Abstract
Background
The BRAF p.V600E genetic variant facilitates the pathogenesis of various tumors by triggering tumor proliferation and progression. The aim of this study was to analyze the prevalence of BRAF p.V600E in benign mixed epithelial and mesenchymal and malignant odontogenic tumors. In addition, we discussed the different detection methods used to assess for aberrant BRAF.
Methods
This systematic review followed the PRISMA guidelines and was registered in Prospero (CRD42023445689). A comprehensive search of the PubMed/MEDLINE, Scopus, Web of Science, and Embase electronic databases was performed to answer the question “What is the prevalence of the BRAF p.V600E mutation in benign mixed and malignant odontogenic tumors?” The methodological quality of the selected studies was assessed using the JBI’s Critical Appraisal Tool.
Results
Initially, 387 records were identified, but only 11 articles met the inclusion criteria. A total of 70 patients with benign mixed epithelial and mesenchymal odontogenic tumors and 63 with malignant odontogenic tumors were included in the analysis. We found that the BRAF p.V600E mutation had a prevalence of 31.42% in mixed tumors and 26.98% in malignant odontogenic tumors. Moreover, immunohistochemistry showed high concordance with DNA-based molecular methods.
Conclusion
In general, the BRAF p.V600E variant exhibited a prominent prevalence in mixed and malignant odontogenic tumors. However, most of the findings are based on small cohorts of patients and further studies with larger cohorts are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Odontogenic tumors (OT) comprise a heterogeneous group that develops from remnants of epithelial or ectomesenchymal tissues associated with odontogenesis. This group encompasses a wide range of lesions, including both benign and malignant neoplasms [1, 2]. The World Health Organization’s (WHO) Classification of Head and Neck Tumors categorizes odontogenic tumors into epithelial, mesenchymal, and mixed epithelial and mesenchymal types based on their tissue of origin. Furthermore, the fifth edition of the WHO Classification of Tumors provides essential and desirable diagnostic criteria for each entity [3]. In certain cases, molecular studies may be required to differentiate between these tumor types.
The pathogenesis of OT is intimately linked to alterations in components of signaling pathway, primarily within the mitogen-activated protein kinase (MAPK) pathway, which may represent a pivotal early event in odontogenic tumorigenesis. [2, 4] This prototypical MAPK cascade, Ras-Raf-MEK-ERK, is frequently dysregulated in various human cancers [5]. Among these components, BRAF stands out as the most potent activator of the MAPK pathway [4, 6]. Ordinarily, BRAF is activated in response to growth signals, initiating a series of molecular events that govern controlled cell proliferation. In the context of BRAF p.V600E, B-Raf becomes hyperactive and remains persistently activated, independent of growth signals. This abnormal activation leads to an excessive flow of signaling through the MAPK pathway, resulting in uncontrolled cell growth, proliferation, and cell survival [7]. The substitution of valine (V) for glutamic acid (E) at codon 600 (BRAF p.V600E) is responsible for approximately 90% of all BRAF gene mutations [4, 8]. This genetic variant has been identified as a broad driver neoplasia, including OT [2, 5].
Initially, the BRAF p.V600E was identified in benign epithelial OT, such as ameloblastoma, the most extensively studied, and adenomatoid odontogenic tumor. This led to the assumption that the mutation was confined to the epithelium [9]. However, recent studies have also revealed the presence of BRAF mutation in the mesenchymal component [10, 11]. Consequently, mixed epithelial and mesenchymal OT have been included in the spectrum of tumors harboring BRAF p.V600E mutation. To the best of our knowledge, there is no comprehensive report in the literature that systematically assesses the incidence of BRAF mutations in these specific groups of mixed and malignant OT.
Hence, the aim of this study is to enhance our comprehension of the occurrence and prevalence of the BRAF p.V600E in patients with benign mixed epithelial and mesenchymal tumors as well as malignant odontogenic tumors. Furthermore, this study outlines the different methods employed for the detection of BRAF p.V600E. The insights gained from this data may pave the way for improved diagnostic and therapeutic strategies.
Materials and Methods
Registry Protocol
The present study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2021 guidelines to identify, select, appraise, and synthesize studies [12]. The methods for this systematic review were recorded on the International Prospective Register of Systematic Reviews (CRD42023445689).
