggested for enzyme cost reduction, which includes increasing enzyme production efficiency, enhancing enzyme-specific activity, and recycling cellulase enzymes for successive hydrolysis [19, 20]. To improve cellulase productivity and cellulase-specific activity, Genencor International and Novozymes successfully achieved a 12–20-fold reduction of enzyme cost through protein engineering and process engineering [21]. They improved enzyme productivity by simplifying the downstream process and using a cheaper carbon source and a more effective inducer. Meanwhile, they enhanced the specific activity of cellulases by directed enzyme evolution [22]. Another potential approach to reduce the enzyme cost is enzyme recovery and recycling on the base of cellulase high stability and affinity for cellulose [23, 24].
Cellulase enzymes have been shown to remain remarkably active after lignocellulosic hydrolysis [20, 23, 25], which enables enzyme recycling feasible for enzyme cost reduction. Lu [26] previously reported free cellulase enzymes in the supernatant after hydrolysis kept 83% of their initial filter paper activity and indicated these enzymes are very active and recoverable. Similar results have reported about recovered enzyme-specific activity after hydrolysis by Xu and Chen [27]; they found recovered cellulase enzymes could maintain 96% of their initial specific activity. Even in the present of products inhibition, we have discovered earlier in our research that >70% of cellulase-specific activity could be maintained in the recovered enzyme fraction [20]. Furthermore, surfactant has been shown no effect on enzyme activity of cellulase [28], which facilitates the application of surfactant in the process of enzyme recycling during lignocellulosic hydrolysis [13, 29].
Previous research work has shown that the addition of non-ionic surfactants, such as Tween 80 or Tween 20, can significantly improve the hydrolysis yield of steam-exploded spruce and lodgepole pine [13, 28, 30]. Non-ionic surfactants have also been shown potentially to promote enzyme recycle during the enzymatic hydrolysis of lignocellulose [13, 28–37]. Following the hydrolysis process, the surfactant along with hydrolysate is carried forward to the subsequent fermentation stage in a separate hydrolysis and fermentation process (SHF). Surfactants have been applied also to the simultaneous saccharification and fermentation (SSF) process [30]. However, questions still remaining are how much of enzyme could be saved with the addition of surfactants and what are the potential economic benefits from the addition of surfactant during the hydrolysis of lignocellulosic substrates.
The objective of this study was to investigate further the potential economic benefits of surfactant on the enzymatic hydrolysis of softwood substrates obtained from steam explosion and organosolv pretreatments. A simple economic analysis of the enzymatic hydrolysis process was undertaken.
Materials and Methods
Pretreated Substrates
Steam-exploded lodgepole pine (Pinus contorts; SELP) was prepared in a 2-L steam explosion vessel (Stake Technology Ltd., Norval, ON, Canada) at the Forest Products Biotechnology Lab at the University of British Columbia (Vancouver, Canada). Briefly, 4 × 4 cm wood chip samples (50 g) were impregnated with 4.0% SO2 (w/w) overnight at room temperature. The samples were loaded into a preheated steam explosion vessel. The pretreatment was then carried out at 200°C for 5 min before releasing the pressure. After pretreatment, the substrates were collected and washed with tap water, and stored at 4°C for subsequent experiments. The chemical composition of Lodgepole pine are Cellulose 47.6%, hemicellulose 22.9%, lignin 26.3%, and extractives 4.7%.
Ethanol-pretreated lodgepole pine (EPLP) was prepared in a rotating digester with four vessels (Aurora Products Ltd., Savona, BC, Canada) as previously described [11]. Wood chip samples (200 g) were loaded into the rotating digester and were treated at 170°C with 1.1% H2SO4 (w/w) and 65% ethanol (v/v) for 60 min. The substrates were collected and washed with aqueous ethanol [11]. The chemical composition of SELP and EPLP is shown in Table 1.
An ethanol-pretreated mixed softwood (EPMS, 6.0% lignin) substrate was prepared by Lignol Innovations Corporation (Vancouver, Canada) from mixed softwood furnish (spruce, pine, and Douglas fir) as previously described [12]. The lignin content of these pulps was determined using the Klason procedure (TAPPI T-222).
