Abstract
Cold tumors lack antitumor immunity and are resistant to therapy, representing a major challenge in cancer medicine. Because of the immunosuppressive spirit of the tumor microenvironment (TME), this form of tumor has a low response to immunotherapy, radiotherapy, and also chemotherapy. Cold tumors have low infiltration of immune cells and a high expression of co-inhibitory molecules, such as immune checkpoints and immunosuppressive molecules. Therefore, targeting TME and remodeling immunity in cold tumors can improve the chance of tumor repression after therapy. However, tumor stroma prevents the infiltration of inflammatory cells and hinders the penetration of diverse molecules and drugs. Nanoparticles are an intriguing tool for the delivery of immune modulatory agents and shifting cold to hot tumors. In this review article, we discuss the mechanisms underlying the ability of nanoparticles loaded with different drugs and products to modulate TME and enhance immune cell infiltration. We also focus on newest progresses in the design and development of nanoparticle-based strategies for changing cold to hot tumors. These include the use of nanoparticles for targeted delivery of immunomodulatory agents, such as cytokines, small molecules, and checkpoint inhibitors, and for co-delivery of chemotherapy drugs and immunomodulatory agents. Furthermore, we discuss the potential of nanoparticles for enhancing the efficacy of cancer vaccines and cell therapy for overcoming resistance to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer immunotherapy is known as an encouraging modality for the eradication of various types of malignancies. However, its success is limited by the lack of immune cell infiltration and over-expression of co-inhibitory mediators [1]. Tumors with these properties are known as cold tumors. Cold tumors are marked by a hostile tumor microenvironment (TME), which suppresses the immune response and promotes tumor growth [2]. Therefore, there is a need for innovative approaches that can convert immunosuppressive environment of cold tumors into an inflammatory milieu, which is known as hot tumor. Hot tumors are marked by a high degree of immune cell infiltration and a high concentration of inflammatory cytokines and anticancer molecules. These tumors are often responsive to immunotherapy and other therapy agents, and have a better prognosis compared to cold tumors [3]. Understanding the properties of hot tumors is critical for developing effective strategies for their treatment. Therefore, several researchers have focused on identifying new targets for immunotherapy and developing new approaches for enhancing the immune response in cold tumors [4].
Administration of different agents such as some specific drugs, adjuvants, and also nanoparticles have been proposed as promising tools for modulating immune responses and shifting cold tumors to hot tumors [5]. Nanoparticles are small, versatile, and can be engineered to target specific cells or tissues. They can also be designed to carry a variety of payloads, including drugs, natural products, and immunomodulatory agents [6]. The ability of nanoparticles to modulate TME and enhance immune cell infiltration makes them an attractive platform for cancer immunotherapy [7]. The usual forms of most chemotherapy drugs, adjuvants, and immunomodulatory agents have low bioavailability, fast clearance, and low penetration ability for solid tumors. Fast clearance can cause some side effects such as hepatotoxicity and nephrotoxicity following using a high concentration of drugs [8, 9]. Therefore, normal tissue side effects can limit the usage of enough concentrations to overcome cancer resistance. Nanoparticles may also be performed for drug delivery of anticancer drugs without inducing serious impacts on critical normal tissues [10].
In this review article, we will explore the mechanisms underlying the ability of nanoformulations to modulate TME and enhance immune cell infiltration. We will also highlight recent advances in the design and development of nanoparticle-based strategies for shifting cold tumors to hot tumors. These include the use of nanoparticles for targeted delivery of immunomodulatory agents, such as cytokines, natural products, vaccines, and checkpoint inhibitors, and for co-delivery of chemotherapy drugs and immunomodulatory agents.
Tumor microenvironment (TME)
TME has substantial impacts on tumor development, therapeutic resistance, and suppression of immune responses. TME is a dynamic environment that consists of cancer cells with different potency, stromal cells, stem cells, extracellular matrix (ECM), vasculature, and different types of immune cells [11]. The interactions among these components are tightly regulated and contribute to the cancer expansion [12]. Cancer cells are the primary component of the TME and are responsible for driving tumor growth and metastasis. These cells have acquired several genetic and epigenetic modifications that allow them to evade immune surveillance and proliferate uncontrollably [13]. Cancer cells also secrete a variety of factors that modulate the TME, including cytokines, chemokines, growth factors, and extracellular vesicles [14, 15]. Stromal cells are another important component of the TME and consist of fibroblasts, myofibroblasts, adipocytes, endothelial cells, and mesenchymal stem cells. These cells provide structural support to the tumor and secrete a variety of factors that expand tumor and render metastasis [16]. Stromal cells also shape the ECM, a network of proteins, such as collagen and fibronectin, and carbohydrates that shape the tumor stroma [17].
In addition to the mentioned stromal components, TME also includes a variety of immune cells, such as diverse subsets of T and B cells, macrophages, dendritic cells (DCs), natural killer (NK) cells, and neutrophils. CD8 + T lymphocytes, DCs, and NK cells are central players in detecting and removing malignant cells [18]. Cytotoxic CD8 + T lymphocytes (CTLs) are effector type of T cells that maintain the heart of adaptive immunity against cancer. These cells identify and destroy neoplastic cells by recognizing specific antigens expressed by these cells. Antigen presentation involves the identification and uptake of tumor antigens by DCs, which then present them to CD8 + T cells. This process is associated with the engagement among the T cell receptor (TCR) on CD8 + T cells and the major histocompatibility complex (MHC) class I molecules on the surface of DCs [19]. After antigen presentation, CD8 + T cells undergo expansion and differentiation into CTLs. This process is regulated by a complex network of cytokines and other signaling molecules, including IL-2, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) [20]. These cytokines promote the activation, proliferation, and differentiation of CD8 + T cells, allowing them to mount an effective immune response against cancer [21]. DCs and also M1 macrophages are specialized antigen-presenting cells (APCs) that capture and present antigen to immature CD8 + T cells [22]. DCs express a variety of pattern recognition receptors (PRRs) that allow them to recognize specific molecular patterns associated with cancer cells, including damage-associated molecular patterns (DAMPs). Toll-like receptors (TLRs) are the best-known RRRs [23, 24]. In addition to antigen presentation, DCs also express and liberate cytokines, such as IL-12, IL-15, and IL-18, which expand the differentiation of CD8 + T cells into CTLs. DCs can also induce the differentiation of CD4 + T helper cells, which provide support for CTLs by liberating IL-2 and IFN-γ [25].
NK cells are the best-known innate immune cells that find and kill tumor cells without the need for APCs. These cells use cytotoxic granules containing perforin and granzymes, as well as the activation of death receptors on the surface of tumor cells [26]. NK cells express a variety of activating and inhibitory molecules that provide the differentiation of usual and abnormal cells [27]. NK cells can be triggered by the lack of MHC I molecules, which is a common mechanism used by cancer cells to evade recognition by CD8 + T cells [28]. The interactions between CD8 + T lymphocytes, DCs, and NK cells in TME are complex and dynamic. These cells work together in a coordinated manner to detect and eliminate tumor cells, and their activity is regulated by numerous cellular and molecular interactions [29]. In TME, these mechanisms can be disrupted or dysregulated, leading to immune evasion and tumor progression. For instance, DCs may express some co-inhibitory molecules, such as indoleamine 2,3-dioxygenase (IDO), programmed death ligand 1 (PD-L1), and some others [30].
