Abstract
Background
Dysregulation of the MAPK pathway appears to exert a pivotal role in the pathogenesis of ameloblastomas, since BRAF p.V600E has been reported in over 65% of the tumors. Therefore, the purpose of this study was to investigate whether the BRAF p.V600E is related to biological behavior and disease-free survival in patients with conventional ameloblastomas.
Methods
This is a retrospective cohort study based on the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations. The study population consisted of individuals treated for conventional ameloblastomas. Clinical, imaging, histomorphological, immunohistochemical (Ki67 and CD138/syndecan-1), and molecular BRAF p.V600E mutation analyses were performed. Bivariate statistical analysis was performed through chi-square and Fisher’s exact tests. Kaplan–Meier analysis with log-rank test and Cox proportional hazards regression were used to identify predictors of disease-free survival, with a significance level of 5%.
Results
Forty-one individuals were included, with a male-to-female ratio of 1.15:1. BRAF p.V600E mutation was identified in 75.6% of the tumors. No association between the BRAF mutational status and other clinical, imaging, histomorphological, and immunohistochemical variables was observed. Only the initial treatment modality was significantly associated with a better prognosis in univariate (p = 0.008) and multivariate (p = 0.030) analyses, with a hazard ratio of 9.60 (95%IC = 1.24–73.89), favoring radical treatment.
Conclusion
BRAF p.V600E mutation emerges as a prevalent molecular aberration in ameloblastomas. Nevertheless, it does not seem to significantly affect the tumor proliferative activity, CD138/syndecan-1-mediated cell adhesion, or disease-free survival outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ameloblastoma is a benign epithelial odontogenic tumor and represents one of the most clinically relevant neoplasms of odontogenic origin [1, 2]. In accordance with the 5th Edition of the Classification of Head and Neck Tumors by the World Health Organization (WHO) [1], this neoplastic entity is categorized into three variants with distinct clinical behaviors: conventional, unicystic, and peripheral. Notably, the conventional ameloblastoma stands as the most prevalent among these variants. Although benign, conventional ameloblastoma is a locally aggressive lesion with the potential to infiltrate and destroy adjacent tissues, and associated with high rates of recurrence [1, 2].
The molecular basis for the pathogenesis of ameloblastomas is not fully understood. Dysregulation of the mitogen-activated protein kinase (MAPK) pathway appears to exert a pivotal role in its development [1,2,3], since a missense activating mutation in the RAF proto-oncogene, specifically identified as BRAF p.V600E, has been reported in over 65% of ameloblastomas [4, 5]. This mutation results in the synthesis of an altered protein that disrupts cell signaling within the MAPK pathway and might compromise important cellular processes, such as growth, proliferation, and differentiation [6]. Interestingly, BRAF p.V600E has emerged as a driver in a number of neoplastic processes, and why this particular variant is such a ubiquitous driver is yet unexplained [4, 6].
Therefore, the purpose of the present study was to investigate whether the BRAF p.V600E mutational status is related to biological behavior and disease-free survival in patients with conventional ameloblastomas.
Materials and Methods
Study Design
This is a retrospective cohort study based on the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations for cohort studies [7] and approved by the Ethics Committee of the University of Pernambuco (#4.309.512; CAAE: 35920620.7.0000.5192).
Study Population
The study population was composed of individuals who underwent surgical treatment for ameloblastomas at the Department of Oral and Maxillofacial Surgery of a tertiary care hospital located in northeast Brazil (Recife, Pernambuco, Brazil), between January 2013 and December 2022. Potential participants were identified from medical records, selected for review, and assessed for eligibility.
This study included individuals of both sexes, all ages, a confirmed diagnosis of conventional-type ameloblastoma, and with a minimum postoperative follow-up period of 24 months to control for possible late recurrence episodes, since ameloblastoma is a slow-growing tumor. Cases in which there was absence or inconsistency in the medical records data, or in which the biological material available was insufficient or inadequate to carry out the proposed molecular and immunohistochemical analyses were excluded from the study.
