Abstract
Objective
The aim of this meta-analysis was to compare the diagnostic value of noninvasive imaging methods computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) in the detection of ovarian cancer (OC).
Methods
PubMed, Embase, and Ovid were comprehensively searched from the date of inception to 31st, March, 2022. Pooled sensitivity, specificity, positive likelihood ratio (+ LR), negative likelihood ratio (- LR), diagnostic odds ratio (DOR), and area under the curve (AUC) of summary receiver operating characteristic (SROC) with their respective 95% confidence intervals (CIs) were calculated.
Results
Sixty-one articles including 4284 patients met the inclusion criteria of this study. Pooled estimates of sensitivity, specificity, and AUC of SROC with respective 95% CIs of CT on patient level were 0.83 (0.73, 0.90), 0.69 (0.54, 0.81), and 0.84 (0.80, 0.87). The overall sensitivity, specificity, SROC value with respective 95% CIs of MRI were 0.95 (0.91, 0.97),0.81 (0.76, 0.85), and 0.90 (0.87, 0.92) on patient level. Pooled estimates of sensitivity, specificity, SROC value of PET/CT on patient level were 0.92 (0.88, 0.94), 0.88 (0.83, 0.92), and 0.96 (0.94, 0.97).
Conclusion
Noninvasive imaging modalities including CT, MRI, PET (PET/CT, PET/MRI) yielded favorable diagnostic performance in the detection of OC. Hybrid implement of different tools (PET/MRI) is more accurate for identifying metastatic OC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is the seventh most common cancer worldwide in the female with the highest mortality rate among gynecologic malignant tumors affecting the female reproductive system [1, 2]. According to available statistics, more than 180 thousand women die of ovarian cancer every year worldwide [3]. Histogenetically, OC is classified into three major subtypes, including epithelial, stromal, or germ cell tumors [4]. Approximately, 90% of ovarian cancers have been found to be epithelial ovarian cancer (EOC) subtypes [5]. Surgical resection and chemotherapy are the standard treatment options [6]. The International Federation of Gynecology and Obstetrics (FIGO) or Union for International Cancer Control (UICC) TNM classifications are referred to as the staging standard of ovarian cancer [7, 8]. Nevertheless, due to the vague and nonspecific symptoms and alike gastrointestinal, genitourinary, and gynecological findings, OCs are difficult to diagnose at early stage, and are often metastatic at the time of presentation, and are involved in a high likelihood of recurrence and poor prognosis [4, 9]. With regard to this, accurate preoperative evaluation, namely the differentiation of benign or malignant diseases, or the detection of nodal, peritoneal or distant diseases, is indispensable to achieve an optimized treatment schedule [10].
A biopsy of tumor tissue from surgery or imaged-guided needle aspiration, to date, is the reference standard to confirm the disease [11]. Nevertheless, this procedure is invasive and carries potential risks of unwanted and unpredictable complications [12, 13]. Transvaginal ultrasonography (TVUS) or abdominal contrast-enhanced computed tomography (CT) is the first imaging modality for the detection of OC [9, 14, 15]. TVUS is currently used for screening of OC and may be associated with discomfort and risk to the vagina [16]. In clinical setting, CT is the most commonly employed imaging method before the staging laparotomy [9]. However, the diagnostic value of CT is limited to depict tumor implants that are 1 cm or smaller the sensitivities of 25–50% in peritoneal metastases [17, 18]. Other modalities are being increasingly used in the management of ovarian cancer including magnetic resonance imaging (MRI), positron emission tomography/computed tomography (PET/CT), or PET alone [9]. MRI provides superior soft-tissue contrast resolution, and it can identify indeterminate lesions seen on CT without exposure to radiation [15, 19]. PET, using 2-Deoxy-2-[18F]fluoro-d-glucose (18F-FDG) as radiotracer, if especially combined with CT, is beneficial for the staging gynecological malignant tumors, particularly lymph node metastasis or recurrent tumors, as both functional and anatomical information are available [20,21,22,23]. Furthermore, 18F-FDG PET/CT is known to have a high sensitivity for detection of OC relapse with a reported pooled sensitivity of 89% when multidisciplinary standard of reference including histology, clinical and imaging follow-up have been utilized [24]. Moreover, reports demonstrated that the combination of 18F-FDG PET and MRI also provided both high anatomical and functional resolution, and it had shown acceptably superior diagnostic performance than 18F-FDG PET/CT in gynecologic malignancies [25, 26].
