Abstract
Purpose
Accurate staging of ovarian cancer is critical to guide optimal management pathways. North American guidelines recommend contrast-enhanced CT as the primary work-up for staging ovarian cancer. This meta-analysis aims to compare the diagnostic accuracy of contrast-enhanced CT alone to PET/CT for detecting abdominal metastases in patients with a new or suspected diagnosis of ovarian cancer.
Materials and methods
A systematic review of MEDLINE, EMBASE, Scopus, the Cochrane Library, and the gray literature from inception to October 2022 was performed. Studies with a minimum of 5 patients evaluating the diagnostic accuracy of contrast-enhanced CT and/or PET/CT for detecting stage 3 ovarian cancer as defined by a surgical/histopathological reference standard ± clinical follow-up were included. Study, clinical, imaging, and accuracy data for eligible studies were independently acquired by two reviewers. Primary meta-analysis was performed in studies reporting accuracy on a per-patient basis using a bivariate mixed-effects regression model. Risk of bias was evaluated using QUADAS-2.
Results
From 3701 citations, 15 studies (918 patients with mean age ranging from 51 to 65 years) were included in the systematic review. Twelve studies evaluated contrast-enhanced CT (6 using a per-patient assessment and 6 using a per-region assessment) and 11 studies evaluated PET/CT (7 using a per-patient assessment and 4 using a per-region assessment). All but one reporting study used consensus reading. Respective sensitivity and specificity values on a per-patient basis were 82% (67–91%, 95% CI) and 72% (59–82%) for contrast-enhanced CT and 87% (75–94%) and 90% (82–95%) for PET/CT. There was no significant difference in sensitivities between modalities (p = 0.29), but PET/CT was significantly more specific than CT (p < 0.01). Presumed variability could not be assessed in any single category due to limited studies using per-patient assessment. Studies were almost entirely low risk for bias and applicability concerns using QUADAS-2.
Conclusion
Contrast-enhanced CT demonstrates non-inferior sensitivity compared to PET/CT, although PET/CT may still serve as an alternative and/or supplement to CT alone prior to and/or in lieu of diagnostic laparoscopy in patients with ovarian cancer. Future revisions to existing guidelines should consider these results to further refine the individualized pretherapeutic diagnostic pathway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the second most common gynecologic malignancy and the most common cause of gynecologic cancer death in resource-rich countries with a lifetime risk of 1 in 75 for people with ovaries [1, 2]. Epithelial ovarian cancers (consisting of serous, endometrioid, clear cell, and mucinous subtypes) account for more than 90% of ovarian malignancies, followed by other cell types, including germ cell and sex cord-stromal tumors [2]. Only 15% of ovarian cancers are detected early (stage 1) and the overall 5-year survival for these patients is less than 50% as recently as 2015 [2].
Accurate staging of ovarian cancer remains critically important for treatment planning with stage 3 disease often requiring a combination of neoadjuvant therapy and/or surgery [3]. Current North American guidelines recommend contrast-enhanced CT as the primary work-up for staging and contrast-enhanced CT, MRI, or PET/CT as the post-primary treatment surveillance for stage 2–4 disease. However, supplementary PET/CT may improve accuracy compared to CT alone for detecting advanced ovarian cancer [4]. Further, CT alone may not be reliable for determining stage 3b disease [5]. To our knowledge, the diagnostic accuracy evidence of CT alone versus PET/CT for characterizing Stage 3 disease has not yet been systematically evaluated. The purpose of this systematic review is to compare the diagnostic accuracy of contrast-enhanced CT versus PET/CT for detecting stage 3 ovarian cancer.
Methods
This systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis—Diagnostic Test Accuracy (PRISMA-DTA) guidelines [6, 7]. The study proposal was submitted to the PROSPERO database prior to initiation of the review (CRD42022371668). Institutional ethics approval was not required as it included only previously published information.