Clinical and Imaging Analysis
Demographic (gender, age, and skin color) and clinical data related to the tumor (associated symptoms, time of evolution, history of treatments, recurrences, and follow-up time) were retrospectively retrieved from medical records. For analytical purposes, surgical procedures were categorized as radical (e.g., marginal, partial or total resections) or conservative (e.g., enucleation and/or curettage, with or without adjuvant or neoadjuvant methods).
Digital images from computed tomography scans and/or panoramic radiographs archived at the hospital records were used for the imaging analysis. The images were analyzed by two oral and maxillofacial surgeons with experience in interpreting head and neck imaging exams (FACA and TFF). The following imaging aspects were assessed: anatomical location and extent of the lesion, locularity, margins, expansion and resorption of cortical bone, displacement or involvement of teeth, and resorption of adjacent tooth roots.
Histomorphological Analysis
For each included case, slides with 5 µm-thick histological sections of formalin-fixed, paraffin-embedded (FFPE) tumor tissue stained with hematoxylin and eosin were analyzed by two researchers with experience in Oral and Maxillofacial Pathology (AVMB and MVC) to confirm the diagnosis. The histological subtype was defined according to WHO criteria [1], and the cases were classified as follicular, plexiform, acanthomatous, granular cell, basal cell or desmoplastic.
Immunohistochemical Analysis of Proliferation and Invasiveness Markers
Immunohistochemical analysis was performed to analyze the expression of Ki67 and CD138/Syndecan-1. For this purpose, 3-µm-thick histological sections of the FFPE tumor tissue were submitted to automated processing for the immunohistochemical reaction on the Ventana BenchMark XT immunostainer (Ventana Medical Systems), using monoclonal primary antibodies specific for the Ki67 (clone 30-9) (Ventana Medical Systems) and CD138/syndecan-1 (clone B-A38) (Cell-Marque) proteins, following the manufacturer's instructions. Diaminobenzidine (DAB) was used as the chromogen for the reaction using a biotin-free detection system, and counterstaining was performed with Mayer's hematoxylin. Positive controls (Ki67: lymph node; CD138/syndecan-1: oral mucosa) and a negative control (omission of the primary antibody) were carried out for each reaction.
For the analysis of Ki67 nuclear protein immunoexpression and calculation of the cell proliferation index, five randomly selected representative fields were photographed at ×400 magnification. The cell proliferation index was calculated for each case as the total percentage of neoplastic cells with positive nuclear immunostaining, counted in the photomicrographs using the cell counting tool in the ImageJ software [8].
The CD138 protein immunoexpression was analyzed semi-quantitatively at a magnification of 100x. The estimated percentage of neoplastic cells and stromal cells that showed positive immunostaining on the cell membrane was used to classify the cases according to the criteria established by Nadalin et al. [9] as: negative, when the percentage of immunostaining was < 5% of the cells; or positive, when there was positive immunostaining in more than 5% of the cells.
BRAF p.V600E Mutation Detection in Ameloblastoma Samples
From each included case, 10 µm sections of FFPE tumor tissue were processed using the MagMax™ FFPE DNA/RNA Ultra Kit (Applied Biosystems Thermo Fisher Scientific™), according to the manufacturer's instructions, to isolate genomic DNA. The double-stranded DNA concentration and purity were measured in NanoDrop Lite spectrophotometer (Thermo Scientific™) and stored at − 20 °C until molecular analysis.
BRAF p.V600E detection in DNA samples was performed by real-time PCR using 40 ng of template DNA combined with castPCR™ somatic mutation detection assays containing TaqMan™ probes specific for the c.1799 T > A mutant BRAF allele (BRAF_476_mu) and for the reference BRAF gene (BRAF_rf) (Applied Biosystems Thermo Fisher Scientific™). The PCR amplification conditions followed the manufacturer's recommendations. Amplification curves of cfDNA and genomic DNA reactions were separated and imported to Mutation Detector™ software (Thermo Fisher Life Technologies), where the mutational status of the BRAF gene was analyzed. Positive control (confirmed BRAF p.V600E template DNA) and No Template Control (NTC) were included in the reactions.