Enzymes
Commercial cellulase preparation (Celluclast 1.5 L) and β-glucosidase preparation (Novozym 188) were from Novozymes (Franklinton, NC, USA). The filter paper activity of Celluclast 1.5 L was 71.7 FPU/mL; protein content, 129.8 mg/mL. The β-glucosidase preparation used was Novozym 188 (Novozymes, Franklinton, NC, USA), 350 IU/mL.
Chemical Analyses
The carbohydrate composition of pretreated substrates was quantified on a Dionex DX 2500 ion chromatography system (Dionex, Sunnyvale, CA, USA) by CarboPac PA-1 anion exchange column (Dionex) [38]. The lignin content was determined using the Klason technique (TAPPI Method T 249 cm-85).
Protein Assay
A protein assay utilizing ninhydrin was used [39], and the protein samples (0.1 mL) were pipetted into glass tubes and placed in an oven at 105 °C for 2 h. After the tubes were completely dry, 0.15 mL 13.5 N NaOH was added to each tube. The tubes were then autoclaved at 121 °C for 20 min. After cooling, the alkali was neutralized by adding 0.25 mL glacial acetic acid. A 0.5-mL amount of 2% solution Ninhydrin reagent (Sigma, St. Louis, MO, USA) was added to each tube and then heated for 20 min in a boiling water bath. After the reaction, the tubes were cooled in an ice water for 10 min, and 2.5 mL 50% ethanol was further added. The tubes were then shaken vigorously on a vortex. Finally, the solution was read at 570 nm on a UV–Vis spectrometer (Perkin-Elmer). Bovine serum albumin (BSA) was used as the protein standard [20].
Enzymatic Hydrolysis of Lignocellulosic Substrates
Unless otherwise stated, all enzymatic hydrolysis experiments were performed in 50 mL of 50 mM acetate buffer (pH 4.8) at a 2% consistency (based on cellulose). The hydrolysis reaction was incubated at 45 °C, with shaking at 150 rpm for 48 h. The cellulase loading was 20 FPU g−1 cellulose, and the β-glucosidase (Novozym 188) loading was 40 IU g−1 cellulose. Samples were removed from the reaction at different times and centrifuged to remove the insoluble materials. The reducing sugar content was measured by high performance liquid chromatography (HPLC) as described previously [11]. The hydrolysis yield of the substrate was calculated from the measured reducing sugar content, as a percentage of the theoretical reducing sugar available in each substrate. The protein content in the supernatant was measured using the ninhydrin assay with BSA as the protein standard (Bio-Rad, USA).
Cellulase Recycling during Lignocellulosic Hydrolysis with the Addition of Surfactants
Cellulase recycling process employed in this study followed the procedure that was described previously [13]. Surfactants were added at a concentration 0.2% w/v to the hydrolysis of the EPLP substrate or the SELP substrate with Celluclast cellulases preparations. The filtrate was recovered from the hydrolyzed samples by filtration using a glass microfiber membrane (Whatman GF/A). The filter cake was rinsed with an additional 10 mL of acetate (pH 4.8) buffer. The free cellulases were then readsorbed from the filtrate at 25 °C for 2.5 h using the same amount of fresh substrate that was employed in the initial hydrolysis. The free cellulases adsorbed onto the fresh substrate were recovered by filtration and resuspended in acetate buffer containing fresh β-glucosidase, (β-glucosidase did not adsorb onto the substrate). A second round of hydrolysis was performed subsequently using the recovered cellulases. Cellulase recycling was carried out for four additional rounds of hydrolysis. The protein content and reducing sugar content in the supernatant was determined by the ninhydrin assay and HPLC respectively, as discussed earlier.
Assumptions for Bioconversion of EPLP Substrate to Ethanol (SHF)
Previous work has shown that it is feasible to use Tween to facilitate the recycling of cellulases during the hydrolysis of an EPLP substrate [13]. In order to assess the value of enzyme recycling strategies, further analysis should be performed, including careful consideration of the costs associated with implementing this technology. This work presented a simple economic analysis of the hydrolysis process, with specific assumptions.