Literature search
A complete database search of MEDLINE, EMBASE, Scopus, and the Cochrane Library (including the Cochrane Central Register of Controlled Trials, the Cochrane Central Register of Protocols, and the Cochrane Database of Systematic Reviews) from inception through to October 30, 2022 was performed to identify studies evaluating the diagnostic accuracy of CT and/or PET/CT to detect stage 3 ovarian carcinoma as compared to a surgical reference standard ± clinical follow-up of at least 12 months. Different title/abstract/keywords and medical subject heading terms were searched with variations of (“computed tomography” OR “positron emission tomography”) AND “ovary” AND “cancer” were searched on an individualized basis in each database. The search strategies applied for each database are shown in Supplementary Information. No language or publication date restrictions were applied. The search was performed according to best practices for electronic search strategies [8]. Articles from each database were subsequently combined and duplicate articles were removed. Title and abstracts were screened for relevance of the study objective. Subsequently, full-text review was performed for potentially relevant studies performed independently by two reviewers with 3 and 6 years of imaging experience (BB and SS). Additionally, gray literature consisting of conference presentations from the Radiological Society of North America (RSNA) between 2020 and 2022, the European Congress of Radiology (ECR) between 2022 and 2023, and the International Society for Gynecologic Cancer (ICGS) from 2022 was performed by one author with 8 years of imaging experience (MPW). If a conference abstract provided sufficient information to meet inclusion criteria, these abstracts were included in the review and authors were contacted for additional information when necessary. Finally, reference lists for key studies were checked and forward searching was performed in Google Scholar.
Selection criteria
Original articles evaluating the diagnostic accuracy of CT and/or PET/CT for detecting stage 3 ovarian malignancy compared to a surgical reference standard ± clinical follow-up of at least 12 months were evaluated in full-text review. For inclusion, studies further required a minimum of 5 patients and sufficient information to reconstruct a 2 × 2 contingency table on a per-patient basis. If studies met all criteria but the latter, the authors were contacted via email for additional information and would be included if sufficient information was supplied. Studies evaluating the accuracy of CT and/or PET/CT by dividing individual patients into multiple regions of the abdomen (a “per-region basis”) were included in the systematic review but not included in the meta-analysis if sufficient data to construct a 2 × 2 contingency table on a per-patient basis could not be obtained. Studies were excluded when (1) CT or PET/CT were not the index test(s); (2) ovarian malignancy was not the target pathology; or (3) surgery was not the principal reference standard. Malignancy was defined by a pathological gold standard within each individual study. Non-original articles including review articles, guidelines, consensus statements, letters, and editorials were excluded.
Data extraction
The relevant data from included studies were extracted independently by two reviewers (BB AND SS). Study, patient, and imaging characteristics were recorded. Study demographics included first author, year of publication, country in which the study was performed, use of a prospective or retrospective study design, data acquisition from a single hospital or multi-hospital (multi-center) setting, reference standard used, number of readers for imaging interpretation, if consensus reading was used, and type of specialist of the reader. Acquired patient information included the total number of patients, mean age of included patients, modality used in that study, and tumor subtypes included in the study. Included imaging characteristics for CT: brand, number of detector rows, slice thickness, presence of contrast, and size threshold used for positivity. Included imaging characteristics for PET/CT: brand, PET-positive criteria, number of detector rows, and slice thickness.
True-positive (TP), false-negative (FN), false-positive (FP), and false-negative (FN) results for the presence of malignancy by size threshold were recorded. If data for different size thresholds was provided within an individual study, these were each recorded separately. If a study did not report these results directly but reported the sensitivity, specificity, sample size, and prevalence, then contingency table results were calculated. For studies with multiple index test readers, a single result was recorded as an average of all reviewers [9]. Following the completion of data acquisition independently by two authors, the contingency tables were reviewed in entirety by three authors (BB, SS, MPW) with re-review of articles where data were inconsistent and consensus agreement was used for the final data file. The third reviewer has 8 years of imaging experience (MPW). Data were collected using Microsoft Excel v15.14 (Microsoft, Redmond, Washington, USA).
Risk of bias assessment
The methodological quality of each study was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [10]. Studies with a high-risk evaluation for any single signaling question in a particular category were considered high risk for that respective domain.