Statistical Analysis
The database was built on the SPSS® software platform in version 20.0.0. Descriptive and inferential statistics were analyzed to associate the BRAF p.V600E mutation with categorical predictive variables using the chi-square test or Fisher’s exact test, when applicable.
Kaplan–Meier survival analysis was used to estimate disease-free survival (DFS) rates, considering the first tumor recurrence as the main event. Patients who were alive and disease-free were censored at the time of the last follow-up contact recorded in the hospital records. For statistical purposes, the quantitative variables were dichotomized based on the median in this analysis. Statistical differences between Kaplan–Meier curves for disease-free survival in relation to BRAF p.V600E mutational status and other clinicopathological characteristics were analyzed using the log-rank test. Prognostic variables with a p value < 0.20 in the univariate analysis were introduced into a multivariate Cox proportional hazards regression model to identify independent predictors of DFS and calculate their hazard ratio (HR) and respective 95% confidence intervals. A statistical significance level of 5% (p ≤ 0.05) was established.
Results
Clinicopathological Characteristics of Study Participants
The flow diagram of the study participants is shown in Fig. 1. This study included 41 individuals diagnosed with conventional ameloblastoma. The mean age at diagnosis was 34.51 ± 17.73 years, ranging from 11 to 74 years. The tumors were more frequent in brown-skinned individuals (73.2%), with a male-to-female ratio of 1.15:1. Most cases of ameloblastomas were asymptomatic (75.6%) and located in the mandible (97.6%), with only one case affecting the maxilla (Table 1).
The imaging, histomorphological, and immunohistochemical characteristics of the cases are described in Table 2. Multilocular appearance, well-defined margins, expansion and disruption of cortical bone, displacement and root resorption of adjacent teeth were common findings in most ameloblastomas.
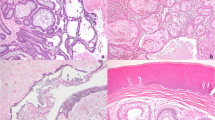
Plexiform and follicular were the most frequent histological subtypes, comprising 36.6% and 31.7% of cases, respectively. The cell proliferation index, calculated according to the nuclear immunoexpression of the Ki67 protein, ranged from 0% to 14.52%, with a mean of 5.07% ± 3.19. In most cases, immunohistochemical expression of the CD138/syndecan-1 protein was positive in the neoplastic epithelium (58.5%) and/or in the tumor stroma (56.1%). Variations in immunoexpression of Ki67 and CD138/syndecan-1 are illustrated in Fig. 2.
Photomicrographs showing variations in the immunoexpression of Ki67 (A, B, C) and CD138/syndecan-1 (D, E, F) in ameloblastomas. A, low (> 1%) Ki67 nuclear immunoexpression (×250). B, medium (between 1 and 10%) Ki67 nuclear immunoexpression (×250). C, High (> 10%) Ki67 nuclear immunoexpression (×250). D, Absence of CD138/syndecan-1 immunoexpression (×250). E, CD138/syndecan-1 immunoexpression in neoplastic epithelial cell membrane (×400). F, CD138/syndecan-1 immunoexpression in tumor stroma (×250)
BRAF p.V600E Mutational Analysis
The BRAF p.V600E mutation was detected in 31 out of 41 ameloblastomas, accounting for 75.6% of the tumors. No association between the mutation and other clinical, imaging, histomorphological, and immunohistochemical variables was observed, as shown in Table 3.
History of Treatments and Clinical Outcomes
Among the included cases, 27 (65.9%) were initially treated with conservative surgical approaches, while the other 14 (34.1%) received radical surgical resection. Nineteen individuals developed tumor recurrence during the follow-up period, with a recurrence rate of 46.3%. However, only one of the individuals treated with radical surgery had recurrence. The time until the first recurrence ranged from 12 to 120 months, with a mean of 46.63 ± 30.63 months. Detailed data on surgical treatment, recurrence and clinical follow-up are available in Tables 1 and 4.