In order to quantify the possible cost benefits of Tween addition, a simple economic analysis of the benefits of using Tween and enzyme recycling on the enzymatic hydrolysis was undertaken. As reported previously, cellulases could be recycled for five consecutive rounds of hydrolysis of EPLP [13]. From these results, it can be inferred that it would be possible to reduce the cost of cellulases fivefold during the hydrolysis of EPLP. However, the impact of the cost of Tween 80 and other necessary process and equipment modifications required to carry out enzyme recycling must also be taken into account. Cellulase enzymes typically cost approximately US $0.40–0.50 per gallon of ethanol production [14], while the cost of Tween 80 was estimated to be over the range of US $0.25–1.00 per kilogram.
The desorption of cellulases from the hydrolysis residue showed a positive correlation with the concentration of Tween 80 in the hydrolysis of organosolv-pretreated softwood [40]. Increasing Tween concentration will therefore increase the concentration of free enzymes in the supernatant after lignocellulosic hydrolysis. In order to truly evaluate the economic impact of adding Tween to the hydrolysis step, it is necessary to carry out a complete economic evaluation of the bioconversion process, including any capital and operation costs that become necessary as a result of Tween addition. However, at this stage of the work, by making a few assumptions, a simplified economic analysis could be made to assess the potential enzyme cost savings that may result by adding Tween 80 to the hydrolysis.
In this initial analysis, it was assumed that the enzyme recycling process does not require additional capital and operational costs. Furthermore, the economic analysis focused mainly on the cellulases without considering β-glucosidase, since it has been shown that β-glucosidase could be effectively “recycled” by immobilization [41]. Based on our preliminary experiments and literature values [42], the cellulose conversion, ethanol stoichiometric yield, and fermentation efficiency are summarized in Table 2. Using this information, it was calculated that the production of 1 gal of ethanol requires 10.6 kg of EPLP substrate. Assuming the hydrolysis of EPLP substrate is performed at a concentration of 6% (w/v) and a Tween 80 concentration of 0.2% (w/v), theoretically, 353 g of fresh Tween 80 would be required for four consecutive enzyme-recycling rounds. Decreasing the concentration of Tween 80 in the hydrolysis system would result in a decrease in the number of effective enzyme recycling rounds (Table 3).
Results and Discussion
Effect of Surfactant on Free Cellulase Distribution
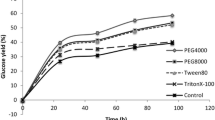
The effects of surfactant on free cellulase (excluding β-glucosidase) concentration after a 24-h hydrolysis of EPMS were evaluated under a series concentration of Tween 80 (Fig. 1). It was apparent that free cellulase in supernatant increased significantly with the addition of Tween 80 during hydrolysis of EPMS. Without the addition of Tween 80, free cellulase in the supernatant accounts for only 55% of initial cellulase after 24 h hydrolysis. With the addition of 0.2% (w/v) of Tween 80, 96.4% of the initial cellulases remained in the liquid phase (supernatant). However, free cellulase quickly reached its maximum when the concentration of Tween 80 increased to 0.5%. Previously, we have demonstrated that 85% of free cellulase could be recovered and recycled for further hydrolysis based on readsorption of free cellulase on fresh substrate [20]. The free cellulase readsorption process was mainly governed by Langmuir adsorption isotherm related to enzyme affinity to cellulose [13, 23]. The results of this experiment indicated that surfactant improved free cellulase by 75% after 24 h hydrolysis of EPMS. As a result, surfactant could considerably facilitate enzyme recycling and reduce enzyme cost during lignocellulosic hydrolysis. However, in order to build a relationship between the addition of Tween 80 and enzyme cost savings from cellulase recycling, a correlation between Tween 80 concentration and free cellulase from 24 h hydrolysis of EPMS should be established, which was used for a simple economic analysis of enzyme cost saving during the hydrolysis of EPLP in this study.
The correlation between Tween 80 concentration and free cellulase content is a logarithmic function (Fig. 1). Using non-linear curve fitting (R 2 = 0.99), a logarithmic function of Tween 80 concentration and the percentage of cellulase in the supernatant was established as following:
where y = percentage of cellulase (%), x = Tween 80 concentration (%), a = 104.86 ± 0.37, b = −5.39 ± 0.26, c = 0.00015 ± 0.00006.
Equation 1 was used to establish a relationship between the proportion of the initial cellulase in the supernatant and the concentration of Tween 80.