Eligibility Criteria
The studies selected for this review met the criteria based on the population, exposure, comparison, outcome, and study design (PECOS) strategy as follows:
-
P: Patients with benign mixed and malignant odontogenic tumor
-
E: Expression of BRAF p.V600E
-
C: No expression of BRAF p.V600E
-
O: The prevalence of BRAF p.V600E
-
S: Observational studies
The inclusion criteria for the studies considered in this systematic review were as follows: (a) human studies; (b) observational studies (case–control, cohort, or cross-sectional) that evaluated for BRAF p.V600E in patients with benign mixed and/or malignant odontogenic tumors; and (c) studies that used immunohistochemistry (IHC) or molecular methods to assess for BRAF p.V600E.
The exclusion criteria were as follows: (a) studies reported in animals; (b) studies reported as review papers, practice guidelines, letters to the editor, editorials, commentaries, case reports, and pilot studies; (c) studies that do not report the diagnostic methods for detecting BRAF p.V600E; and (d) studies that did not report data relevant for the purpose of this study.
Information Sources and Search Strategy
A comprehensive search of studies published up to August 15, 2023 was performed by two independent authors (RJGSL and CPC), in the PubMed/MEDLINE, Scopus, Web of Science, and Embase electronic databases and ProQuest platform (non-peer-reviewed literature). No restrictions on language or publication date were applied. The search strategy applied in each database is described in Table 1.
After searching each database, duplicates were removed using a software (Rayyan Management Software). The two authors listed and screened the publications based on title and abstracts and assessed their eligibility. Each potentially eligible article was then read in its entirety. Disagreements were resolved through analysis by a third author (MVC), and consensus was reached through discussion.
The same authors conducted a manual search for articles in specific oral pathology journals, including Head and Neck Pathology, Journal of Oral Pathology and Medicine, Journal of Oral Medicine and Oral Surgery, and Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology. Additionally, the authors reviewed the reference list of included studies to identify other relevant studies.
Data Collection Process
Data from the included studies were collected by one author (RJGSL) and cross-checked by the second author (CPC) to ensure the accuracy and completeness of the contents. The collected data included authorship, year of publication, study design, sex and age of the patients, pathology diagnosis, sample size, place of the lesion, method used to detect BRAF p.V600E, and expression of BRAF p.V600E.
Quality Assessment of Included Studies
To assess the methodological quality of the included studies, two review authors (RJGSL and CPC), working independently and blinded to each other, used the JBI’s Critical Appraisal Tool for Analytical Cross-Sectional Studies. Any disagreements were resolved through discussion between the two review authors and, if necessary, by involving a third review author (MVC).
The tool covers important domains such as assessing the definition of inclusion criteria, providing descriptions of study subjects and setting, measuring exposure validity, controlling for confounding factors, measuring outcomes, and conducting statistical analysis, in eight questions. These questions should be answered as either “Yes,” “No,” “Unclear,” or “Not applicable.”
Additional Analysis
An assessment of inter-rater agreement (Kappa coefficient) [13] was conducted during the inclusion of studies. The obtained scores were analyzed as follows: 0 (no agreement), < 0.8 (moderate agreement), or ≥ 0.8 (near perfect agreement). Any disagreement between the investigators were resolved through discussion to reach a consensus.
Results
Literature Search
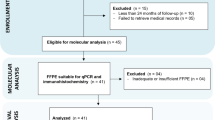
The initial search of the databases identified 387 articles, including 65 in PubMed/MEDLINE, 215 in Scopus, 26 in Web of Science, 17 in Embase, and 64 in ProQuest. After removing duplicate articles, 224 articles remained. The titles and abstracts were read, and the eligibility criteria were applied, resulting in the analysis of 14 articles. After full-text reading, three articles were excluded for the following reasons: no assessment for BRAF p.V600E (n = 1) and no benign mixed or malignant OT (n = 2). Thus, 11 articles were included in this systematic review. A flowchart detailing the search strategy is presented in Fig. 1.
Cohen’s Kappa coefficient was used to calculate the inter-rater agreement during the article selection phase, and it showed “near perfect agreement” between the reviewers RJGSL and CPC (kappa = 1.00).
Description of the Studies
Details about the 11 included studies in this systematic review are presented in Table 2. All included studies were cross-sectional that assessed BRAF p.V600E expression in benign mixed epithelial and mesenchymal or malignant OT using IHC and/or DNA-based molecular methods. A total of 133 patients were evaluated, with 70 having benign mixed epithelial and mesenchymal OT and 63 having malignant OT.