Disease-Free Survival Analysis
All cases were included in the survival analysis. The estimated 5-year DFS rate was 59.5%. Only the initial treatment modality was associated with DFS, in both univariate (p = 0.08) and multivariate analyses (p = 0.030) (Table 5), with a HR of 9.60 (95%IC = 1.24–73.89) favoring radical treatment. Multivariate Cox regression showed no statistically significant differences in the DFS curves regardless of the BRAF mutational status, and other clinical, imaging, histopathological and immunohistochemical predictive variables (Fig. 3).
Discussion
While numerous clinical and molecular parameters have been proposed to potentially correlate with the prognosis of ameloblastomas, their implementation in clinical practice remains very limited [10, 11]. The recent identification of a heightened frequency of BRAF mutations in ameloblastomas raises pertinent questions about the possible impacts of this molecular alteration on the clinical behavior of these tumors. This prompts consideration of the utility of BRAF p.V600E mutation as a reliable prognostic marker and its potential implications for clinical decision-making in the management of ameloblastomas [5, 10, 12, 13].
Although the 5th Edition of the Classification of Head and Neck Tumors considered that BRAF p.V600E mutation could be associated with later recurrence [1], a recent systematic review and meta-analysis [4] showed that the BRAF p.V600E mutation alone seems to not affect the global recurrence of ameloblastomas, in accordance with the results presented in the current study. However, most of the studies included in the review presented major limitations in patient follow-up, and the reported results for recurrence outcomes varied widely among them. In fact, only a few studies suggested that the presence of the BRAF p.V600E mutation in ameloblastomas could be associated with higher recurrence rates and tumor aggressiveness [10, 14]. Additional biological modulatory roles for BRAF p.V600E, such as cell proliferation and invasiveness, were not further explored and remain to be better elucidated [10, 15,16,17,18].
The Ki67 nuclear antigen is a protein expressed during active phases of the cell cycle and is absent in quiescent cells. The assessment of the cell proliferation index based on Ki67 immunoreactivity is valuable for the diagnosis and prediction of the prognosis of various neoplasms, as disruptions in the cellular proliferation process are essential events in oncogenesis [19, 20].
Recent studies have shown that the presence of BRAF p.V600E was associated with high proliferative activity in thyroid carcinomas and congenital melanocytic nevi [21, 22]. Nevertheless, to the authors’ knowledge, only one available study conducted by Fregnani et al. [10] assessed the relationship between BRAF p.V600E and cell proliferation in ameloblastomas. They found that all tumors with high Ki67 proliferation index (> 11%) harbored BRAF p.V600E. In the present study, however, it was observed that although the mean of Ki67 proliferation index in BRAF-mutant ameloblastomas was slightly higher than in BRAF wild-type ameloblastomas (5.44 ± 3.14 vs. 3.91 ± 3.21, respectively), this difference was not statistically significant. Thus, the role of BRAF p.V600E in mediating proliferative activity remains unclear.
The loss of cell adhesion is a molecular event associated with tissue invasiveness and tumor growth in epithelial neoplasms. CD138/syndecan-1 is a transmembrane proteoglycan that plays an important role in the regulation of several biological processes, such as cytoskeleton organization and cell adhesion [23, 24]. Physiological expression of CD138/syndecan-1 was observed in several epithelial tissues, including in those of odontogenic origin [25]. Compared to normal epithelial tissue and odontogenic cysts, ameloblastomas may present a marked reduction in CD138/syndecan-1 immunoexpression in neoplastic cells, associated with variable immunoexpression in the tumor stroma [24, 25]. Although the present survival analysis suggests a trend which may not have reached statistical significance due to the limited sample size, the role of the CD138/syndecan-1 in the pathogenesis of ameloblastoma and other epithelial odontogenic tumors is still unclear, and its immunoexpression levels seem to have little or no correlation with the presence of BRAF p.V600E or with tumor biological behavior.