Data analysis
Meta-analysis was performed on studies that reported the results per patient, not per radiographic region. A bivariate meta-analysis model that addresses the correlation between sensitivity and specificity was used to evaluate the accuracy of CT and PET/CT, each compared to a reference standard. A generalized linear mixed model for the binomial family with a logit link was used as implemented in package [metadata]. Analysis was performed using STATA software (StataCorp, 2019, Stata Statistical Software: Release 17, College Station, Tx: StataCorp LP). Studies evaluating the accuracy of CT and/or PET/CT on a per-region basis were summarized using descriptive statistics (median and range). Variability was assumed based on current best-practice recommendations by the PRISMA-DTA group for bivariate models and a funnel plot and associated tests were not performed to explore small study effects [6]. A subgroup analysis of both CT and PET/CT groups was planned to evaluate for causes of presumed variability which may include one or more features of study, imaging, and/or patient-specific characteristics.
Results
Literature search
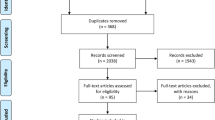
The literature search PRISMA flow diagram is shown in Fig. 1. A total of 3701 titles and abstracts were assessed, with 43 articles reviewed in full text. Twenty-eight studies were excluded flowing full-text review for reasons outlined in Fig. 1. A total of 15 studies with 918 patients were included in the systematic review [4, 11,12,13,14,15,16,17,18,19,20,21,22,23,24] with six studies evaluating the accuracy of CT on a per-patient basis [4, 11, 13, 16, 17, 24] and six studies evaluating the accuracy of CT on a per-region basis throughout the abdomen [12, 15, 18, 19, 21, 23]. Eleven studies evaluated PET/CT [4, 11, 13,14,15, 17, 18, 20,21,22, 24] with seven studies evaluating the accuracy of PET/CT on a per-patient basis [4, 11, 13, 17, 20, 22, 24] and four studies evaluating the accuracy of PET/CT on a per-region basis throughout the abdomen [14, 15, 18, 21]. For studies evaluating contrast-enhanced CT and PET/CT within the same study, readers were blinded to the separate modality interpretation during the evaluation of their given modality.
Study, patient, and imaging characteristics
Study features and patient characteristics are shown in Table 1. Most studies used a prospective study design (9/15) and included single-center data (12/15). The studies were conducted in countries in North America, Europe, and Asia. All but one reporting studies used consensus review for image interpretation with most readers being either a radiologist and/or a nuclear medicine physician. Most studies included between 40 and 120 patients with two studies including less than 20 patients. The mean age of included patients for reporting studies ranged from 51 to 65 years. Most studies included only subtypes of epithelial ovarian cancers.
Tables 2 and 3 summarize the CT characteristics and PET/CT characteristics by study, respectively. Most reporting studies used a lymph node size threshold of 1 cm for positive results in contrast-enhanced CT reporting. PET/CT-positive criteria was variably described with positive results predominantly involving abnormal FDG uptake in lymph nodes, although only two studies described their diagnostic SUV criteria [11, 14].
Diagnostic accuracy of contrast-enhanced CT and PET/CT
Individual study performance reported for patient-based and region-based CT and PET/CT is shown in Table 4. Forest plots for sensitivity and specificity for patient-based CT and PET/CT analysis are shown in Fig. 2. Receiver operating characteristic (ROC) curves for CT and PET/CT are shown in Fig. 3. The sensitivity and specificity for contrast-enhanced CT on a per-patient basis is 82% (67–91%, 95% CI) and 72% (59–82%), respectively. The median and range sensitivity and specificity for contrast-enhanced CT on a per-region basis is 72% (35–96%) and 93% (69–98%), respectively.
The sensitivity and specificity for PET/CT on a per-patient basis is 87% (75–94%) and 90% (82–95%), respectively. The median and range sensitivity and specificity for PET/CT on a per-region basis are 73% (62–95%) and 83% (49–97%), respectively. On a per-patient basis, there is no significant difference in sensitivity between contrast-enhanced CT versus PET/CT (p = 0.29), but PET/CT is significantly more specific (p < 0.01). There were an insufficient number of studies reporting both contrast-enhanced CT and PET/CT on a per-patient basis to perform a subgroup analysis in either group.
Risk of bias assessment
Risk of bias by study domain is shown in Supplementary Information. Risk of bias was low for almost all studies with only one study demonstrating a high risk of bias for patient selection on account of non-consecutive patient selection (excluding patients who received therapy at outside institutions) [18] and one study demonstrating a high risk of bias for index test due to limited clarity for diagnostic PET-positive criteria [13]. One study had an unclear risk for patient selection [15] and two studies had an unclear risk for reference standard [12, 23]. Applicability concerns were low for all included studies.