In the benign mixed OT group, the ages ranged from 3 to 23 years. In addition, 15 of the patients were female, 14 were male, and for 41 cases, this data was not reported. The location of the tumors was reported in 29 cases, with the mandible being the most frequent site in 22 cases (31.43%).
In the malignant OT group, the ages ranged from 14 to 91 years. There were 19 cases in females, 31 in males, and in 13 cases, the sex was not reported. As in the previous group, the location of the malignant tumors was reported with a higher incidence in the mandible in 36 cases, while 12 were reported in the maxilla and 15 did not report this data. Furthermore, 47 of the cases were malignant tumors of epithelial origin and 16 of mixed origin.
Among the 11 included studies, only 6 provided information on the recurrence of these tumors, with 5 in malignant OT, namely clear cell odontogenic carcinoma (CCOC), ameloblastic carcinoma (AC), and the odontogenic sarcoma subtype: ameloblastic fibrosarcoma (AFS). Bologna et al. were the only ones to report this information in a mixed OT, the primordial odontogenic tumor (POT), in which recurrence was negative [14].
Expression of BRAF p.V600E and Detection Method
Of the 11 included studies, 6 reported the assessment of BRAF p.V600E using DNA-based molecular diagnostic methods [5, 10, 11, 15,16,17], 1 using IHC alone [14], and 4 reported using both methods [2, 4, 8, 18]. The monoclonal antibody used in IHC was VE1, with only one study by Bologna et al. [14] using a different antibody, RM8. Detailed results of this assessment are presented in the Supplementary Material.
In the benign mixed epithelial and mesenchymal OT group, BRAF p.V600E was reported in ameloblastic fibroma (AF), ameloblastic fibrodentinoma (AFD), ameloblastic fibro-odontoma (AFO), odontoameloblastoma (OA), odontoma (Od), and primordial odontogenic tumor (POT). IHC was performed in 4 tumor types (AF, AFO, Od, and POT), out of the 6 reported. BRAF p.V600E by IHC was detected in 2 of them, AF (20%), and AFO (10%). Meanwhile, by molecular methods, it was expressed in 48.1% of AF, 60% of AFD, and 26.3% of AFO, as well as in the only case of OA, while in the 17 cases of Od, the mutation was wild-type Table 3.
Moreover, when BRAF p.V600E was evaluated by both methods, only Oh et al.[2] reported differences. In the case of AF, the same result was obtained in 11 of the 15 reported cases, while the other 4 cases were negative by IHC and mutant type by molecular methods. Similarly, in the AFO, out of 10 cases in which both methods were performed, 2 cases showed discordant results, negative by IHC and mutant type by molecular method.
In the malignant OT group, BRAF p.V600E was reported in clear cell odontogenic carcinoma (CCOC), ameloblastic carcinoma (AC), ghost cell odontogenic carcinoma (GCOC), and the odontogenic sarcoma subtype: ameloblastic fibrosarcoma (AFS). IHC was performed in 3 tumor types (CCOC, AC, and AFS). BRAF p.V600E was detected by IHC in 22.2% of AC and in 50% of AFS. On the other hand, by molecular methods, it was expressed in CCOC in 14.2%, AC in 41.6%, and AFS in 81.6%, while the only 2 reported cases of GCOC were wild type. No differences were reported when the mutation was evaluated by both methods. A summary of the 11 selected studies and their association with the BRAF p.V600E variant is summarized in Table 3.
Quality Assessment of Studies Included
The risk of bias was analyzed using the JBI’s Critical Appraisal Tool. According to this assessment, the overall quality of the 11 included studies was generally good, with almost all the records scoring “yes” for most domains, related to participant selection, exposure measurement, and outcome assessment. In two studies, Oh et al. [8] and Togni et al. [4] confounding factors were identified, and strategies to address them were described. However, Brown et al. [11] and Oh et al. [2, 8] did not provide information on patient details, such as age, sex, and the location of the lesion. All studies showed “Not applicable” in terms of statistical analysis used. JBI’s assessment of cross-sectional studies for risk of bias and concerns regarding the applicability of studies for this systematic review are available in Table 4.