Tregs are the suppressive subset of CD4 + T cells that restrain immune responses. These cells can recruited to the TME following the liberation of some specific chemoattractants [31]. MDSCs are other prominent suppressor cells in TME. These cells can also be recruited to the TME after the liberation of some chemokines [32]. Similar to these cells, some forms of neutrophils and macrophages can repress immune responses by NK cells and CTLs. Neutrophils and macrophages have dual effects on tumor growth. M1 alternative macrophages and N1 neutrophils can render immune activity by liberating inflammatory cytokines and phagocytosis of malignant cells [33, 34]. However, tumor-associated neutrophils (TANs) and tumor-associated macrophages (TAMs) repress the immune system responses and expand to the proliferation of malignant cells by releasing anti-inflammatory cytokines and growth factors [35].
Vasculature is another substantial component of the TME, providing essential nutrients and oxygen to the tumor. However, the vasculature in tumors is often abnormal, with leaky vessels and irregular blood flow [36]. This can lead to hypoxia, which is a hallmark of the TME. Hypoxia expands tumor growth and metastasis by rendering angiogenesis, altering gene expression, and suppressing the immune response [37, 38]. Hypoxia is associated with the regulation of hypoxia-inducible factors (HIFs), which regulate a wide range of metabolic pathways and neovascularization in the tumor. Hypoxia and also irregular metabolism of cancer cells, which is known as the Warburg effect, render the production of acidic metabolites such as lactate [38]. However, the regulation of some transporters such as proton pumps in the tumors can also enhance the acidity of TME [39].
Cold tumors
Cold tumors are a subset of tumors that are characterized by an absence of an inflammatory response. These tumors are often resistant to immunotherapy, chemotherapy, and radiotherapy, and have a poor prognosis [4]. Understanding the properties of cold tumors is critical for developing effective strategies for their treatment. One of the key properties of cold tumors is their lack of immune cell infiltration. This is due to several factors, including the absence of tumor antigens that can be recognized by immune cells, the presence of immunosuppressive factors in TME, and defects in the recruitment of inflammatory cells [40]. Tumor antigens are proteins that are regulated by tumoral cells and can be recognized by immune cells, as explained earlier. However, many cold tumors do not express these antigens, making them invisible to the immune system. This is often due to defects in the antigen processing and presentation machinery, which is responsible for presenting tumor antigens to immune cells [41]. Cold tumors also express a variety of immunosuppressive factors that interfere with the function of CTLs. These factors include cytokines such as TGF-β and IL-10, which promote the differentiation and activation of Tregs and MDSCs [42]. Another factor that contributes to the lack of immune cell infiltration in cold tumors is defects in chemokine expression or defects in the adhesion molecules that are crucial for immune cell trafficking. In addition, cold tumors often lack the inflammatory signals that are essential for NK cell activation [43]. High infiltration of Tregs and MDSCs is associated with overexpression of co-inhibitory molecules, such as immune checkpoints, such as PD-L1 [44]. Stroma rigidity and stiffness in tumors can also create a barrier and cause T-cell exclusion [45]. On the other hand, resident NK cells and CD8 + T cells may be remodeled by the released growth factors and suppressive cytokines such as TGF-β. A high presence of TGF-β in the tumors may reprogram NK cells and T cells toward pro-angiogenic cells. These cells also can’t attack immune cells and aren’t able to release enough inflammatory cytokines, such as TNF-α and IFN-γ [46]. As infiltrated effector T cells and NK cells are crucial for the response of tumors to immunotherapy, cold tumors have a low response to immunotherapeutic modalities such as ICIs [47]. In addition to their resistance to immunotherapy, cold tumors also have a poor prognosis. This is due to their aggressive behavior and their ability to invade to distant organs [48]. Cold tumors often have a high mutation rate, which can lead to the development of drug resistance and the emergence of new tumor clones [49].
Some types of the cold tumors include pancreatic cancer, prostate cancer, ovarian cancer, and glioblastoma [50]. Prostate cancer is common in males, with a unique profile that often evades immune detection [51]. The prevalence of prostate cancer correlate with lifestyle factors, such as smoking, which are more common in men [52]. Ovarian cancer is a female-specific cold tumor that is notorious for its silent progression and immune evasion [4]. Glioblastoma also occurs more frequently in males, with incidence peaking in the elderly population [53]. These patterns underscore the importance of considering gender-specific factors in the study and treatment of cold tumors. Hormonal influences, genetic predispositions, and environmental exposures contribute to the complex interplay that determines the epidemiology of these cancers [54, 55].
Hot tumors
Hot tumors are another shape of tumors that are known by a high infiltration of NK cells and CTLs that provide an inflammatory milieu. These tumors are often responsive to immunotherapy and have a better prognosis compared to cold tumors [56]. One of the key properties of hot tumors is their high degree of infiltration of NK cells and CTLs [57]. These tumors are recognized by the activity of DCs and the presence of tumor antigens that can be recognized by immature CD8 + T cells [58]. On the other hand, the expression of immunosuppressive factors in hot tumors is lower compared to cold tumors [59]. The infiltration of NK cells and effector T lymphocytes in hot tumors provides an inflammatory environment that promotes the recruitment and activation of more anticancer immune cells. This is due to the secretion of specific chemoattractants and cytokines such as IFN-γ, IL-12, IL-2, TNF-α, etc. [60]. Hot tumors have often suitable response to immunotherapy due to high frequency of NK cells and CTLs [61]. In these tumors, ICIs can target the immune checkpoint molecules such as PD-1 and CTLA4 that are expressed on the surface of infiltrated immune cells to prolong the generation of IFN-γ and TNF-α. By blocking these molecules, ICIs can unleash the antitumor immunity to remove malignant cells [62, 63].
In addition to their responsiveness to immunotherapy, hot tumors also have a better prognosis compared to cold tumors. This is due to their lower mutation rate and their lower propensity for metastasis [64]. Hot tumors often have a lower mutation rate compared to cold tumors, which reduces the likelihood of drug resistance and the emergence of new tumor clones. In addition, hot tumors often have a more localized growth pattern, which reduces the likelihood of metastasis to other parts of the body [65].
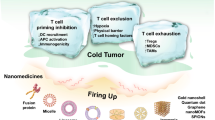
Examples of hot tumors include melanoma, non-small cell lung cancer (NSCLC), bladder cancer, and head and neck tumors [66]. Melanoma is known for its high mutation load and rapid proliferation [67]. NSCLC is characterized by a significant presence of immune cells [68]. Bladder cancer exhibits a strong immune response due to its neoantigen profile [69]. Head and neck cancers are often responsive to immunotherapy due to high T-cell infiltration [70]. The prevalence of these hot tumors varies widely. Melanoma and NSCLC, for example, are more common in populations with high exposure to risk factors, such as UV radiation and smoking, respectively [71, 72]. Bladder cancer and head and neck cancers also have lifestyle and environmental risk factors, such as exposure to certain chemicals, smoking, and human papillomavirus (HPV) infection [73, 74]. Rates of melanoma are slightly higher in males compared to females, possibly due to behavioral differences in sun exposure and protection [75]. NSCLC is more common in males, but the gap is closing as smoking rates in females have increased over time [76]. Bladder cancer is predominantly affects males, with a male-to-female ratio of about 3:1 [77]. Head and neck cancers are also more common in males, which may be linked to higher rates of tobacco and alcohol use [78]. (Fig. 1).