Effect of Surfactant on Enzyme Recycling During the Hydrolysis of EPLP and SELP
Steam explosion and organosolv pretreatment processes resulted in significantly different chemical composition of pretreated substrates (Table 1). High-lignin content of SELP substrate pretreated at medium severity showed lower hydrolysis yield (70–80%) without the addition of surfactant, when compared to the hydrolysis of EPLP substrate [28, 43]. Moreover, the high-lignin content of SELP substrate resulted in poor performance for enzyme recycling; it was shown that the addition 0.2% Tween 80 permitted the recycling of cellulases in five rounds of hydrolysis of EPLP substrate, while cellulase only could be recycled once during the hydrolysis of SELP substrate (Fig. 2). The results also revealed that hydrolysis yield of EPLP substrate remained high and dropped slowly and in a linear pattern over the five rounds of consecutive hydrolysis. However, the hydrolysis yield of SELP showed an exponential drop (Fig. 2). It indicated that free cellulase recovery from the hydrolysis of EPLP was mainly controlled by one factor, Langmuir adsorption isotherm, and the cellulase recovery from the hydrolysis of SELP probably was governed by two factors, non-productive binding from lignin and Langmuir adsorption isotherm. Although the addition of surfactant significantly reduced the non-productive binding between lignin and enzyme, 45% of lignin in the SELP substrate still contributed considerable amount of effect on non-productive binding even in the presence of surfactant. Previously, we have demonstrated that the addition of Tween 80 could only reduce about 50% of adsorbed cellulase on isolated cellulolytic enzyme lignin [40], but the adsorbed cellulase on lignin was not reduced correspondingly, even with the further increase of surfactant concentration. In the subsequent work, we elaborated the free cellulase content after each 24-h hydrolysis over various concentration of Tween 80 (0.2–0.005%; Table 3).
The values calculated using Eq. 1 were shown in Table 3. Previously, free cellulase recovery by readsorption on fresh substrates was 85% from the Langmuir model analysis [20]. In the present study, with the addition of 0.2% Tween 80, 96.4% of the free cellulases remain in the supernatant after the first round hydrolysis, of which 81.9% (96.4 × 85%) could be recovered for use in a second round of hydrolysis (Table 3). The third round of free cellulase content was 79% (81.9% × 96.4%).
Effect of Surfactant on Enzyme Cost Savings During Hydrolysis of EPLP
Based on the results shown in Table 3, Tween 80 at a 0.2% (w/v) resulted in five rounds of hydrolysis, which suggested that the possible threshold amount of free cellulases necessary to carry out an additional successful round of hydrolysis could be set at greater than or equal to 53% (Table 3, entry 5). According to this criterion, the addition of 0.15% or 0.10% Tween 80 enables four successful recycling rounds, while 0.05% or 0.025% Tween 80 would attain three successful recycling rounds. Using this information, the cost of cellulase enzymes in the entire process can be estimated from dividing the cost of cellulase enzymes (US $0.50/gal ethanol) by the number of possible hydrolysis rounds. Considering the total costs of enzyme and Tween 80, the potential cost savings as a result of enzyme recycling using a range of Tween concentrations is shown in Table 4. Without the addition of Tween 80, the total initial cost of enzymes was set to US $0.50 per gallon ethanol. Increasing the Tween 80 concentration from 0.005% to 0.20% allows the number of effective hydrolysis rounds to be increased from two to five. As a result, the cost of enzymes could potentially be decreased further from $0.25 to $0.10. However, the cost of Tween will also increase from $0.01 to $0.35. Therefore, the highest enzyme cost savings that can be realized was 58% using 0.025% Tween 80 under the price of $1.0 per kilogram.
Alkasrawi et al. [30] performed a similar economic evaluation of the effect of Tween 20 on the production cost of ethanol in the simultaneous saccharification and fermentation process (SSF). In their evaluation, it was suggested that the maximum allowable price for Tween 20 could be US $0.429/kg for one enzyme recycling round in SSF. Since a precise industrial price for Tween 80 is unavailable, we have assumed the price of Tween 80 is in the range of $0.25–1.00/kg. Following the same approach as above, the enzyme cost savings were determined using Tween prices of $0.25/kg and $0.50/kg (Fig. 3).