In this scenario, none of the clinical, imaging, histomorphological, or immunohistochemical features analyzed in this study were associated with the BRAF p.V600E nor with the DFS in ameloblastomas, thus possessing limited value in predicting its prognosis. Nevertheless, individuals with ameloblastomas who were subjected to conservative surgical treatment exhibited a significantly higher risk of recurrence compared to those treated with radical surgical procedures, representing the only variable that reliably predicted DFS in these tumors. Despite this, it is imperative to emphasize that therapeutic decisions for ameloblastomas should not solely rely on the tumor's recurrence potential, as radical surgical treatment is linked to a higher incidence of postoperative complications and sequelae, significantly impacting quality of life [26,27,28].
Given that this is a single-center retrospective study with a limited sample size, it is susceptible to specific limitations inherent in its methodological design. Consequently, the results should be taken in the context of these limitations. The collection of secondary data from hospital records may introduce biases related to the type and quality of available data, which included the impossibility to assess reliable data to calculate tumor size. For this reason, this variable was not included in the present study. Furthermore, a considerable heterogeneity in the total follow-up time among patients was observed, which can distort the recurrence analysis. To control for this limitation, a minimum follow-up period of 24 months was established as eligibility criteria for this study, given that ameloblastoma is a tumor characterized by slow growth and progression, with recurrence possible even decades after initial treatment [2].
In conclusion, the BRAF p.V600E mutation emerges as the predominant molecular aberration in ameloblastomas, with promising diagnostic and therapeutic implications for clinical practice. Nevertheless, it does not seem to exert a significant influence on the tumor proliferative activity and CD138/syndecan-1-mediated cell adhesion. Furthermore, the tumor recurrence rates were not affected by BRAF mutational status, thereby suggesting that the BRAF p.V600E mutation lacks a significant effect on disease-free survival outcomes in patients with ameloblastomas.
Data Availability
All data supporting the findings of this study are available within the paper.
References
WHO Classification of Tumours Editorial Board (2022) Head and neck tumours. Lyon (France): International Agency for Research on Cancer. https://publications.iarc.fr/. Accessed 20 Dec 2023
Effiom OA, Ogundana OM, Akinshipo AO, Akintoye SO (2018) Ameloblastoma: current etiopathological concepts and management. Oral Dis 24(3):307–316. https://doi.org/10.1111/odi.12646
McClary AC, West RB, McClary AC, Pollack JR, Fischbein NJ, Holsinger CF, Sunwoo J, Colevas AD, Sirjani D (2016) Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol 273(7):1649–1661. https://doi.org/10.1007/s00405-015-3631-8
Martins-de-Barros AV, Silva CCG, Gonçalves KKN, Almeida RAC, Silva EDO, Araújo FAC, Robinson L, van Heerden WFP, Carvalho MV (2023) Does BRAF V600E mutation affect recurrence rate of ameloblastomas? Systematic review and meta-analysis. J Oral Pathol Med 52(8):701–709. https://doi.org/10.1111/jop.13458
Kurppa KJ, Catón J, Morganv PR, Ristimäki A, Ruhin B, Kellokoski J, Elenius K, Heikinheimo K (2014) High frequency of BRAF V600E mutations in ameloblastoma. J Pathol 232(5):492–498. https://doi.org/10.1002/path.4317
Cantwell-Dorris ER, O’Leary JJ, Sheils OM (2011) BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther 10(3):385–394. https://doi.org/10.1158/1535-7163.MCT-10-0799