Schematic of cold tumors vs hot tumors. Cold tumors have low numbers of CTLs and high expression of immune checkpoints and pro-angiogenic factors. However, high numbers of CTLs in hot tumors can cause the release of antitumor cytokines and pro-apoptotic molecules for malignant cells. (Created with BioRender.com)
Treatment options for rendering inflammatory responses in the tumor
As explained, cold tumors have a low number of inflammatory cells, such as Th1 and CD8 + T lymphocytes. However, anti-inflammatory cells such as Tregs, M2 macrophages, MDSCs, Th2 cells, and CAFs have a high activity in these tumors. Some therapeutic agents such as radiotherapy, some particular chemotherapy drugs, ICIs, and also photodynamic and photothermal therapy may cause inflammatory responses in the tumors. ICIs, particularly anti-PD-L1 and anti-PD-1 agents, can repress the PD-L1/PD-1 axis, leading to more liberation of IFN-γ and TNF-α by CTLs [79]. The release of these cytokines not only renders apoptosis and tumor regression but also triggers other inflammatory cells, including Th1 cells, M1 macrophages, and NK cells [80]. However, experimental studies have suggested that treatment with an ICI such as an anti-PD-L1 or anti-PD-1 antibody may stimulate the regulation of other co-inhibitory mechanisms such as CTLA4 and TIM-3 by DCs or other immune cells [81]. Therefore, some experiments have suggested dual blockade of immune checkpoints for improving the activity of CTLs and more release of inflammatory cytokines [82].
Radiotherapy and certain chemotherapy drugs such as cisplatin, anthracyclines, oxaliplatin, and cyclophosphamide have been observed to induce immunogenic cell death (ICD) and inflammatory responses in tumors, which can generate a "hot" environment within the tumor [83]. Radiotherapy and also the mentioned drugs act by producing ROS and damaging the DNA and other critical components of the cancer cells. These damages can induce various forms of regulated cell death, including apoptosis, ferroptosis, necrosis, and autophagy. Interestingly, these cell death mechanisms can trigger ICD, which involves the release of DAMPs from dying cancer cells [84, 85]. DAMPs act as danger signals, alerting the immune system to the presence of cellular damage [86]. One key DAMP that is released during ICD is high mobility group box 1 (HMGB1). HMGB1 binds to receptors on DCs, macrophages, NK cells, and T lymphocytes, leading to more antigen presentation and higher inflammatory responses [87, 88].
Photodynamic therapy and photothermal therapy are other agents that can render ICD and maturation of DCs and CTLs in solid cancers. Photothermal and photodynamic therapy are both forms of cancer treatment that utilize light energy to induce cell death in tumors. Although they primarily target tumor cells, they can also induce inflammatory responses in the TME [89, 90]. Photodynamic therapy uses photosensitizing agents that accumulate selectively in tumor tissues. Upon activation with light of a specific wavelength, photosensitizing agents generate a high concentration of ROS, such as singlet oxygen, which can cause direct damage to tumor cells or initiate a cascade of events leading to their destruction. However, severe oxidative stress and necrosis following this form of therapy can render ICD and inflammatory responses [91]. Photothermal therapy uses photothermal agents, which generate heat upon exposure to specific wavelengths of light. These photothermal agents can be delivered to the tumor site through various methods, such as nanoparticle-based systems or laser-activatable molecules. When illuminated with laser light, photothermal agents convert light energy into heat, leading to thermal ablation of tumor cells. The heat generated by photothermal therapy can significantly increase the local temperature within the tumor. This thermal effect can cause stress and damage to tumor cells, leading to cell death. In addition, elevated temperatures can induce heat shock proteins and the release of DAMPs [92]. Both mentioned modalities can render inflammatory responses and also amplify the antitumor efficacy of other treatment options such as chemotherapy and immunotherapy [93, 94].
Nanoparticles in cancer therapy; types and properties for targeting tumors
Nanotechnology has suggested numerous intriguing approaches for the diagnosis, treatment, and prevention of cancer [95]. Nanoparticles, characterized by their dimensions in the nanometer range, have demonstrated significant potential in cancer therapy owing to their distinctive attributes such as high surface area, reactivity, and the ability to traverse biological membranes [95]. This versatility and unique properties make nanoparticles an encouraging mean in cancer therapy, with vast potential for further exploration and innovation. As research in this domain progresses, it is anticipated that there will be more innovative applications of nanoparticles in cancer treatment. This section aims to explore various types of nanoparticles that can be harnessed in cancer therapy, encompassing inorganic nanoparticles such as metal nanoparticles, mesoporous nanoparticles, carbon nanoparticles, and organic nanoparticles including micelles, polymeric nanoparticles, lipid nanoparticles, exosomes, dendrimers, and nanoemulsions.
Inorganic nanoparticles, a subset of nanocarriers, find utility in biomedicine, particularly for drug delivery. These nanoparticles are crafted from diverse inorganic agents such as metals, ceramics, and carbon [96]. Gold nanoparticles (AuNPs) stand out as one of the most renowned and applicable metal nanoparticles in biomedicine. AuNPs have garnered significant attention in cancer therapy because of their safety and distinctive physical characteristics [97]. They can be readily synthesized and modified with different molecules such as antibodies, peptides, or drugs for targeted delivery into tumors [98]. Moreover, they can elaborate radiotherapy and photothermal therapy efficacy by absorbing light rays and converting them into heat to selectively destroy adjacent malignant cells [99]. In addition, AuNPs serve imaging purposes, enabling the detection and monitoring of tumors [100]. Iron oxide nanoparticles (IONPs) represent another category of metal nanoparticles that have been extensively examined in cancer research studies. IONPs can be employed for magnetic resonance imaging (MRI) to detect tumors and monitor treatment responses [101]. They can also be utilized for magnetic hyperthermia, where an alternating magnetic field is applied to generate high temperatures within tumors and selectively eliminate malignant cells [102]. Furthermore, IONPs can be loaded with drugs or genes and targeted to specific tumor sites using magnetic targeting techniques [103]. Other metal nanoparticles such as silver and titanium nanoparticles have been developed for targeting cancer cells [104].
Mesoporous nanoparticles (MSNs) are silica-based nanomaterials with a high surface area and large pore volume that exhibit compelling properties for delivering anticancer agents [105]. The substantial surface area also allows for the adsorption of proteins, enzymes, or nucleic acids, further enhancing their therapeutic potential [106]. MSNs feature numerous pores on their surface, which can be modified to open under specific conditions. For instance, MSNs can be functionalized to release anticancer agents in a controlled manner in the low pH environment of tumors. The delivery of anticancer drugs using these nanoparticles can reduce the toxicity to other organs and release a high concentration of drugs within the tumor stroma [107]. Carbon nanoparticles, including carbon nanotubes, carbon dots, fullerenes, and graphene oxide, are among the major inorganic forms of nanoparticles and possess unique physicochemical properties that make them attractive for delivering cancer drugs [108]. Carbon nanotubes can be employed for targeted drug delivery and photothermal therapy [109]. They can also be functionalized with specific molecules or RNAs to target antigens expressed by neoplastic cells [110]. On the other hand, graphene oxide has demonstrated promise in gene delivery and photodynamic therapy, generating ROS upon irradiation with light to eliminate cancer cells [111].