Discussion
This meta-analysis shows no statistical difference in sensitivity between contrast-enhanced CT alone and PET/CT for detecting stage 3 disease of ovarian cancer but does show statistical improvement in specificity and a trend toward improved overall accuracy when using PET/CT on a per-patient basis. The study supports existing North American guidelines which indicate that contrast-enhanced CT alone is a suitable method for initially screening patients with ovarian cancer. However, supplemental PET/CT in these patients may play an important role for improving detection of abdominal metastasis given the improved specificity and trend toward improved overall accuracy when compared to CT alone.
The standard supplementary use of PET/CT in the initial staging of patients with ovarian cancer may be limited by modality access relative to CT alone. In one study comparing contrast-enhanced CT, MRI, and FDG PET/CT for detecting peritoneal carcinomatosis in patients with ovarian cancer, PET/CT showed the highest specificity, while MRI showed the highest sensitivity, although no statistical differences in overall accuracy were identified between the modalities and multidetector CT was recommended as the initial staging modality as it was the “fastest, most economical, and most widely available modality” [21]. Even in this study, however, the additional potential value of PET/CT was emphasized. In fact, four of five studies comparing contrast-enhanced CT alone to PET/CT on a per-patient basis recommend the use of PET/CT, particularly for the utility of PET/CT in detecting lymph node metastases [4, 11, 13, 17]. A further two of the three studies comparing contrast-enhanced CT alone to PET/CT on a per-region basis also came to the same conclusion [15, 18]. The supplementary use of PET/CT may be particularly important for patients undergoing alternative or systemic therapies prior to or even in lieu of a diagnostic laparoscopy as the existing surgical gold standard has limitations in performance despite having near perfect specificity in the regions that are visualized and evaluated [25].
While serving as the first known systematic review evaluating the accuracy of contrast-enhanced CT alone versus PET/CT for ovarian cancer staging, this systematic review and meta-analysis is subject to several limitations. One principal limitation may be the use of consensus reading in nearly all reporting studies for both CT and PET/CT evaluation. This may not reflect a “real-world” scenario where the initial study is more often read by a single reader at most institutions in North America, particularly for CT, and the ability to detect small lesions throughout the abdomen may be lessened than when read by consensus. Further complicating this, is that a surgical gold standard is inherently imperfect, particularly with respect to sensitivity, given the potential for non-visualization of small lesions and particular regions (such as the retroperitoneum) with diagnostic laparoscopy [25]. In our opinion, this would likely have a more detrimental effect on the sensitivity of contrast-enhanced CT compared to PET/CT, supported by a recent prospective cohort study showing that PET/MRI has an improved sensitivity compared to CT for detecting peritoneal carcinomatosis of general primary abdominopelvic malignancies, despite the current meta-analysis not identifying statistically improved sensitivity with PET/CT [26]. Conversely, PET/CT has been known to have false positives in primary mucinous carcinoma subtypes on account of increased mucin content and decreased tumor cellularity, although the potential effect of cellularity on disseminated abdominal metastases for this subtype is not clearly defined [27]. Several other sources of variability both within and between modality assessments may also have an unrecognized effect on the results of our study including individual study, patient-, and/or imaging-specific parameters, although there were an insufficient number of studies evaluating each modality on a per-patient basis to undertake a planned subgroup analysis. By way of example, the nuanced differences in the acquisition of the CT portion of a PET/CT (such as slice thickness and contrast enhancement) may have an important but undefined difference in diagnostic accuracy within the PET/CT modality.
Given the limitations in the existing literature and recognized potential benefit for supplementary imaging beyond CT alone for staging patients with ovarian cancer, this study outlines several avenues for future research. First, further comparative analysis of contrast-enhanced CT versus PET/CT reporting accuracy on a per-patient basis would help improve the precision of this comparative analysis. Future systematic reviews should also include MRI or even PET/MRI studies in their comparative analysis. For patients evaluated with FDG PET/CT, a subgroup analysis of threshold values for positivity may help clarify the optimal SUV values for diagnosis given limited clarity and some variability for positive thresholds in the current existing publications. Finally, comparing new and emerging modalities for staging in these patients to CT and/or FDG PET/CT may provide alternative approaches to imaging these patients. As an example, in one recent publication, Gallium-68-labeled fibroblast activation protein inhibitor ([68Ga]-FAPI-04 PET/CT) was found to have a higher sensitivity than FDG PET/CT for detecting lymph node and peritoneal metastasis of epithelial ovarian cancers [28]. Future national and international guidelines should be informed by this and future analyses as the literature further evolves both within the existing modalities and expands on emerging modalities.