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61(4):344–349
Fulawka L, Halon A (2016) Proliferation index evaluation in breast cancer using ImageJ and ImmunoRatio applications. Anticancer Res 36(8):3965–3972
Nadalin MR, Fregnani ER, Silva-Sousa YTC, Perez DEC (2011) Syndecan-1 (CD138) and Ki-67 expression in odontogenic cystic lesions. Braz Dent J 22(3):223–229. https://doi.org/10.1590/s0103-64402011000300008
Fregnani ER, Perez DEC, Almeida OP, Fonseca FP, Soares FA, Castro-Junior G, Alves FA (2017) BRAF-V600E expression correlates with ameloblastoma aggressiveness. Histopathology 70(3):473–484. https://doi.org/10.1111/his.13095
Yang YC, Wang JJ, Huang Y, Cai WX, Tao Q (2021) Development and validation of a prognostic nomogram for postoperative recurrence-free survival of ameloblastoma. Cancer Manag Res 13:4403–4416. https://doi.org/10.2147/CMAR.S307517
Heikinheimo K, Kurppa KJ, Elenius K (2015) Novel targets for the treatment of ameloblastoma. J Dent Res 94(2):237–240. https://doi.org/10.1177/0022034514560373
Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, Elenitoba-Johnson KSJ, Betz BL (2014) Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 20(21):5517–5526. https://doi.org/10.1158/1078-0432.CCR-14-1069
Martins-de-Barros AV, Anjos RS, Silva CCG, Silva EDO, Araújo FAC, Carvalho MV (2022) Diagnostic accuracy of immunohistochemistry compared with molecular tests for detection of BRAF V600E mutation in ameloblastomas: systematic review and meta-analysis. J Oral Pathol Med 51(3):223–230. https://doi.org/10.1111/jop.13278
Fuji S, Ishibashi T, Kokura M, Fujimoto T, Matsumoto S, Shidara S, Kurppa JK, Pape J, Caton J, Morgan PR, Heikinheimo K, Kikuchi A, Jimi E, Kiyoshima T (2022) RAF1-MEK/ERK pathway-dependent ARL4C expression promotes ameloblastoma cell proliferation and osteoclast formation. J Pathol 256(1):119–133. https://doi.org/10.1002/path.5814
Kondo S, Ota A, Ono T, Karnan S, Wahiduzzaman M, Hyodo T, Rahman ML, Ito K, Furuhashi A, Hayashi T, Konishi H, Tsuzuki S, Hosokawa Y, Kazaoka Y (2020) Discovery of novel molecular characteristics and cellular biological properties in ameloblastoma. Cancer Med 9(8):2904–2917. https://doi.org/10.1002/cam4.2931
Oh KY, Cho SD, Yoon HJ, Lee JI, Ahn SH, Hong SD (2019) High prevalence of BRAF V600E mutations in Korean patients with ameloblastoma: clinicopathological significance and correlation with epithelial-mesenchymal transition. J Oral Pathol Med 48(5):413–420. https://doi.org/10.1111/jop.12851
Diniz MG, Guimarães BVA, Pereira NB, Menezes GHF, Gomes CC, Gomez RS (2017) DNA damage response activation and cell cycle dysregulation in infiltrative ameloblastomas: a proposed model for ameloblastoma tumor evolution. Exp Mol Pathol 102(3):391–395. https://doi.org/10.1016/j.yexmp.2017.04.003
Jabbarzadeh M, Hamblin MR, Pournaghi-Azar F, Saatloo MV, Kouhsoltani M, Vahed N (2021) Ki-67 expression as a diagnostic biomarker in odontogenic cysts and tumors: a systematic review and meta-analysis. J Dent Res Dent Clin Dent Prospects 15(1):66–75. https://doi.org/10.34172/joddd.2021.012
Whitfield ML, George LK, Grant GD, Perou CM (2006) Common markers of proliferation. Nat Rev Cancer 6(2):99–106. https://doi.org/10.1038/nrc1802
Minh TBD, Duc TN, Thanh VPN, Le TD, Tong MD, Nguyen TH, Tuan AL, Nguyen KX, Viet TT, Ta TB, Nguyen ST, Vu HA, Nguyen BV, Ngoc DNT, Quoc VT, Duc TB (2023) Relationships of BRAF V600E gene mutation with some immunohistochemical markers and recurrence rate in patients with thyroid carcinoma. Clin Med Insights Oncol 28(17):11795549231203504. https://doi.org/10.1177/11795549231203503