In addition to inorganic nanoparticles, various forms of organic nanoparticles are employed for cancer therapy. These nanocarriers are crafted from biodegradable molecules such as lipids and polymers [112]. Micelles, a prevalent form of organic nanoparticles, consist of amphiphilic molecules containing a hydrophobic core and a hydrophilic shell, enabling the encapsulation of water-insoluble anticancer drugs [113]. Micelles provide a high solubility for these drugs, prolong the circulation time, and augment accumulation in tumors through the enhanced permeability and retention (EPR) effect [114]. Polymeric nanoparticles are produced from natural or artificial polymers and can be tailored to provide requirements for drug delivery [115]. These nanoparticles can encapsulate both hydrophobic and hydrophilic drugs and exhibit controlled release properties [116].
Lipid nanoparticles, such as liposomes and solid lipid nanoparticles (SLNs), are among the most valuable organic nanoparticles for drug delivery [117]. Liposomes are vesicular structures composed of lipid bilayers that can encapsulate both hydrophobic and hydrophilic drugs [118]. SLNs, on the other hand, are solid particles composed of lipids that can carry hydrophobic agents [119]. They offer improved stability and controlled release properties compared to liposomes [120]. Nanoemulsions represent another form of organic nanoparticles. Nanoemulsions are containing colloidal dispersions of oil and water. They have small size and high stability, which facilitate high capacity for hydrophobic or hydrophilic agents. Nanoemulsions can enhance the solubility and bioavailability of drugs and improve their targeting of tumors through passive or active targeting strategies [121].
Exosomes and dendrimers serve as other organic nanocarriers for drug delivery. Exosomes, naturally occurring extracellular vesicles that participate in intercellular communication [122]. They can be isolated from various cell types, including cancer cells, and loaded with anticancer drugs or therapeutic nucleic acids [123]. Exosomes have shown promise in targeted drug delivery and immunotherapy, as they can be modified to specifically target cancer cells and deliver therapeutic payloads [124]. Dendrimers, highly branched nanoparticles with a well-defined structure, can be synthesized from various polymers, such as polyamidoamine (PAMAM) or polyethyleneimine (PEI) [125]. Dendrimers can encapsulate drugs, genes, or imaging agents and be functionalized with targeting ligands for specific delivery to cancer cells. They have also shown potential in photodynamic therapy and gene therapy [126] (Fig. 2).
Strategies for shifting “cold” to “hot” tumors: potential of nanoparticles, products, and drugs
Shifting cold tumors to hot tumors is a major goal in cancer immunotherapy, as it can increase the efficacy of existing immunotherapies and expand the number of patients who can benefit from these treatments. In this section, we overview the different mechanisms for shifting cold to hot tumors and the potential of nanotechnology for this aim.
Inducing immunogenic cell death (ICD)
ICD is a form of cell death that elicits an immune response, leading to the activation of inflammatory processes. It is a crucial process in cancer therapy as it helps to enhance the efficacy of treatment and improve long-term outcomes [127]. Radiotherapy and chemotherapy drugs have been shown to induce ICD [128], therefore, understanding the cellular and molecular mechanisms behind this process in TME is essential for developing novel therapeutic strategies. The cellular and molecular mechanisms underlying immunogenic cell death in TME involve the interaction between dying cancer cells, immune cells, and TME components [129]. During ICD, dying cancer cells release DAMPs, including calreticulin, ATP, and HMGB1 [130]. Calreticulin is a key molecule involved in the recognition of dying cells by DCs. It is normally located in the endoplasmic reticulum (ER) and plays a role in protein folding and calcium homeostasis. However, during ICD, calreticulin can translocate to the cell surface, which facilitates their recognition and uptake by DCs [131]. ATP and other extracellular DAMPs also act as a "find me" signal, attracting DCs to the site of cell death, promoting the maturation of DCs. This activation leads to the upregulation of co-stimulatory molecules and the production of pro-inflammatory cytokines, which are essential for the activation of T cells [132].
Radiotherapy is a commonly used treatment modality for cancer. In addition to apoptosis, high doses of radiotherapy can induce ICD [133]. Nanoparticles may be utilized for rendering ICD by radiotherapy. Metal nanoparticles are promising agents for inducing ICD in combination with radiotherapy. These nanoparticles, such as gold, iron, and hafnium oxide nanoparticles, have high atomic numbers, which cause more absorption of X-rays and more production of ROS by radiotherapy [134, 135]. Radiotherapy in combination with hafnium oxide nanoparticles has been shown to induce immunogenic responses through a phenomenon called the abscopal effect [136]. This effect is triggered following ICD and the liberation of DAMPs in the tumor. The abscopal effect is associated with infiltration of CTLs in the tumors and also distant metastasis in other organs [137, 138]. These nanoparticles have shown safety and good treatment efficacy in clinical trial studies for both male and female [139, 140].
Several chemotherapy drugs have also been found to induce ICD. Anthracyclines such as doxorubicin and daunorubicin are common drugs for various cancers [141]. These drugs induce DNA damage and disrupt cellular homeostasis, leading to the release of DAMPs [142]. Another example is oxaliplatin, a platinum-based chemotherapy drug used in the treatment of colorectal cancer. Oxaliplatin induces ICD through the activation of calreticulin [143]. One approach to utilizing nanoparticles for inducing ICD involves their use as carriers for chemotherapy drugs. Traditional chemotherapy drugs can induce ICD in certain cases, but their effectiveness is often limited by poor tumor targeting and systemic toxicity. By encapsulating these drugs within nanoparticles, their delivery to the tumor site can be improved, resulting in higher concentrations of the drug within the tumor and reduced exposure to healthy tissues [144]. This targeted delivery can augment ICD by increasing the concentration of the drug within cancer cells, leading to more efficient cell death and release of DAMPs [145].
Various nanoformulations are available for the delivery of chemotherapy drugs into tumor stroma. These nanoformulations have been examined in combination with immunotherapy to amplify immune system activation by ICIs. This combination can also provide prolonged antitumor immunity by blocking immune checkpoints after activation and maturation of DCs and CTLs [146]. Nanoparticles can be designed to concentrate in the cancers through EPR effects and also activate targeting by functionalizing these nanoparticles by specific ligands [147]. Nanoparticles can also deliver a combination of these drugs to amplify cancer cell killing without serious side effects in normal tissues. Guo et al. developed a polymeric nanocomplex containing fluorouracil (5-Fu) and oxaliplatin for rendering ICD and better elimination of colorectal cancer cells. They showed that this nanocomplex renders ROS generation and ICD in tumors, thereby inducing a synergistic effect on the treatment outcome. In addition, this nanocomplex could significantly improve the therapy efficacy of anti-PD-L1 therapy [148]. Similar findings have been reported after treatment with doxorubicin–poly(lactic-co-glycolic acid (PLGA)–polyethylene glycol (PEG) and anti-PD-L1 therapy [149]. This synergistic effect has also been reported following co-delivery of doxorubicin and a TLR7/8 agonist [150].