With the addition of Tween in enzyme recycling, 60% of the total enzyme cost (including cost of Tween $0.25/kg) could be saved at concentrations in the 0.025% to 0.2% range. However, if Tween 80 costs $1.00/kg, the potential cost savings decreases from 58% to 10% as the Tween concentration is raised from 0.025% to 0.2% (Fig. 3).
Effect of Surfactant on Enzyme Cost Savings During Hydrolysis of SELP
As for the potential enzyme cost savings for SELP substrate with the addition of surfactant, we made the same assumptions about cellulase enzymes cost. Cellulase enzymes cost approximately US $0.50 per gallon of ethanol production, while the cost of Tween 80 was estimated to be around US $0.50 per kilogram [30]. As reported earlier, cellulases could be recycled for two consecutive rounds of hydrolysis of SELP substrate. From these results, it can be inferred that it would be possible to reduce the cost of cellulases by 50% during the hydrolysis of SELP substrate.
In this initial analysis, it was assumed that the enzyme recycling process does not require additional capital and operational costs. Furthermore, the economic analysis focused mainly on the cellulases without considering β-glucosidase. Based on our experiments and literature values [42], the cellulose conversion (69% without Tween and 91% with Tween), ethanol stoichiometric yield (0.51), and fermentation efficiency (0.75) are assumed in Table 2. Using this information, it was calculated that the production of 1 gal of ethanol requires 17.6 kg of pretreated SELP substrate. Assuming the hydrolysis of SELP is performed at a concentration of 6% (w/v) and a Tween 80 concentration of 0.2% (w/v), theoretically, 588 g of fresh Tween 80 would be required for producing 1 gal of ethanol. Lignocellulosic ethanol production cost was $3.14 from the initial process. With the addition of Tween 80, the cost did not change much ($2.12). However, with the further enzymes recycling, the ethanol production cost could go down to $2.87 per gallon. Apparently, the estimate based on the increase in product yield justifies the cost of the addition of Tween, because the increase of hydrolysis yield significantly reduced the material cost by 24% for producing 1 gal of ethanol (Tables 5 and 6). Approximately 50% of enzyme could be recycled, and the ethanol production cost could be reduced by 8.6% with the addition of Tween and enzymes recycle. However, it is recognized that the benefits of Tween 80 will be heavily dependent on the price of Tween and cellulase enzymes. In the long term, the enzyme cost could be reduced considerably, and the benefits of surfactants have to be reconsidered in the bioconversion process.
Concept of Enzyme Recycling and Recirculation of Process Streams
From the economic analysis, it is apparent that although the addition of Tween 80 will result in savings in enzyme costs, Tween itself imposes an additional cost on the process. However, it may be possible to recycle both cellulase enzymes and Tween. A process was envisioned where the ethanol production cost could be reduced significantly by recirculating either the SSF product stream or the post-distillation stream in order to re-use buffer or water [44]. It is likely that Tween 80 could be recycled as part of the product stream after distillation. Alkasrawi et al. [44] compared ethanol yields and productivity of SSF with recirculation of the product stream after distillation to the same process without recirculation. Using recirculation to recycle Tween, the ethanol production cost was reduced by 17% [44]. Therefore, it is possible that the inclusion of surfactant recycling may partially offset the cost of Tween addition.
In contrast to the study of Alkasrawi, the enzyme recycling protocol proposed here was based on an SHF. As shown in the schematic of Fig. 4, our recycling protocol consisted of a separate hydrolysis followed by readsorption of cellulases onto fresh substrates for enzyme recovery. The hydrolysis products were subsequently fermented to ethanol. Tween 80 could be recovered in the distillation step for subsequent re-use in a similar fashion as that shown in Fig. 4.
The potential to recycle both cellulases and Tween 80 enables a further reduction in Tween cost, assuming the process does not require additional capital and operation costs. If Tween 80 could be recycled for five rounds by recirculation during the bioconversion process, the cost of Tween would be $0.07 to produce 1 gal of ethanol from the EPLP substrate (Table 5). Performing another cost analysis with integration of Tween 80 recycling, a 66% reduction in total enzyme cost could potentially be achieved under the assumed conditions.