Chen J, Zhang G, Liu X, Tu P (2023) The association of BRAF V600E gene mutation with proliferative activity and histopathological characteristics of congenital melanocytic nevi in children. An Bras Dermatol 98(4):498–505. https://doi.org/10.1016/j.abd.2022.01.016,2023
Etemad-Moghadam S, Alaeddini M (2017) A comparative study of syndecan-1 expression in different odontogenic tumors. J Oral Biol Craniofac Res 7(1):23–26. https://doi.org/10.1016/j.jobcr.2016.11.001
Carreón-Burciaga RG, González-González R, Molina-Frechero N, López-Verdín S, Pereira-Prado V, Bologna-Molina R (2018) Differences in E-cadherin and syndecan-1 expression in different types of ameloblastomas. Anal Cell Pathol (Amst) 2018:9392632. https://doi.org/10.1155/2018/9392632
Bologna-Molina R, Mosqueda-Taylor A, Lopez-Corella E, Almeida OP, Carrasco-Daza D, Garcia-Vazquez F, Farfan-Morales JE, Irigoyen-Camacho ME, Damián-Matsumura P (2008) Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol 44(8):805–811. https://doi.org/10.1016/j.oraloncology.2007.10.007
Menon S, Kumar V, Archana S, Nath P, Shivakotee S, Hoda M (2019) Ameloblastoma management: “horses for courses” protocol. J Maxillofac Oral Surg 18(3):400–404. https://doi.org/10.1007/s12663-019-01189-x
Hresko A, Burtyn O, Pavlovskiy L, Snisarevskyi P, Lapshyna J, Chepurnyi Y, Kopchak A, Karagozoglu KH, Forouzanfar T (2021) Controversies in ameloblastoma management: evaluation of decision making, based on a retrospective analysis. Med Oral Patol Oral Cir Bucal 26(2):e181–e186. https://doi.org/10.4317/medoral.24104
Bonacina R, Indi A, Massazza G, Rulli E, Gianatti A, Mandalà M, Ameloblastoma Cooperative Group (2022) Correlation of BRAF mutational status with clinical characteristics and survival outcomes of patients with ameloblastoma: the experience of 11 Italian centres. J Clin Pathol 75(8):555–559. https://doi.org/10.1136/jclinpath-2021-207527
Acknowledgements
The authors declare that this study was supported by grants from the Research Support Foundation of the State of Pernambuco, Brazil (FACEPE, Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco) and from the Coordination for the Improvement of Higher Education Personnel (CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Funding
This work was supported by grants from the Research Support Foundation of the State of Pernambuco, Brazil (FACEPE, Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco) and from the Coordination for the Improvement of Higher Education Personnel (CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
The authors AVMB, FACA and MVC contributed to the study conception and design. Data collection from medical records was performed by AVMB, TFF, FACA, and AATA; histomorphological and immunohistochemical analysis were performed by AVMB and MVC; mutation detection and molecular analysis were performed by AGBN, HAMS, ELSL, and MTCM; statistical and survival analysis were performed by AVMB. The first draft of the manuscript was written by AVMB and EDOS. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
This study was approved by the Ethics Committee of the University of Pernambuco (#4.309.512; CAAE: 35920620.7.0000.5192).
Informed Consent
The Ethics Committee of the University of Pernambuco granted an exemption for patient consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martins-de-Barros, A.V., da Costa Araújo, F.A., Faro, T.F. et al. BRAF p.V600E Mutational Status Does Not Correlate with Biological Behavior in Conventional Ameloblastomas: A Disease-Free Survival Analysis. Head and Neck Pathol 18, 23 (2024). https://doi.org/10.1007/s12105-024-01621-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12105-024-01621-w