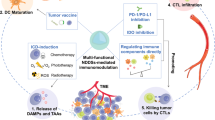
Photodynamic and photothermal therapies are other modalities that by which nanoparticles can induce ICD and subsequent immune responses [90]. Both these modalities offer the potential for targeted tumor therapy and alleviating damage to surrounding organs [151]. Nanoparticles used in these therapies can be designed with tailored properties to enhance their light absorption capacity, stability, biocompatibility, and targeted delivery to TME [152]. For instance, gold nanoparticles have demonstrated exceptional ability to absorb light and convert it into heat for photothermal therapy [153], while photosensitizers such as porphyrins can be delivered by different nanocarriers such as quantum dots, metal nanoparticles, graphene oxide, and MSNs have shown promise in photodynamic therapy [154]. Notably, the use of nanoparticles in photothermal therapy and photodynamic therapy has been shown to potentiate the induction of ICD, thus priming the immune system to recognize and attack cancer cells, which can significantly augment the efficacy of immunotherapy [155, 156] (Fig. 3).
Amplification of radiotherapy, chemotherapy and photodynamic or photothermal therapy by nanoparticles can render ICD in cold tumors. ICD is associated with the liberation of DAMPs and overexpression of TLRs, leading to the maturation of DCs and CTLs. The delivery of ICIs by nanoparticles can also enhance the efficacy of ICD by more inducing CTLs. (Created with BioRender.com)
Toll-like receptor (TLR) agonists
The utilization of TLR agonists represents a compelling strategy in cancer immunotherapy aimed at triggering potent inflammatory responses by activating NK cells and CTLs [157]. TLR agonists have garnered significant interest due to their potential to induce a pro-inflammatory milieu within the TME and to bolster antitumor immune responses mediated by NK cells and CTLs [158]. One of the key mechanisms through which TLR agonists exert their effects involves the activation of DCs [159]. Upon recognition of TLR agonists, DCs undergo maturation, leading to increased antigen presentation. This maturation process primes the immune system for a heightened response, facilitating the activation and recruitment of NK cells and CTLs to the TME [160]. TLR agonists, by promoting DC maturation and enhancing antigen presentation, effectively prime CD8 + T lymphocytes for recognizing tumor-specific antigens and mounting robust immune responses. This activation and amplification of CD8 + T lymphocytes within the TME drive the targeted killing of cancer cells, ultimately contributing to the containment and eradication of the tumor [161]. Some TLR agonists have been tested and utilized in preclinical and clinical studies. Imiquimod is a synthetic TLR7 agonist that has been investigated for its immunostimulatory properties in cancer therapy. Imiquimod has been utilized in the treatment of skin cancers, where it induces ICD and triggers antitumor immune responses within the TME. Resiquimod is a TLR7/8 agonist that acts by inducing the production of pro-inflammatory molecules, leading to the maturation of DCs and the activation of CTLs. Poly-ICLC, a TLR3 agonist, is another TLR synthetic agonist that has demonstrated immunomodulatory effects. By engaging TLR3 on immune cells, poly-ICLC induces the production of IFN-γ, contributing to the activation of DCs and the enhancement of antigen presentation [162].
Numerous experiments have examined the potential of nanoparticle-loaded TLR agonists for targeting tumor stroma. These experiments suggest that nanoparticle-loaded TLR agonists can induce antitumor immunity and remodel tumor stroma by hampering immunosuppressive TME. For instance, nanoparticle-loaded resiquimod has been shown to sensitize tumors to cisplatin and doxorubicin by remodeling antitumor immunity. It has been uncovered that cisplatin and doxorubicin can render ICD and initiate inflammatory responses in the tumor. On the other hand, nanoparticle-loaded resiquimod can amplify immune responses by rendering DC maturation and activation of CTLs [163]. In addition, biodegradable and tumor-sensitive nanoparticles have shown the potential to deliver TLR agonists into tumors without significant systemic toxicity. In mice-bearing colon tumors, ROS-sensitive MSN-loaded resiquimod has shown the potential to eradicate tumors by inducing inflammatory responses such as the maturation of DCs and CTLs and subsequent releasing TNFα and IL-12 [164]. Some other findings are explained in Table 1.
Immune checkpoint inhibitors (ICIs)
ICIs are drugs that target and block specific immune checkpoints, allowing the immune system to trigger effective antitumor responses [176]. PD-L1 is often overexpressed on cancer cells, and its interaction with PD-1 on CTLs inhibits their activity, leading to immune suppression and tumor escape [177]. By blocking the PD-1/PD-L1 interaction using ICIs, such as pembrolizumab or nivolumab, the inhibitory signals are removed, and CTLs can regain their ability to recognize tumoral cells [178]. This can lead to tumor repression and improved patient prognosis in various types of malignancies such as lung cancer [179]. CTLA4 is another immune checkpoint that can be blocked by ICIs for rendering CTLs [180]. However, T cell immunoreceptor with Ig and ITIM domains (TIGIT) can be suggested for recovering the function of NK cells [181]. The precise delivery of ICIs to the tumor stroma is a critical challenge in cancer therapy [182]. Systemic administration of ICIs can result in off-target effects such as brain, lung, and cardiovascular toxicity, and limited penetration into TME [183]. Nanoparticles can help to overcome these limitations and improve the delivery of ICIs to the TME. By encapsulating ICIs within nanoparticles and engineering them to target specific cells or tissues, their delivery to the tumor site can be enhanced, resulting in higher concentrations and reduced systemic toxicity [184].
Numerous experiments have designed nanoparticles for the delivery of ICIs. An experiment showed that an anti-PD-1 nanoformulation has a higher efficacy compared to a free form of anti-PD-1. This study exhibited that anti-PD-1-loaded PEG–PLGA can penetrate and distribute into TME more effectively compared to free anti-PD-1. Image analysis of mice-bearing tumors confirmed more accumulation of nanodrugs in the tumor and very lower distribution in normal tissues. This was associated with more effective infiltration of CTLs against head and neck cancer cells [185]. Similar findings were reported following administration of PLGA-anti-PD-1 for mice-bearing melanoma tumors [186].
Silencing PD-1/PD-L1 gene expression using specific small molecules or siRNAs is another approach for targeting ICIs by nanoparticles. A study by Wu et al. investigated the capacity of two nanoparticles for PD-1 gene silencing. They utilized layered double hydroxide and lipid-coated calcium phosphate nanoparticles for delivering PD-1 siRNA to T lymphocytes. The findings revealed that both nanoparticles effectively delivered PD-1 siRNA into T lymphocytes, resulting in a significant knockdown of PD-1 expression. However, lipid-coated calcium phosphate nanoparticles demonstrated superior transfection efficiency, higher gene silencing efficacy, and cellular uptake compared to layered double hydroxide nanoparticles [187]. Another study developed lipid-coated calcium phosphate nanoparticles to deliver both PD-1 and PD-L1 siRNAs to MCF-7 breast cancer cells and T lymphocytes. The findings showed that these nanoparticles can effectively repress the regulation of PD-L1 in cancer cells. On the other hand, a significant augment in the INF-γ production was reported [188]. A study by Jeong et al. utilized a β-hairpin peptide isolated from an engineered PD-1 protein to target PD-L1 in tumors. They developed to conjugate β-hairpin peptides with dendrimer nanoparticles to enhance their stability and multimerize their binding sites. The study used biophysical and cellular assays to evaluate the stability, binding affinity, and efficacy of the peptide–nanoparticle conjugates in blocking PD-1/PD-L1 interactions. The results demonstrated that the dendrimer conjugation significantly stabilized the β-hairpin peptides and improved their binding affinity towards PD-L1. Furthermore, the multivalency of the peptide–nanoparticle conjugates enhanced their blocking efficacy, leading to more effective inhibition of PD-1/PD-L1 interactions. This study highlights the potential of nanoparticle conjugation as a strategy to augment the stability and targeting ability of β-hairpin peptides in targeting the PD-1/PD-L1 pathway and suggests their potential application in cancer immunotherapy [189].