Discussion
This systematic review included articles that analyzed BRAF p.V600E in patients with benign mixed epithelial and mesenchymal and malignant odontogenic tumors and the methods by which the mutation is detected. Considering the inherent genomic stability of benign tumors, it is possible to identify remarkably distinct genetic fingerprints that identify specific molecular changes that are most likely responsible for tumor development or it can shed light on important aspects of neoplastic progression [19].
Overall, we found a prevalence of the BRAF mutation in mixed OT of 31.42% out of the 70 cases included and 26.98% in malignant OT out of the 63 cases included in this study. This suggests a possible association with more aggressive behavior since the mutation induces an increase in cell proliferation, tumor invasion, and progression. However, no clinical information has been reported in the studies analyzed. Among OT, benign epithelial tumors were the first to report this mutation. Ameloblastoma, being the most studied OT in relation to BRAF p.V600E, with a reported prevalence between 60 and 80%, predominantly in the mandible [20]. To the best of our knowledge and research, we are not aware of any reported cases of mesenchymal odontogenic tumors harboring BRAF p.v600E in the literature. Therefore, it was thought that the mutation was limited to epithelial tissue only. However, Brown et al. reported the mutation for the first time in a benign mixed epithelial and mesenchymal tumor. This prompted further investigations to identify tumors with this mutation, and indeed, additional data are needed due to the rarity of these lesions. It should be noted that within malignant OT, there are those of epithelial, mesenchymal, and mixed origin, so finding the mutation in these tumors was also an important factor in understanding their pathogenesis. Further, these findings raise the possibility of modified, targeted therapeutic options for patients harboring mutated BRAF.
Odontogenesis is controlled by reciprocal signaling between epithelium and ectomesenchyme and is entirely dependent on MAPK/ERK, WNT/ β-catenin, and Sonic Hedgehog signaling pathways. Since the majority of pathogenic mutations in OT disrupt these pathways, it is likely that persistent activation of these pathways plays a role in the tumorigenesis of these lesions [1, 19, 21]. Although the molecular basis of the pathogenesis of OT remains poorly understood, studies in recent years have described pathogenic mutations in components of the MAPK pathway cascade in OT. The success of neoplastic cells may be dependent on changes in the MAPK pathway since it is closely involved in the control of key cellular activities, such as proliferation, survival, growth, metabolism, migration, and differentiation [21].
The MAPK signaling pathway is activated by the B-Raf protein, which is encoded by the BRAF gene, the only RAF gene family member that is regularly mutated in human neoplasia [21]. OT have been shown to harbor a high frequency of BRAF p.V600E, which induces cell proliferation and is capable of promoting transformation, and is therefore classified as an oncogene. Oncogenic mutations, long thought to be exclusive to cancer, can be detected in benign and potentially malignant tumors [7, 19].
Brunner et al. reported that a BRAF mutation was consistently absent in stromal components, suggesting that the mutation appeared to be exclusive to epithelial component [9]. However, this has been strongly refuted, as new studies have shown the presence of a concomitant mutation in the mesenchymal component [2, 4, 8, 10, 11, 14, 17, 18].
Regarding benign mixed epithelial and mesenchymal odontogenic tumors, AF is a rare neoplasm, representing less than 2% of all OT cases. Based on our analysis, it showed 20% positivity for the BRAF p.V600E by IHC, while the DNA-based molecular methods yielded a higher percentage, 48.1%. However, it should be noted that the sample size for the molecular method was almost twice as large, which could explain these results. The mutation has usually been reported as limited to the mesenchymal component, but Coura et al. detected it in the epithelial component in one case. However, the authors suggested that the apparent epithelial mutation could probably be explained by contamination with mesenchymal tissue and the high sensitivity of the qPCR assay, supporting that only the mesenchymal component harbors the BRAF mutation and suggesting that both components be evaluated separately [10].
Based solely on histopathological features, distinguishing between AF and early-stage Od is not possible until they differentiate and mature. This differentiation is important to avoid potentially destructive, unnecessary surgery. Thus, the detection of BRAF p.V600E is important for the differential diagnosis [7]. On the other hand, lesions previously diagnosed as AFD and AFO have previously been classified as developing stages of Od. The histological and molecular overlap makes it unclear whether AFD and AFO are separate entities, intermediate lesions, or a mixture of developing Od and AF [3, 22]. The status of these tumors has been debated for decades, and the current classification as Od is not consistent with the presence of BRAF p.V600E, which suggests a relationship with AF rather than with Od, that lacks this mutation.