For decades, a great number of studies on the diagnostic performance of noninvasive imaging modalities (CT, MRI, PET, PET/CT, and PET/MRI) aforementioned have been performed. The corresponding results varied on account of the study design, sample size, baseline characteristics, type of disease, and etc. The aim of this meta-analysis was to provide a broad overview of the diagnostic value of noninvasive imaging methods in the detection of ovarian cancer, and to come up with more evidenced-based findings for decision and strategy making on a clinical basis.
Materials and methods
This study was performed and reported in accordance with the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (The PRISMA-DTA Statement) [27].
Database search and study selection
Three electronic databases including PubMed, Embase, and Ovid were systematically searched from the date of inception to 31st, March, 2022. Only records in English language were considered for potential inclusion. Moreover, the bibliographies of reviews and studies included were manually screened in order to retrieve additional studies that met the inclusion criterion of the current study. The following medical subject headings and search terms were used for the database search: “magnetic resonance imaging”, “positron emission tomography”, “computed tomography”, “MRI”, “CT”, “PET”, “PET/CT”, “PET/MRI” and “Ovarian cancer”. The records searched from the aforementioned databases were altogether exported into Endnote software (Version 9.3.3; Thomson Corporation, Stanford, USA) for duplicates removing and further screening. The processes with regard to the literature search and study screening were performed by two independent investigators. Any disagreement was assessed by a third reviewer until the final consensus was achieved.
Studies were included if they met all the following criteria: (1) studies used MRI, CT, PET, PET/CT, or PET/MRI as detection modalities for the presence of ovarian cancer, regardless of tumor types; (2) reference standard was explicitly documented in the study; (3) absolute numbers of patients with true positive (TP), false positive (FP), true negative (TN), false negative (FN) outcomes, or they can be derived based on the presented data. Animal studies, case reports, reviews, comments, conference abstracts or records without extractable data were excluded in this study.
Data extraction and quality assessment
The following information in each study enrolled were identified and extracted in a predesigned table: name of the first author, year of publication, country or district where the study was conducted, absolute number of participants, age, gender, prevalence of OC, patient selection (suspected or confirmed OC), type of OC (primary, relapsed, or metastatic), reference standard, modality used, variables including TP, FP, FN, TN, and analytical level (patient-based or lesion based). Two reviewers independently performed the data extraction, and discrepancies were addressed through discussion with a third reviewer.
The revised tool for the quality assessment of diagnostic accuracy studies 2 (QUADAS-2) was utilized to evaluate the methodological quality of each included study [28]. This 14-item tool is comprised of 2 components, namely risk of bias and applicability concerns. As for the assessment of risk of bias, 4 aspects including patient selection, index test, reference standard, and flow and timing were considered. Patient selection, index test, and reference standard were assessed, respectively, in the evaluation of applicability concerns. Each item was rated as ‘yes’, ‘no’, or ‘unclear’. Two independent authors scored the included studies using the Review Manager software (Version 5.3; Cochrane Collaboration, Oxford, UK). Disagreements were resolved by discussion with a third reviewer.
Statistical analysis
Based on the data extracted, pooled estimates for diagnostic indicators, such as sensitivity, specificity, positive likelihood ratio (+ LR), negative likelihood ratio (- LR), diagnostic odds ratio (DOR), and area under the curve (AUC) of summary receiver operating characteristic (SROC) with corresponding 95% confidence intervals (CIs) were synthesized using the random effects models. The I2 static tests were used to test heterogeneity between the studies, the value of I2 > 50% and p value < 0.05 were considered to be statistically significant heterogeneity [29, 30]. Furthermore, subgroup meta-analysis and meta-regression were performed to investigate the underlying source of heterogeneity [31,32,33]. We employed Deeks’ asymmetry test and funnel plot to detect potential publication bias in included studies [34]. The Stata software (Version 16.0; StataCorp, College Station, TX, USA) and R (Version 4.1.2; Comprehensive R Archive Network) were used for data.