The results of the aforementioned economic evaluation for enzyme recycling with Tween 80 suggest that the addition of Tween could reduce the total enzyme costs for the overall process. Evidently, the cost of Tween is an important factor governing the maximum achievable enzyme cost savings obtained during enzyme recycling. The possibility of recycling Tween 80 using a recirculation step improves the prospect for practical application of the enzyme recycling strategies developed in this work. Furthermore, the costs of immobilization of β-glucosidases will also need to be considered in the overall recycling scheme. Once the specific capital and operational costs of enzyme recycling are established, a thorough economic analysis must be performed considering the impact of enzyme recycling on the entire bioconversion process.
Conclusions
The recycling of cellulase enzymes was accomplished on ethanol-pretreated EPLP substrate with a 14.5% Klason lignin content with the addition of surfactants and subsequent readsorption of desorbed cellulases onto fresh substrates. Comparisons of various surfactants revealed that Tween 80 was the most effective detergent used in enzyme recycling. The efficient recycling of cellulases from Trichoderma was shown to be due to their high affinity for the EPLP substrate which facilitated subsequent readsorption to fresh substrate. The inability to effectively recycle cellulases during the hydrolysis of high-lignin content SELP substrate indicated the detrimental effect which lignin had on the proposed cellulase recycling strategies. The results from this study indicate that cellulase recycling is feasible for organosolv-pretreated lignocellulosics with the addition of surfactant, as cellulases could be recycled and used for a total of five hydrolysis rounds. Based on a simple economic analysis, the addition of Tween 80 could save 60% of the total enzyme cost at concentrations in the 0.025% to 0.2% range (assumed price of Tween 80 is US $0.25/kg) assuming there are no additional capital or operation costs. However, it is recognized that the benefits of Tween 80 will be heavily dependent on the price of Tween and cellulase enzymes.
References
Kurabi, A., Berlin, A., Gilkes, N., Kilburn, D., Bura, R., Robinson, J., et al. (2005). Applied Biochemistry and Biotechnology, 121, 219–230.
Robinson, J., Keating, J. D., Boussaid, A., Mansfield, S. D., & Saddler, J. N. (2002). Applied Microbiology and Biotechnology, 59, 443–448.
Robinson, J., Keating, J. D., Mansfield, S. D., & Saddler, J. N. (2003). Enzyme and Microbial Technology, 33, 757–765.
Duff, S. J. B., & Murray, W. D. (1996). Bioresource Technology, 55, 1–33.
Galbe, M., & Zacchi, G. (2002). Applied Microbiology and Biotechnology, 59, 618–628.
Wyman, C. E. (1999). Annual Review of Energy and the Environment, 24, 189–226.
Chum, H. L., Johnson, D. K., Black, S., Baker, J., Grohmann, K., Sarkanen, K. V., et al. (1988). Biotechnology and Bioengineering, 31, 643–649.
Saha, B. C., Iten, L. B., Cotta, M. A., & Wu, Y. V. (2005). Process Biochemistry, 40, 3693–3700.
Alizadeh, H., Teymouri, F., Gilbert, T. I., & Dale, B. E. (2005). Applied Biochemistry and Biotechnology, 121, 1133–1141.
Ballesteros, M., Oliva, J. M., Negro, M. J., Manzanares, P., & Ballesteros, I. (2004). Process Biochemistry, 39, 1843–1848.
Pan, X. J., Gilkes, N., Kadla, J., Pye, K., Saka, S., Gregg, D., et al. (2006). Biotechnology and Bioengineering, 94, 851–861.
Pan, X. J., Arato, C., Gilkes, N., Gregg, D., Mabee, W., Pye, K., et al. (2005). Biotechnology and Bioengineering, 90, 473–481.
Tu, M. B., Chandra, R. P., & Saddler, J. N. (2007). Biotechnology Progress, 23, 1130–1137.
Steele, E., Raj, S., Nghiem, J., & Stowers, M. (2005). Applied Biochemistry and Biotechnology, 121, 901–910.
Gregg, D. J., Boussaid, A., & Saddler, J. N. (1998). Bioresource Technology, 63, 7–12.
Lynd, L. R., Weimer, P. J., van Zyl, W. H., & Pretorius, I. S. (2002). Microbiology and Molecular Biology Reviews, 66, 506–577.