In accordance with our results, BRAF p.V600E has been detected in AFD (3/5) and AFO (5/16) by molecular methods and only one case of AFO was positive by IHC (1/10). On the other hand, all cases of Od analyzed by both methods did not harbor the mutation [2, 8, 10]. Odontomas are the second most common tumor, without gender predilection [3]. However, the molecular processes involved in its pathogenesis have not yet been elucidated, with no reported genetic alterations.
OA is another entity that has been excluded from the prior WHO classification of Head and Neck Tumors. Historically, odontoameloblastoma was so-named because it was reported that ameloblastoma could arise in association with an odontoma. However, the WHO considers OA to represent a histologic variant of conventional ameloblastoma [23]. Furthermore, the only case reported by Brown et al. in which BRAF p.V600E was assessed by allele-specific PCR was wild-type [11]. A mutated BRAF (p.V600E) has been reported in 60–80% of conventional ameloblastoma, mostly in the mandible [3]. However, in the aforementioned case of OA, no further epidemiological information has been reported, so no further analysis can be performed and will require additional studies with a larger cohort of this rare, ameloblastoma subtype.
POT is a rare tumor, which was first described in 2014, and subsequently included in the WHO Classification of Head and Neck Tumors in the group of benign mixed epithelial and mesenchymal neoplasms. The name was coined due to its possible development from the early stages of odontogenesis [3, 14]. No mutations were identified by NGS of 151 cancer-associated genes and 42 odontogenesis-associated genes and therefore, their molecular basis remains to be clarified [1, 3]. Furthermore, this tumor does not harbor BRAF p.V600E in the 4 cases evaluated by Bologna et al.[14] It was the only study where other than monoclonal antibody VE1 was used, the RM8, which has few studies in the literature and for which no data on its sensitivity or specificity are not yet available [24]. The BRAF mutation was negative in both the mesenchyme and the epithelial component. Therefore, the absence of BRAF p.V600E positions POT in a different category with respect to ameloblastic lesions.
Comprehensive molecular investigation of malignant OT is hampered by their rarity, even though they are the most clinically important because of their increased morbidity and mortality. In recent studies, malignant OT have also been included in the spectrum of tumors harboring BRAF p.V600E. However, most findings are based on isolated cases or small cohorts of patients.
CCOC is a malignant tumor with a high recurrence rate of 40% [3]. However, of the 11 included cases, BRAF p.V600E was only identified in 1 case by qPCR and confirmed through Sanger sequencing by Diniz et al. [5] On the other hand, the same author evaluated the rare malignant GCOC, of which only 50 cases have been described in the literature, most of them in the Asian population. BRAF mutation was only evaluated in 2 cases by molecular method, which showed the mutation as wild-type [5].
The new edition of the WHO Classification of Tumors positions AC as an entity unrelated to ameloblastoma [3]. However, AC also harbors BRAF p.V600E like other ameloblastoma-related tumors, ranging from 22.2 to 41.6%, by IHC and DNA-based molecular methods, respectively. It is also noteworthy that the sample size evaluated by molecular methods is almost 3 times larger and that the results obtained by IHC coincide with those obtained by PCR and Sanger sequencing. Furthermore, in the studies by Diniz et al. [5] and Niu et al. [16], 87.5% of BRAF mutation-positive cases were in the mandible and among males. Ameloblastic carcinomas frequently exhibit locally aggressive growth [3], which means that they can infiltrate and destroy surrounding structures in the mandible or maxilla. In addition, they might be capable of metastatic dissemination. Treatment of ameloblastic carcinomas usually involves extensive surgery to remove the tumor and nearby affected tissue. Therefore, BRAF mutation-related findings may encourage targeted therapy as an innovative approach in future.
It has been reported that the Odontogenic sarcoma subtype AFS could be caused by malignant transformation of AF [10, 18]. This is a rare, aggressive neoplasm characterized by an ameloblastic epithelial component and a malignant mesenchymal spindle cell stroma [3]. According to our analysis, BRAF p.V600E appeared restricted to the sarcomatous area, ranging from 50 to 81.8%, by IHC- and DNA-based molecular methods, respectively [2, 4, 10, 17, 18]. Coura et al. [10] reported a case where an AFS containing a benign AF region was examined. Both (AFS and AF) included the BRAF mutation in their mesenchymal components, supporting a malignant development from a benign AF precursor, as well as the limitation of the mutation to the mesenchymal component. The molecular data supported the long-held view that the mesenchymal component causes the growth of mixed odontogenic tumors.