Results
Baseline information on included studies
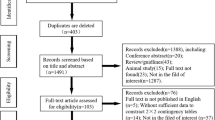
A total of 2406 citations were yielded through original literature search. Altogether 368 duplicates were removed using both Endnote software and manual identification. After title and abstract screening of the remaining 2038 records, 1943 articles were excluded. Ninety-five citations were reviewed in full text. Finally, 61 records (83 studies) including 4284 patients were deemed eligible for this study. The flow of literature search is displayed in Fig. 1. Enrolled citations included 18 prospective and 39 retrospective studies, 4 studies did not clarify their study design. The numbers of studies with regard to imaging modalities including CT, MRI, PET, PET/CT, and PET/MRI were 22, 11, 5, 43, and 2, respectively. The radiotracer used in PET imaging was 18F-FDG. Detailed information on the characteristics in provided in Supplementary Table 1. Most studies were rated as low risk of bias and low applicability concerns. High risk of bias or applicability concerns was not notified in included studies (Fig. 2 and Supplementary Fig. 1).
Diagnostic performance of CT
Pooled estimates of sensitivity, specificity, + LR, -LR, DOR, and AUC of SROC with respective 95% CIs of CT on patient level were 0.83 (0.73, 0.90), 0.69 (0.54, 0.81), 2.7 (1.8, 4.0), 0.25 (0.17, 0.37), 11 (6, 19), and 0.84 (0.80, 0.87) with substantial heterogeneity among studies (I2 = 91.87% for sensitivity, I2 = 86.66% for specificity) (Table 1, Supplementary Fig. 2). On lesion level, the pooled sensitivity, specificity, + LR, -LR, DOR, and AUC of SROC with corresponding 95% CIs of CT were 0.69 (0.51, 0.83), 0.88 (0.73, 0.95), 5.8 (2.7, 12.2), 0.35 (0.22, 0.55), 17 (8, 36), and 0.86 (0.82, 0.89), respectively. Heterogeneity was detected (I2 = 93.73% for sensitivity, I2 = 96.84% for specificity) (Table 1, Supplementary Fig. 3). Results of meta-regression and subgroup analysis manifested insignificant affection of baseline characteristics on the pooled results (Table 2).
Diagnostic performance of MRI
The overall sensitivity, specificity, + LR, -LR, DOR, and AUC of SROC with respective 95% CIs of MRI were 0.95 (0.91, 0.97), 0.81 (0.76, 0.85), 4.9 (3.8, 6.3), 0.07 (0.04, 0.12), 72 (36, 147), and 0.90 (0.87, 0.92) on patient level (I2 = 62.82% for sensitivity, I2 = 45.13% for specificity) (Table 1, Supplementary Fig. 4). The pooled estimates on lesion-based MRI were not performed due to limited number of studies. Five included studies used DWI examination, 3 used conventional MRI, and 2 studies utilized either conventional or contrast-enhanced MRI. Results of meta-regression showed significant impact of characteristics including study design, type of examination, Tesla level, age, and number of patients on the overall outcomes (Table 3).
Diagnostic performance of PET, PET/CT, PET/MRI
Results of studies investigating the diagnostic performance of PET/MRI were not pooled due to the limited number of studies included. The reported sensitivities and specificities were 91–97% and 86–87% in these studies. The overall sensitivity, specificity, SROC value of PET on patient level were 0.81 (0.71, 0.88), 0.81 (0.58, 0.93), and 0.82 (0.78, 0.85) (Table 4, Supplementary Fig. 5). Pooled estimates of sensitivity, specificity, and SROC value of PET/CT on patient level were 0.92 (0.88, 0.94), 0.88 (0.83, 0.92), and 0.96 (0.94, 0.97) (Table 4, Supplementary Fig. 6). The lesion-based overall sensitivity, specificity, and SROC value of PET/CT were 0.82 (0.71, 0.89), 0.94 (0.88, 0.97), and 0.95 (0.92, 0.96) (Table 4, Supplementary Fig. 7). Meta regression resulted in significant affection of study design, year of publication, and type of analysis on the pooled estimates of parameters.
Publication bias
Deeks’ funnel plot asymmetry tests yielded p values of 0.83, 0.77, 0.31, and 0.53 for CT, MRI, PET, and PET/CT studies (Fig. 3).
Discussion
Ovarian cancer remains one of the leading causes of mortality of gynecologic malignancies [35]. Metastases are often presented at the time of OC diagnosis, and high rate of relapse and poor prognosis are involved regardless of optimized management [36]. Timely and accurate detection of OC plays a significant role in treatment improvement and prognosis appraisal [21]. Imaging is critical for ovarian cancer management.