The development of nanoparticles for the delivery of clustered regularly interspaced short palindromic repeat (CRISPR) is another approach for targeting immune checkpoints. Zhang et al. developed a dual-responsive polymeric nanoparticle to deliver CRISPR/Cas13a to tumors by responding to the unique environment of TME. Nanoparticles consisted of a core–shell structure, where the CRISPR/Cas13a encapsulated inside the core of nanocarriers. The CRISPR/Cas13a system was carefully monitored to target PD-L1 in melanoma-bearing mice. The findings of this experiment suggested successful disruption of the PD-1/PD-L1 axis and infiltration of CTLs [190]. Another study showed successful delivery and activation of antitumor immunity by delivering ROS-responding nanoparticles loaded with CRISPR/Cas13a. Nanoparticles have been designed to deliver both CRISPR/Cas13a and SN38 as an immunomodulatory prodrug. The CRISPR/Cas13a component was designed to specifically target and edit the RNA of PD-L1. The findings demonstrated that the CRISPR/Cas13a-loaded nanoparticles effectively edited the RNA of PD-L1, leading to downregulation of PD-L1 in the tumor. This knockdown of PD-L1 was associated with a significant augment in the infiltration of CTLs and the liberation of antitumor molecules by these cells. Furthermore, the SN38 component of the nanoparticles demonstrated long-lasting release kinetics, sustaining the activation of T cells by rendering ICD in the tumor. The treatment led to a significant reduction in tumor volume and prolonged survival compared to control groups [191].
Co-delivery of anti-PD-1 and other antitumor agents such as chemotherapy drugs and photosensitizers is another intriguing approach for remodeling TME by nanoparticles-loaded ICIs. An experiment by Lan et al. developed pH-sensitive lipid nanoparticles loaded with PD-1 antibody and cisplatin against skin and oral tumor xenografts. The findings revealed a significant augment in the infiltration of CTLs and concentration of IFN-γ following treatment with the mentioned nanoformulation. Co-delivery of anti-PD-1 and cisplatin was also more effective in removing cancer cells and repressing tumor volume. Nanoparticles could also ameliorate side effects associated with antitumor drugs in normal tissues [192]. Similar synergistic effects have been reported following treatment with polymeric nanoparticles loaded with anti-PD-1 and docetaxel, paclitaxel, or doxorubicin [193, 194]. Smart nanoparticles such as pH-sensitive nanoparticles loaded with anti-PD-1 have also been shown promising to target TME and increase the efficacy of chemotherapy [195]. Nanoparticles loaded with anti-PD-1 have also been investigated for their synergistic effects in combination with ICD inducers, and hypoxia and angiogenesis inhibitors [196,197,198,199].
Stimulating antigen-presenting cells (APCs) and antigen presentation
As explained, one of the key factors that determine whether a tumor is hot or cold is the level of antigen presentation. Tumors with high levels of antigen presentation are more likely to be removed by CTLs. However, tumors have developed intricate pathways to evade immune surveillance, containing impairing antigen presentation, thus hampering an effective antitumor immune response [200]. Therefore, enhancing antigen presentation is a promising strategy for shifting cold to hot tumors [201]. One way to enhance antigen presentation is to increase the expression of MHC I on malignant cells [202]. Several studies have revealed that augmenting MHC I expression on tumor cells can enhance the efficacy of immunotherapy in animal models and human patients [200]. Another way to enhance antigen presentation is to augment the uptake and processing of antigens by DCs. Numerous approaches have been developed to trigger DC function, including the use of TLR agonists, cytokines, and adjuvants [203].
One promising approach involves leveraging the potential of nanoparticles loaded with tumor antigens or other agents to facilitate enhanced antigen presentation, leading to a robust antitumor immunity. Zwiorek et al. developed one of the first nanodrugs for the delivery of tumor antigens. In this study, the researchers used cationic gelatin nanoparticles to deliver cytidine phosphate guanosine (CpG) oligodinucleotides (ODN; TLR9 agonist) to DCs. The primary findings exhibited that these nanodrugs can remarkably penetrate murine DCs and render the maturation of these cells. These effects were associated with the production of IL-12 and TNF-α [204]. CpG-loaded Pluronic F127-stabilized poly(propylene) sulfide nanoparticles are another form of CPG nanodrugs that have been used for tumor targeting. Adjuvant delivery particles can also induce the maturation of DCs to increase efficiency for tumor-draining lymph node delivery. These nanoparticles have shown the ability to render the function of CTLs while reducing Treg numbers in melanoma tumors [205]. Some experiments have been conducted to control the freedom of CpG antigens from nanoparticles for efficient immunization of tumors. For instance, an experiment developed AuNPs loaded CPG using spacers to facilitate the liberation of CpG from nanoparticles. In this experiment, the size of nanoparticles was reduced to improve the immunization of tumors by increasing the infiltration and antitumor activity of macrophages and DCs. The CpG-AuNPs exhibited more efficacy on immune cells to release antitumor molecules, such as TNF-α. These immunomodulatory impacts led to obvious tumor retardation and improved survival rate in mice-bearing melanoma compared to mice that received free CpG [206].
In addition to TLR agonists, tumor antigens and neoantigens are other important mediators for tumor vaccination and inducing inflammatory responses in the tumor. Neoantigens are a class of tumor antigens that arise from somatic mutations in malignant cells. These antigens are unique to each patient's tumor and are identified as foreign by CTLs [207]. As such, neoantigens have achieved remarkable attention in cancer immunotherapy due to their capacity to induce antitumor responses. By presenting neoantigens to the immune system, either through vaccination or checkpoint blockade, researchers aim to trigger potent antitumor immunity within the TME [208]. By leveraging the immunogenicity of shared tumor-associated antigens (TAAs), researchers seek to stimulate broad anti-tumor immune responses within the TME, potentially conferring protection against a range of cancer types [209].
Some studies have investigated the capacity of nanoparticles loaded with tumor antigens. A study explored the potential use of polymer nanoparticles for carrying TAAs and also and whole tumor cells. The researchers observed that the immune cells stimulated with the nanoparticles showed an increased production of cytokines and activation markers, indicating an enhanced immune response against the cancer cells. To examine the immunomodulatory impacts of nanoparticles on TME, female C57BL/6 mice were treated with either the nanoparticles containing TAAs, whole tumor cells, or a control group receiving no treatment. The results demonstrated an obvious tumor size drop in mice that treated with nanoparticles compared to non-treated mice. In addition, the nanoparticles induced the infiltration of immune cells, indicating an enhanced immune response by CTLs against cancer cells [210]. Another experiment examined the potential of PLGA-loaded TAA and tumor lysates on DCs and head and neck squamous carcinoma tumors. DCs exposed to nanoparticles loaded with tumor lysate antigens demonstrated enhanced activation, as evidenced by increased expression of activation markers and secretion of pro-inflammatory cytokines. The loaded DCs also displayed improved antigen uptake and presentation, leading to enhanced T-cell activation.