In summary, this meta-analysis shows no statistical difference in sensitivity between contrast-enhanced CT and PET/CT for detecting stage 3 ovarian cancer, supporting current guidelines indicating contrast-enhanced CT as an initial staging test for these patients. However, PET/CT is significantly more specific, potentially more accurate, and may serve an alternative and/or supplementary role to CT alone in these patients prior to and/or in lieu of diagnostic laparoscopy. Future research comparing SUV thresholds for FDG PET/CT, including MRI and/or PET/MRI, and evaluating the utility of emerging PET radiotracers may help advance the overall accuracy of PET imaging for the diagnosis of stage 3 ovarian cancer. Future revisions to existing guidelines should be informed by this study and emerging literature to further refine the pretherapeutic diagnostic pathway on an individualized basis.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87-108. DOI: https://doi.org/10.3322/caac.21262.
Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: A review. Cancer Biol Med 2017;14(1):9-32. DOI: https://doi.org/10.20892/j.issn.2095-3941.2016.0084.
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw 2021;19(2):191–226. DOI: https://doi.org/10.6004/jnccn.2021.0007.
Yoshida Y, Kurokawa T, Kawahara K, et al. Incremental benefits of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. AJR Am J Roentgenol 2004;182(1):227-233. DOI: https://doi.org/10.2214/ajr.182.1.1820227.
Onda T, Tanaka TO, Kitai S, et al. Stage 3 disease of ovarian, tubal and peritoneal cancers can be accurately diagnosed with pre-operative CT. Japan Clinical Oncology Group Study JCOG0602. Jpn J Clin Oncol 2021;51(2):205–212. doi: https://doi.org/10.1093/jjco/hyaa145
McInnes MDF, Moher D, Thombs BD, McGrath T, Bossuyt PM, and the PRISMA-DTA Group. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018;23;319(4):388–396. DOI: https://doi.org/10.1001/jama.2017.19163.
McGrath TA, Bossuyt PM, Cronin P, Salameh JP, Kraaijpoel N, Schieda N, et al. Best practices for MRI systematic reviews and meta-analyses. J Magn Reson Imaging 2019;49:e51-e64. DOI: https://doi.org/10.1002/jmri.26198.
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol 2009;62:944-952. DOI: https://doi.org/10.1016/j.jclinepi.2008.10.012.
McGrath T, McInnes MDF, Langer FW, et al. Treatment of multiple test readers in diagnostic accuracy systematic reviews – meta-analyses of imaging studies. Eur J Radiol 2017;93:59-64. doi: https://doi.org/10.1016/j.ejrad.2017.05.032
Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155(8):529-536. DOI: https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
Castellucci P, Perrone AM, Picchio M, et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: Correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun 2007;28(8):589-595. DOI: https://doi.org/10.1097/MNM.0b013e3281afa256.
Choi HJ, Lim MC, Bae J, et al. Region-based diagnostic performance of multidetector CT for detecting peritoneal seeding ovarian cancer patients. Arch Gynecol Obstet 2011;283(2):353-360. DOI: https://doi.org/10.1007/s00404-010-1442-0.
Dauwen H, Van Calster B, Deroose CM, et al. PET/CT in the staging of patients with pelvic mass suspicious for ovarian cancer. Gynecol Oncol 2013;131(3):694-700. DOI: https://doi.org/10.1016/j.ygyno.2013.08.020.
De laco P, Musto A, Zamagni C, et al. PET/CT in advanced ovarian cancer staging: value and pitfalls in detecting lesions in different abdominal and pelvic quadrants compared with laparoscopy. Eur J Radiol 2011;80(2):e98–103. DOI: https://doi.org/10.1016/j.ejrad.2010.07.013.