In the current study, we evaluated the most commonly utilized noninvasive imaging modalities in the detection of OC via the conduction of a meta-analysis. The diagnostic performance of CT, MRI, PET, along with hybrid imaging modalities, including PET/CT and PET/MRI were investigated. Results revealed that MRI manifested the highest overall sensitivity (0.95) and PET/CT showed the highest pooled specificity (0.88) on patient level. In general, PET/CT demonstrated the most superior diagnostic performance with an SROC value of 0.96 on a patient basis. Of note, only 2 PET/MRI studies were included in this study, the corresponding results were not meta-analyzed, sensitivities and specificities in these studies ranged from 91 to 97% and 86% to 87%, respectively. The combination of PET with CT or MRI can provide hybrid anatomical and functional imaging information so as to improve the detection accuracy [35, 37, 38]. Furthermore, results of meta-regression showed insignificant affection of baseline characteristics on the heterogeneity among CT studies. For MRI studies, study design, type of examination, Tesla level, age, and number of patients were detected to be potential sources of heterogeneity by meta-regression. Study design, year of publication, and the detection of metastatic OC were deemed as causes of heterogeneity among PET (PET/CT) studies. Due to limited number of covariates extracted from each enrolled study and insufficient numbers of studies in certain subgroups, meta-regression was performed based on available parameters, other possible sources of heterogeneity among studies should be further explored in the future.
Subgroup analyses were carried out to investigate the diagnostic value of these imaging modalities in specific subgroups. CT revealed the highest sensitivity in the detection of primary OC and showed superior specificity in metastases. High-field MRI produces better image quality compared with the lower field strength 1.5 T magnetic resonance MRI machines because it has a better signal-to-noise ratio (SNR) [39]. However, MRI performed on 1.5 T showed better diagnostic performance than on 3.0 T in this study. The probable reasons may be the differences on characteristics of study population, prevalence of OC, and the subjectivity of imaging reading between included studies. More well-designed relevant studies are warranted to further explore the impact of field strength on the diagnostic ability of OC. DWI examination demonstrated superior diagnostic ability than conventional or contrast-enhanced MRI. This combination of high contrast, millimeter level spatial resolution, robust performance, and short imaging time enables DWI to depict small tumor deposits in large volumes in a clinically timely manner [40]. PET/CT showed superior specificity but inferior sensitivity in the detection of metastasis compared to qualitative diagnosis. The possible reason may be the limited number of enrolled studies with regard to the detection of metastasis, more related studies are needed to verify the diagnostic performance of PET/CT in detecting metastasis. In addition, two reports enrolled in this study revealed that 18F-FDG PET/MRI yielded better diagnostic accuracy for the detection of metastatic ovarian cancer but did not offer significant additional information for the diagnosis primary OC [10, 41].
The current meta-analysis focused on the assessment of diagnostic performance of noninvasive imaging modalities on OC detection despite the cancer stages. It also provided considerable updates to previous meta-analyses on individual OC stage or imaging technique [11, 24, 42,43,44]. First, electronic database was systematically searched using relevant keywords and comprehensive search strategies; second, the processes of the literature screening, quality assessment, and data extraction were performed by two independent reviewers to minimize objective bias. Finally, statistical approaches were employed to detect potential heterogeneity and publication bias in included records. Publication bias of included studies was not indicated by the Deeks’ funnel plot asymmetry test. Nevertheless, as similar to any meta-analysis, heterogeneity is inevitable and the source of this heterogeneity is not sorted out thoroughly. The results should be interpreted with caution.
Conclusion
This meta-analysis revealed that noninvasive imaging modalities including CT, MRI, PET (PET/CT, PET/MRI) yielded favorable diagnostic performance in the detection of OC. A combination of different tools (PET/MRI) was deemed to be more accurate for identifying metastatic OC. Professional and economic issues should be considered by practitioners in the real-world clinical setting.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- OC:
-
Ovarian cancer
- EOC:
-
Epithelial ovarian cancer
- CT:
-
Contrast-enhanced computed tomography
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- 18F-FDG:
-
2-Deoxy-2-[18F]fluoro-d-glucose
- PRISMA-DTA:
-
The Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies
- TP:
-
True positive
- FP:
-
False positive
- TN:
-
True negative
- FN:
-
False negative
- QUADAS:
-
The quality assessment of diagnostic accuracy studies
- + LR:
-
Positive likelihood ratio
- – LR:
-
Negative likelihood
- DOR:
-
Diagnostic odds ratio
- SROC:
-
Summary receiver operating characteristic
- CI:
-
Confidence interval
References
Hu X, Li D, Liang Z, Liao Y, Yang L, Wang R, et al. Indirect comparison of the diagnostic performance of 18F-FDG PET/CT and MRI in differentiating benign and malignant ovarian or adnexal tumors: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):1080.
Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151–6.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Virarkar M, Ganeshan D, Gulati AT, Palmquist S, Iyer R, Bhosale P. Diagnostic performance of PET/CT and PET/MR in the management of ovarian carcinoma-a literature review. Abdominal Radiol. 2021;46(6):2323–49.
Forstner R. Early detection of ovarian cancer. Eur Radiol. 2020;30(10):5370–3.
Orr B, Edwards RP. Diagnosis and treatment of ovarian cancer. Hematol Oncol Clin North Am. 2018;32(6):943–64.
Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133(3):401–4.
Huang SH, O’Sullivan B. Overview of the 8th editionTNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18(7):40.
Kemppainen J, Hynninen J, Virtanen J, Seppänen M. PET/CT for evaluation of ovarian cancer. Semin Nucl Med. 2019;49(6):484–92.
Tsuyoshi H, Tsujikawa T, Yamada S, Okazawa H, Yoshida Y. Diagnostic value of [(18)F]FDG PET/MRI for staging in patients with ovarian cancer. EJNMMI Res. 2020;10(1):117.
Van’t Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol. 2020;30(6):3101–12.
Weber S, McCann CK, Boruta DM, Schorge JO, Growdon WB. Laparoscopic surgical staging of early ovarian cancer. Rev Obstet Gynecol. 2011;4(3–4):117–22.
Chi DS, Abu-Rustum NR, Sonoda Y, Ivy J, Rhee E, Moore K, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol. 2005;192(5):1614–9.
Marzola MC, Chondrogiannis S, Rubello D. Fludeoxyglucose F 18 PET/CT assessment of ovarian cancer. PET Clin. 2018;13(2):179–202.
Javadi S, Ganeshan DM, Qayyum A, Iyer RB, Bhosale P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. AJR Am J Roentgenol. 2016;206(6):1351–60.
Campbell S, Gentry-Maharaj A. The role of transvaginal ultrasound in screening for ovarian cancer. Climacteric. 2018;21(3):221–6.
Pannu HK, Horton KM, Fishman EK. Thin section dual-phase multidetector-row computed tomography detection of peritoneal metastases in gynecologic cancers. J Comput Assist Tomogr. 2003;27(3):333–40.
Tempany CM, Zou KH, Silverman SG, Brown DL, Kurtz AB, McNeil BJ. Staging of advanced ovarian cancer: comparison of imaging modalities–report from the Radiological Diagnostic Oncology Group. Radiology. 2000;215(3):761–7.
Forstner R, Hricak H, Occhipinti AK, Powell BC, et al. Ovarian cancer: staging with CT and MR imaging. Radiology. 1995;197(3):619–26.
Alt CD, Brocker KA, Eichbaum M, Sohn C, Arnegger FU, Kauczor H-U, et al. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT. Strahlenther Onkol. 2011;187(11):705–14.
Friedman SN, Itani M, Dehdashti F. PET imaging for gynecologic malignancies. Radiol Clin North Am. 2021;59(5):813–33.
Shaaban A, Rezvani M. Ovarian cancer: detection and radiologic staging. Top Magn Reson Imaging. 2010;21(4):247–59.
Khiewvan B, Torigian DA, Emamzadehfard S, Paydary K, Salavati A, Houshmand S, et al. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur J Nucl Med Mol Imaging. 2017;44(6):1079–91.
Massollo M, Treglia G, Trimboli P, Fiz F, Ugolini M, Piccardo A. Head-to-head comparison between 18F-FDG PET/low-dose CT and 18F-FDG PET/contrast-enhanced CT in relapsing ovarian carcinoma: a systematic review and meta-analysis. Clin Transl Imaging. 2021;9(1):73–81.