Hayward, T. K., Hamilton, J., Tholudar, A., & McMillan, J. D. (2000). Applied Biochemistry and Biotechnology, 84–6, 859–874.
Zhang, Y. H. P., Himmel, M. E., & Mielenz, J. R. (2006). Biotechnology Advances, 24, 452–481.
Merino, S. T., & Cherry, J. (2007). Advances in Biochemical Engineering/Biotechnology, 108, 95–120.
Tu, M. B., Chandra, R. P., & Saddler, J. N. (2007). Biotechnology Progress, 23, 398–406.
Jensen, J. K. (2003). Palo Alto: Genencor International, Inc.
Cherry, J. R., & Fidantsef, A. L. (2003). Current Opinion in Biotechnology, 14, 438–443.
Castanon, M., & Wilke, C. R. (1980). Biotechnology and Bioengineering, 22, 1037–1053.
Reese, E. T., & Mandels, M. (1980). Biotechnology and Bioengineering, 22, 323–335.
Knutsen, J. S., & Davis, R. H. (2002). Applied Biochemistry and Biotechnology, 98, 1161–1172.
Lu, Y. P., Yang, B., Gregg, D., Saddler, J. N., & Mansfield, S. D. (2002). Applied Biochemistry and Biotechnology, 98, 641–654.
Xu, J., & Chen, H. Z. (2007). Applied Biochemistry and Biotechnology, 143, 93–100.
Eriksson, T., Borjesson, J., & Tjerneld, F. (2002). Enzyme and Microbial Technology, 31, 353–364.
Castanon, M., & Wilke, C. R. (1981). Biotechnology and Bioengineering, 23, 1365–1372.
Alkasrawi, M., Eriksson, T., Borjesson, J., Wingren, A., Galbe, M., Tjerneld, F., et al. (2003). Enzyme and Microbial Technology, 33, 71–78.
Duff, S. J. B., Moritz, J. W., & Casavant, T. E. (1995). Biotechnology and Bioengineering, 45, 239–244.
Helle, S. S., Duff, S. J. B., & Cooper, D. G. (1993). Biotechnology and Bioengineering, 42, 611–617.
Kaar, W. E., & Holtzapple, M. T. (1998). Biotechnology and Bioengineering, 59, 419–427.
Kim, S. B., & Chun, J. W. (2004). Applied Biochemistry and Biotechnology, 113–116, 1023–1031.
Ooshima, H., Sakata, M., & Harano, Y. (1986). Biotechnology and Bioengineering, 28, 1727–1734.
Park, J. W., Takahata, Y., Kajiuchi, T., & Akehata, T. (1992). Biotechnology and Bioengineering, 39, 117–120.
Yang, B., & Wyman, C. E. (2006). Biotechnology and Bioengineering, 94, 611–617.
Keating, J. D., Panganiban, C., & Mansfield, S. D. (2006). Biotechnology and Bioengineering, 93, 1196–1206.
Starcher, B. (2001). Analytical Biochemistry, 292, 125–129.
Tu, M. (2007). In The Faculty of Graduate Studies (p. 195). Vancouver: University of British Columbia.
Tu, M. B., Zhang, X., Kurabi, A., Gilkes, N., Mabee, W., & Saddler, J. (2006). Biotechnological Letters, 28, 151–156.
Demirbas, A. (2005). Energy Sources, 27, 327–337.
Ewanick, S. M., Bura, R., & Saddler, J. N. (2007). Biotechnology and Bioengineering, 98, 737–746.
Alkasrawi, M., Galbe, M., & Zacchi, G. (2002). Applied Biochemistry and Biotechnology, 98, 849–861.
Wingren, A., Galbe, M., Roslander, C., Rudolf, A., & Zacchi, G. (2005). Applied Biochemistry and Biotechnology, 121, 485–499.
Acknowledgements
We would like to thank Novozymes North America Inc. for providing the enzymes. The funding was provided by Natural Resources Canada and the National Science and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tu, M., Saddler, J.N. Potential Enzyme Cost Reduction with the Addition of Surfactant during the Hydrolysis of Pretreated Softwood. Appl Biochem Biotechnol 161, 274–287 (2010). https://doi.org/10.1007/s12010-009-8869-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8869-4