Drieskens O, Stroobants S, Gysen M, et al. Positron emission tomography with FDG in the detection of peritoneal and retroperitoneal metastases of ovarian cancer. Gynecol Obstet Invest 2003;55(3):130-134. DOI: https://doi.org/10.1159/000071525.
Forstner R, Hricak H, Occhipinti KA, et al. Ovarian cancer: Staging with CT and MR imaging. Radiology 1995;197(3):619-626.
Kim HW, Won KS, Zeon SK, et al. Peritoneal carcinomatosis in patients with ovarian cancer: enhanced CT versus 18F-FDG PET/CT. Clin Nucl Med 2013;38(2):93-97. DOI: https://doi.org/10.1097/RLU.0b013e31826390ec.
Kitajima K, Murakami K, Yamasaki E, et al. Diagnostic accuracy of integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer: comparison with enhanced CT. Eur J Nucl Med Mol Imaging 2008;35(10):1912-1920. DOI: https://doi.org/10.1007/s00259-008-0890-2.
Metser U, Jones C, Jacks LM, Bernardini MQ, Ferguson S. Identification and quantification of peritoneal metastases in patients with ovarian cancer with multidetector computed tomography: correlation with surgery and surgical outcome. Int J Gynecol Cancer 2011;21(8):1391-1398. DOI: https://doi.org/10.1097/IGC.0b013e31822925c0.
Nam EJ, Yun MJ, Oh YT, et al. Diagnosis and staging of primary ovarian cancer: correlation between PET/CT, doppler US, and CT or MRI. Gynecol Oncol 2010;116(3):389-394. DOI: https://doi.org/10.1016/j.ygyno.2009.10.059.
Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med 2015;40(5):371-377. DOI: https://doi.org/10.1097/RLU.0000000000000768.
Tardieu A, Ouldamer L, Margueritte F, et al. Assessment of lymph node involvement with PET-CT in advanced epithelial ovarian cancer. A FRANCOGYN Group Study. J Clin Med 2021;10(4):602. DOI: https://doi.org/10.3390/jcm10040602.
Trempany CM, Zou KH, Silverman SG, et al. Staging of advanced ovarian cancer: comparison of imaging modalities – report from the Radiological Diagnostic Oncology Group. Radiology 2000;215(3):761-767. DOI: https://doi.org/10.1148/radiology.215.3.r00jn25761.
Uysal NE, Bakir MS, Birge O, et al. Prediction of lymph node involvement in epithelial ovarian cancer by PET/CT, CT and MRI imaging. Eur J Gynecol Oncol 2021;42(3):506-511. DOI: https://doi.org/10.31083/j.ejgo.2021.03.2340.
Rutten MJ, Leeflang MMG, Kenter GG, Mol MWJ, Buist M. Laparoscopy for diagnosing resectability of disease in patients with advanced ovarian cancer. Cochrane Database Syst Rev 2014(2):CD009786. DOI: https://doi.org/10.1002/14651858.CD009786.pub2.
Furtado FS, Wu MZ, Esfahani SA, et al. Positron emission tomography/Magnetic resonance imaging (PET/MRI) versus the standard of care imaging in the diagnosis of peritoneal carcinomatosis. Ann Surg 2023;277(4):e893-e899. DOI: https://doi.org/10.1097/SLA.0000000000005418.
Marko J, Marko KL, Pachigolla SL, Crothers BA, MAttu R, Wolfman DJ. Mucinous neoplasms of the ovary: Radiologic-pathologic correlation. RadioGraphics 2019;39:982–997. DOI: https://doi.org/10.1148/rg.2019180221.
Chen J, Xu K, Li C, et al. [68Ga]Ga-FAPI-04 PET/CT in the evaluation of epithelial ovarian cancer: comparison with [18F]F-FDG PET/CT. Eur J Nucl Med Mol Imaging 2023;50(13):4064-4076.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilson, M.P., Sorour, S., Bao, B. et al. Diagnostic accuracy of contrast-enhanced CT versus PET/CT for advanced ovarian cancer staging: a comparative systematic review and meta-analysis. Abdom Radiol 49, 2135–2144 (2024). https://doi.org/10.1007/s00261-024-04195-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-024-04195-x