Zheng M, Xie D, Pan C, Xu Y, Yu W. Diagnostic value of 18F-FDG PET/MRI in recurrent pelvis malignancies of female patients: a systematic review and meta-analysis. Nucl Med Commun. 2018;39(6):479–85.
Queiroz MA, Kubik-Huch RA, Hauser N, Freiwald-Chilla B, von Schulthess G, Froehlich JM, et al. PET/MRI and PET/CT in advanced gynaecological tumours: initial experience and comparison. Eur Radiol. 2015;25(8):2222–30.
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Luo Q, Luo L, Tang L. A network meta-analysis on the diagnostic value of different imaging methods for lymph node metastases in patients with cervical cancer. Technol Cancer Res Treat. 2018;17:1533034617742311.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Spineli LM, Pandis N. Exploring heterogeneity in meta-analysis: subgroup analysis. Part 1. Am J Orthod Dentofacial Orthop. 2020;158(2):302-4.e1.
Spineli LM, Pandis N. Exploring heterogeneity in meta-analysis: meta-regression analysis. Am J Orthod Dentofacial Orthop. 2020;158(4):623–5.
Spineli LM, Pandis N. Exploring heterogeneity in meta-analysis: subgroup analysis. Part 2. Am J Orthod Dentofacial Orthop. 2020;158(3):462–3.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93.
Virarkar M, Ganeshan D, Gulati AT, Palmquist S, Iyer R, Bhosale P. Diagnostic performance of PET/CT and PET/MR in the management of ovarian carcinoma-a literature review. Abdom Radiol (NY). 2021;46(6):2323–49.
Forstner R, Meissnitzer M, Cunha TM. Update on imaging of ovarian cancer. Curr Radiol Rep. 2016;4:31.
Ohliger MA, Hope TA, Chapman JS, Chen LM, Behr SC, Poder L. PET/MR imaging in gynecologic oncology. Magn Reson Imaging Clin N Am. 2017;25(3):667–84.
Ponisio MR, Fowler KJ, Dehdashti F. The emerging role of PET/MR imaging in gynecologic cancers. PET Clin. 2016;11(4):425–40.
Booth SJ, Turnbull LW, Poole DR, Richmond I. The accurate staging of ovarian cancer using 3T magnetic resonance imaging–a realistic option. BJOG. 2008;115(7):894–901.
Michielsen KL, Vergote I, Dresen R, de Beeck Op K, Vanslembrouck R, Amant F, et al. Whole-body diffusion-weighted magnetic resonance imaging in the diagnosis of recurrent ovarian cancer: a clinical feasibility study. Br J Radiol. 2016;89(1067):20160468.
Kawahara K, Yoshida Y, Kurokawa T, Suzuki Y, Nagahara K, Tsuchida T, et al. Evaluation of positron emission tomography with tracer 18-fluorodeoxyglucose in addition to magnetic resonance imaging in the diagnosis of ovarian cancer in selected women after ultrasonography. J Comput Assist Tomogr. 2004;28(4):505–16.
Yuan Y, Gu ZX, Tao XF, Liu SY. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: a meta-analysis. Eur J Radiol. 2012;81(5):1002–6.
Limei Z, Yong C, Yan X, Shuai T, Jiangyan X, Zhiqing L. Accuracy of positron emission tomography/computed tomography in the diagnosis and restaging for recurrent ovarian cancer: a meta-analysis. Int J Gynecol Cancer. 2013;23(4):598–607.
Dodge JE, Covens AL, Lacchetti C, Elit LM, Le T, Devries-Aboud M, et al. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol. 2012;126(1):157–66.
Acknowledgements
We are extremely grateful to the authors of all the included papers for proving suitable data for analysis.
Funding
There is no fund support for this work.
Author information
Authors and Affiliations
Contributions
XL and LW: developed the concept, designed the study, and prepared the manuscript. LW and YZ: conducted the literature search. XL and PG: acquired the data, LW and QS: analyzed the data. CC and YZ: controlled quality of the work.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declared no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Wang, L., Guo, P. et al. Diagnostic performance of noninvasive imaging using computed tomography, magnetic resonance imaging, and positron emission tomography for the detection of ovarian cancer: a meta-analysis. Ann Nucl Med 37, 541–550 (2023). https://doi.org/10.1007/s12149-023-01856-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01856-7