Odontogenic tumors, among other lesions, have significant genetic patterns that have been identified due to rapid advances in DNA sequencing technologies. However, IHC cannot be neglected, as it remains an accessible method with a high concordance rate with molecular methods. Furthermore, the use of formalin-fixed, paraffin-embedded tissues is a way to circumvent the challenges posed by these lesions, as these samples are widely available. Moreover, in recent decades, improved techniques have been developed to assess DNA, RNA, proteins, and metabolites in FFPE tissues.
This systematic review provides a comprehensive analysis of odontogenic tumors offering insights into their genetic changes and potential diagnostic and prognostic markers. However, it is essential to acknowledge the study’s limitations. The rarity of these tumors restricted the cohort size, preventing in-depth statistical analysis. Furthermore, variations in BRAF mutation assessment methods and the number of assessors interpreting IHC results may introduce potential biases to be considered in future investigations. In contrast, the studies reviewed consistently demonstrated good quality according to the JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies. This systematic review encompassed internationally recognized databases, enhancing its credibility by presenting a comprehensive overview of the literature. Additionally, each study involved more than one pathologist with expertise in the diagnosis of oral pathology, particularly when addressing rare malignant tumor cases, like CCOC. All included studies have been published in reputable journals and further underscore the study’s reliability of this review. Nevertheless, the study’s limitations stem from the small sample sizes due to the rarity of the tumors, restricting the inclusion of images for all cases. Future research endeavors with more extensive sample sizes are warranted to further explore the implications for BRAF p.V600E in OT. These genetic markers associated with such lesions have the potential to provide clinicians with invaluable insights to improve prognostic accuracy, refine diagnoses, and facilitate informed decision-making, ultimately resulting in improved patient outcomes.
Conclusion
In summary, OT are rare lesions that remain poorly understood and require further investigation. The data analyzed in this systematic review revealed that the BRAF p.V600E variant exhibits a significant prevalence in benign mixed and malignant odontogenic tumors.
Data Availability
The manuscript has data included as electronic supplementary material.
Code Availability
The manuscript has data included as electronic supplementary material.
References
Gomes IP, Bastos VC, Guimarães LM, Gomes CC (2023) The molecular basis of odontogenic cysts and tumours. J Oral Pathol Med. https://doi.org/10.1111/jop.13401
Oh KY, Kim JH, Cho SD et al (2022) BRAF V600E and previously unidentified KRAS G12C mutations in odontogenic tumors may affect MAPK activation differently depending on tumor type. Genes Chromosomes Cancer 61:481–490. https://doi.org/10.1002/gcc.23040
WHO Classification of Tumours Editorial Board (2022) Head and neck tumours, 5th edn. International Agency for Research on Cancer, Lyon
Togni L, Zizzi A, Mazzucchelli R et al (2022) Identification of BRAF V600E mutation in odontogenic tumors by high-performance MALDI-TOF analysis. Int J Oral Sci. https://doi.org/10.1038/s41368-022-00170-8
Diniz MG, Gomes CC, Guimarães BVA et al (2015) Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumor Biol 36:5649–5653. https://doi.org/10.1007/s13277-015-3238-0
Seki-Soda M, Sano T, Ito K et al (2020) An immunohistochemical and genetic study of BRAFV600E mutation in Japanese patients with ameloblastoma. Pathol Int 70:224–230. https://doi.org/10.1111/pin.12899
Buettner R, Gültekin SE (2022) Molecular diagnostics in odontogenic tumors. Pathologie 43:81–85. https://doi.org/10.1007/s00292-022-01152-7
Oh KY, Cho SD, Yoon HJ et al (2021) Discrepancy between immunohistochemistry and sequencing for BRAF V600E in odontogenic tumours: comparative analysis of two VE1 antibodies. J Oral Pathol Med 50:85–91. https://doi.org/10.1111/jop.13108
Brunner P, Bihl M, Jundt G et al (2015) BRAF p.V600E mutations are not unique to ameloblastoma and are shared by other odontogenic tumors with ameloblastic morphology. Oral Oncol. https://doi.org/10.1016/j.oraloncology.2015.07.010