Some other experiments have developed combinational modalities to render the activity of antitumor immunity following antigen delivery. In an experiment, the researchers designed and synthesized lipid-enveloped polymeric nanoparticles that encapsulate different antigen peptides. These nanoparticles were made of a polymeric core from biodegradable polymers and then coated with a lipid envelope to enhance stability and facilitate cellular uptake. Nanoparticles carrying both TAA and immune-stimulating peptides showed significantly higher immunogenicity compared to nanoparticles carrying either peptide alone. This suggests that the combinatorial delivery of multiple peptides can enhance the immune response against tumor antigens. The combinational delivery induced long-term antitumor memory responses, leading to prolonged tumor growth inhibition and survival rate in mice-bearing melanoma tumors [211]. In another study, PLGA nanoparticles have been utilized to co-deliver tumor antigens, STAT3 siRNA, and imiquimod to DCs and tumors. The nanoparticles successfully presented antigenic peptides to DCs, leading to enhanced antigen-specific immune responses. The antigen-loaded nanoparticles effectively activated T cells and promoted their proliferation and secretion of cytokines.
An investigation examined the potential of the nanoparticle-delivered TGF-β siRNA in combination with a melanoma vaccine. They administered the nanoparticles to tumor-bearing mice, followed by vaccination with a melanoma antigen-specific vaccine. They monitored the tumor growth, survival rate, and immune responses of the mice to investigate the effectiveness of the combined treatment. The findings exhibited the successful delivery of TGF-β siRNA using nanoparticles, resulting in efficient knockdown of TGF-β gene expression in melanoma cells. This led to the suppression of TGF-β signaling and subsequent alterations in the tumor stroma. This was accompanied by infiltration of CTLs and NK cells, and reduction of Tregs and MDSCs [212].
Nanovaccines containing neoantigens alone or in combination with other immunostimulatory agents such as TLR agonists are other interesting adjuvants for cancer immunotherapy. Arbelaez et al. developed a complex containing G12D KRAS mutations and CpG for stimulating the immune system in mice-bearing lung adenocarcinoma. In this experiment, G12D KRAS mutations acted as neoantigens, and CpG was utilized as an adjuvant in liposomes. Administration of this nanocomplex could significantly repress tumors by rendering CD4 + and CD8 + T lymphocytes [213]. Another experiment exhibited that treatment with a nanocomplex containing TLR7/8 agonist R848, TLR9 CpG agonists, and a peptide neoantigen loaded with polymeric nanoparticles for targeting colorectal tumors. The findings demonstrated a remarkable immune system awakening following treatment with this nanocomplex. In addition, treatment with this nanocomplex in combination with anti-PD-1 therapy led to 70% tumor regression without tumor recurrence. The findings suggested that tumor antigens can wake DCs to present antigens to CD8 + T cells while anti-PD-1 therapy prevented the exhaustion of CTLs [214].
In addition to the mentioned nanocomplexes, some nanoparticles alone may trigger immune responses against tumors. Fullerenes are nanocarriers for the delivery of different adjuvants, however, they can modulate immune system responses. It has been revealed that polyhydroxylated fullerenes can activate APCs and render the liberation of TNF-α, thereby reducing tumor volume in animal models [215, 216] (Fig. 4).
Targeting of tumor stroma and rigidity
One of the major challenges in treating cold tumors is the dense network of tumor stroma. This rigid environment consists of some proteins and fibers that surround and support malignant cells against the penetration of various agents [217]. This stroma develops a barrier that reduce the infiltration of CTLs and NK cells. In addition, the rigid environment and abnormal vascular structure can prevent the penetration of drugs into TME [218]. Interstitial fluid pressure (IFP) arises from imperfect vascular structure within tumors. The high hydrostatic pressure created by IFP can compress blood vessels, compromising blood flow and reducing the delivery of therapeutic agents to the tumor. This limited drug penetration hinders the effective targeting of cancer cells, reducing treatment efficacy [219]. Furthermore, elevated IFP can hamper antitumor immunity and the efficacy of drugs [220]. To overcome this barrier, researchers have been exploring ways to target tumor rigidity to facilitate the infiltration of CTLs and entrance of anticancer drugs.
Nanoparticles have interesting properties to overcome these barriers. They can be designed to release enzymes that break down the ECM. By breaking down this matrix, nanoparticles can increase the permeability of the tumor vasculature, allowing immune cells to infiltrate the tumor and attack cancer cells. By binding to these cells, nanoparticles can disrupt their function and reduce their ability to support cancer cell growth. Some experiments have shown the potential of nanoparticles loaded with some enzymes for the degradation of ECM. For instance, MSN-loaded bromelain (a proteolytic agent) has been shown to enhance the diffusion of nanoparticles into TME [221]. Some nanoparticle-loaded ECM remodeling agents such as lysyl oxidase, cyclopamine, and CLT1 (anti-fibronectin) have shown the ability to repress ECM, thereby hindering tumor growth [222,223,224]. Some other nanoformulations have been developed to reduce the expression of ECM molecules, such as collagen. For instance, nanogels containing metformin have been shown to deplete ECM by downregulating the concentration of TGF-β and its downstream signaling pathway [225]. The pulsed high-intensity focused ultrasound is another agent that can remodel ECM to enhance the penetration of anticancer drugs [226]. Treatment with anti-ECM-loaded nanoparticles has also been shown to sensitize tumors to antitumor agents, such as radiotherapy, chemotherapy, and photothermal therapy [227, 228].
Inhibiting and reprogramming immunosuppressive cells
Inhibiting immunosuppressive cells or reprogramming tumor-promoting immune cells is another strategy to propel the shifting cold into hot tumors. One of the pivotal players in tumor immunosuppression is TAMs, which have been known to polarize towards an immunosuppressive phenotype, contributing to the establishment of an immune-evasive TME [229]. By targeting TAMs and reprogramming their polarization from an immunosuppressive M2 phenotype to an inflammatory M1 phenotype, it is possible to tip the balance within the tumor stroma, fostering an inflammatory milieu that facilitates the activation of CTLs and other effector immune cells essential for combating the tumor [230]. Tregs and MDSCs are other important immune cells that participate in dampening the antitumor immune response by suppressing CTLs. Inhibiting these cells is effective in promoting an inflammatory and immune-activating TME [231]. Nanoparticles can be utilized for depleting these cells or reprogramming their function by changing the tumor's immunosuppressive environment [155].
An experiment by Zhang et al. reported the beneficial impacts of PLGA nanoparticles on primary breast tumors and metastasis. They injected nanoparticles intravenously and detected the frequency of TAMs and MDSCs in the circulation and tumors. The findings exhibited that cargo-free nanoparticles can significantly repress the expression of CCL2 by tumors, leading to a reduction in the number of circulating and intratumoral MDCSs and TAMs. These changes were associated with increased expression of TNF-α by more than twofold. The findings also suggested that the injection of these cargo-free nanoparticles can augment the efficacy of anti-PD-1 therapy [232]. Another study by Sun et al. reported interesting effects of MSNs on immunosuppressive TME in melanoma. They showed that MSNs trigger the expression of CCL5 and CXCL9/10 by rendering the TLR4–NFκB axis in macrophages, thereby enhancing the infiltration of CTLs into tumors. The regulation of macrophages by MSNs led to the sensitization of tumors to anti-PD-1 therapy [233].