Coura BP, Bernardes VF, de Sousa SF et al (2020) Targeted next-generation sequencing and allele-specific quantitative PCR of laser capture microdissected samples uncover molecular differences in mixed odontogenic tumors. J Mol Diagn 22:1393–1399. https://doi.org/10.1016/j.jmoldx.2020.08.005
Brown NA, Rolland D, McHugh JB et al (2014) Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 20:5517–5526. https://doi.org/10.1158/1078-0432.CCR-14-1069
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. https://doi.org/10.2307/2529310
Bologna-Molina R, Mikami T, Pereira-Prado V et al (2017) Primordial odontogenic tumor: An immunohistochemical profile. Med Oral Patol Oral Cir Bucal 22:e314–e323. https://doi.org/10.4317/medoral.21859
Diniz MG, Duarte AP, Villacis RA et al (2017) Rare copy number alterations and copy-neutral loss of heterozygosity revealed in ameloblastomas by high-density whole-genome microarray analysis. J Oral Pathol Med 46:371–376. https://doi.org/10.1111/jop.12505
Niu Z, Li Y, Chen W et al (2020) Study on clinical and biological characteristics of ameloblastic carcinoma. Orphanet J Rare Dis. https://doi.org/10.1186/s13023-020-01603-5
Magalhães MCSV, Felix FA, Guimarães LM et al (2022) Interrogation of TERT promoter hotspot mutations in ameloblastoma and ameloblastic carcinoma. J Oral Pathol Med. https://doi.org/10.1111/jop.13364
Agaimy A, Skalova A, Franchi A et al (2020) Ameloblastic fibrosarcoma: clinicopathological and molecular analysis of seven cases highlighting frequent BRAF and occasional NRAS mutations. Histopathology 76:814–821. https://doi.org/10.1111/his.14053
Diniz MG, Gomes CC, de Sousa SF et al (2017) Oncogenic signalling pathways in benign odontogenic cysts and tumours. Oral Oncol 72:165–173. https://doi.org/10.1016/j.oraloncology.2017.07.021
Martins-de-Barros AV, Silva CCG, Gonçalves KKN et al (2023) Does BRAF V600E mutation affect recurrence rate of ameloblastomas? Systematic review and meta-analysis. J Oral Pathol Med 52:701–709
Guimarães LM, Coura BP, Gomez RS, Gomes CC (2021) The molecular pathology of odontogenic tumors: expanding the spectrum of MAPK pathway driven tumors. Front Oral Health. https://doi.org/10.3389/froh.2021.740788
Soluk-Tekkesin M, Wright JM (2022) The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2022 (5th) edition. Turk Patoloji Derg 38:168–184. https://doi.org/10.5146/tjpath.2022.01573
Soluk-Tekkeşin M, Wright JM (2018) The world health organization classification of odontogenic lesions: a summary of the changes of the 2017 (4th) edition. Turk Patoloji Derg 34:1–18. https://doi.org/10.5146/tjpath.2017.01410
Martins-de-Barros AV, dos Anjos RS, Silva CCG et al (2022) Diagnostic accuracy of immunohistochemistry compared with molecular tests for detection of BRAF V600E mutation in ameloblastomas: systematic review and meta-analysis. J Oral Pathol Med 51:223–230. https://doi.org/10.1111/jop.13278
Acknowledgements
The authors declare that this study received financial support and that this manuscript, or any part of it, has not been submitted or published and will not be submitted elsewhere for publication while being considered by the Head and Neck Journal.
Funding
This work was supported by grants from the Coordination for the Improvement of Higher Education Personnel (CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
RJGSL contributed to conception, design, data acquisition, analysis, and interpretation and then drafted and critically revised the manuscript. MVC and CPC contributed to conception, design, data acquisition, and interpretation and critically revised the manuscript. SLDM, BCEV and EPP contributed to conception, design, and data analysis and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study does not contain any studies with human participants performed by the author, so no ethical approval was necessary for the work undertaken.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Severino-Lazo, R.J.G., de Vasconcelos Carvalho, M., Campello, C.P. et al. Prevalence of BRAF p.V600E and Detection Methods in Benign Mixed and Malignant Odontogenic Tumors: A Systematic Review. Head and Neck Pathol 17, 1000–1010 (2023). https://doi.org/10.1007/s12105-023-01601-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01601-6