Some other experiments have been conducted to remodel immunosuppressive cells by delivering small molecules or antitumor products. The colony-stimulating factor-1 (CSF-1) is one of the well-known factors that participate in tumor immunosuppression by rendering the infiltration of TAMs [234]. The blockade of CSF-1 is one of the interesting immunotherapeutic approaches for remodeling immunosuppressive TME and boosting the therapeutic efficacy of other anticancer agents [235, 236]. A study suggested that a single blockade of CSF-1R is associated with more infiltration of MDSCs following the activation of CAFs [237]. Therefore, it seems that combination modalities can be more effective for better remodeling TME and elimination of malignant cells. For instance, the blockade of CSF-1 can increase the efficacy of radiotherapy against cancer [238]. Nanoparticles can effectively deliver CSF-1 inhibitors alone or in combination with other agents to repress TAMs and remodel TME effectively [239,240,241]. Selective blockade of some other molecules such as chemokines may also be effective for repressing TAMs. For instance, targeting CCL2/CCR2 can effectively reduce the infiltration of macrophages and boost immune system activity following treatment with some agents such as radiotherapy and immunotherapy [128, 242]. Nanoparticles such as plasmid encoding CCL2 or lipid nanoparticles containing CCL2 inhibitors have been developed for the blockade of macrophage infiltration [243, 244].
Phytochemical nanoformulations are other adjuvants that can be utilized for remodeling immunosuppressive TME. Curcumin, resveratrol, apigenin, and some approved drugs such as paclitaxel are well-known natural-derived products that have been shown to be effective in remodeling TME. Some experiments have also developed nanoparticles to deliver and improve the bioavailability of these agents in tumor models. Micelles, liposomes, SLNs, and polymeric nanoparticles such as PEG and PLGA are appropriate candidates for the delivery of these agents into tumors. However, metal nanoparticles are useful candidates for the delivery of these agents in combination with photothermal/photothermal therapy [245]. Phytochemical nanoparticles such as curcumin and resveratrol nanoformulations have been shown to render the differentiation of CD4 + T cells into Th1 while reducing the population of Th2 and Tregs [246,247,248]. Furthermore, these nanoformulations have been shown to increase the accumulation of CTLs by hampering immunosuppressive cells [249]. Phytochemical nanoformulations have successfully been shown to increase the antitumor potency of photothermal and photodynamic therapy by repressing immunosuppressive cells [250, 251]. Furthermore, smart nanoparticles such as ROS and pH-sensitive nanoparticles have been developed to release these agents in the tumor selectively [252, 253] (Fig. 5).
Nanoparticles can be utilized for the delivery of different agents such as specific inhibitors and phytochemicals. Some phytochemicals such as curcumin and resveratrol can suppress immunosuppressive cells to restore the activity of CTLs and NK cells. In addition, small molecules can be utilized to repress the recruitment of immunosuppressive cells such as TAMs
Cytokines
Cytokines are small molecules that trigger immune cell infiltration and activation. IFN-γ, IL-2, IL-15, and IL-12 are examples of cytokines that have been used in cancer treatment [254]. IL-2 and IL-12 can activate CD8 + T cells and NK cells, which can remove neoplastic cells. These cytokines stimulate the activity of CTLs and NK cells to release antitumor molecules, such as IFN-γ [255]. IFN-γ is a cytokine that stimulates the immune system to induce apoptosis in cancer cells. IFN-γ has been used in the treatment of melanoma, renal cell carcinoma, and chronic myeloid leukemia. However, the systemic administration of cytokines can lead to off-target effects and toxicity [256]. Some other studies have investigated the potential of cytokine therapy. For instance, IL-2 therapy has been shown promising results for pancreatic and colon tumor-bearing mice [257]. The efficacy and reversed toxicity of IL-2 and IL-19 combination for patients with advanced renal cell carcinoma have been reported [258]. Another clinical study showed more survival rate for melanoma patients that received intratumoral L19IL2 + L19TNF modality. However, severe reactions in the site of injection were reported [259].
Nanoparticles can carry cytokines and deliver them specifically to the TME. This localized delivery can enhance the therapeutic efficacy of adoptively transferred cells and minimize systemic toxicity [260]. Numerous experiments have examined the capacity of nanocarriers for the delivery of IL-2 and IL-12. It has been revealed that nanocarriers, such as polymeric and lipid nanoparticles, and also nanogels can maintain sustained release of these cytokines to reduce systemic toxicity and local delivery into tumors [260,261,262,263,264,265]. Nanoparticles can also deliver the RNAs for the regulation of these cytokines to render antitumor immunity in TME [266,267,268]. These cytokines and their RNAs can be delivered for inducing a synergic response in combination with other therapeutic agents. An experiment by Zhao et al. examined the potential of thermosponge nanoparticles for co-delivery of IL-2 and paclitaxel. The results showed that a low dose of paclitaxel remodels the immunosuppressive environment by triggering DCs and subsequent activation of CTLs. Then, IL-2 could amplify the activity of CTLs against tumor cells. Nanoparticles could improve the pharmacokinetics properties of IL-2 and paclitaxel to augment their synergistic effect against primary tumors and metastasis in female mice-bearing tumors. The findings uncovered that mice that received nanoformulation of drugs have remarkably higher survival compared to mice that received free-form drugs [269]. Another experiment showed the synergic impact of IL-12 RNA delivery with ultrasound-induced necroptosis. The delivery of calcium carbonate nanoparticles containing IL-12 RNA was shown to successfully cross the blood–brain barrier and increase immunogenicity in glioblastoma TME [270]. A clinical trial study evaluated the potential of PEG–PEI–cholesterol lipopolymer loaded with IL-12 in combination with PEG–liposomal doxorubicin for patients with advanced ovarian malignancies. Treatment with these agents led to an augment in therapy response and a remarkable increase in the antitumor cytokines including IL-12, IFN-γ, and TNF-α was observed [271].
Oncolytic viruses
Oncolytic viruses have suggested as a transformative modality in the realm of cancer therapy, exhibiting profound potential in harnessing the tumor stroma to trigger robust inflammatory responses and transform cold tumors into hot ones. One of the crucial mechanisms through which oncolytic viruses exert their effects within the tumor stroma involves selective infection and replication within cancer cells, leading to their destruction [272]. Simultaneously, the viral infection triggers the liberation of tumor antigens and DAMPs, serving as beacons for the immune system to attack malignant cells [273]. This process activates various immune cells within the tumor stroma, including DCs, macrophages, and other innate immune effectors, resulting in the production of pro-inflammatory cytokines and the recruitment of CTLs and NK cells to the TME [274, 275]. Moreover, the immunomodulatory properties of oncolytic viruses extend to the local and systemic activation of CTLs and NK cells, representing key effectors in the immune reactions. The release of TAAs during viral infection, coupled with the induction of DAMPs and pro-inflammatory cytokines, triggers the activation and priming of CTLs and NK cells [276]. This activation results in the targeted destroying of cancer cells within the TME.
Some different types of viruses can be utilized for remodeling tumor stroma. Talimogene laherparepvec (T-VEC), Reovirus (Pelareorep), Newcastle disease virus, Maraba virus, and vesicular stomatitis virus (VSV) are designed viruses to activate DCs and the recruitment of CTLs to the TME by rendering ICD, the release of DAMPs, and antigen presentation [277,278,279,280,281]. It has been revealed that IONPs loaded with oncolytic Newcastle viruses can rapidly render pyroptosis in the tumor, leading to immunogenic responses by antitumor immunity. This was associated with tumor repression and increased survival of mice-bearing tumors. IONPs could improve tumor targeting and uptake of oncolytic viruses by tumor cells [282]. AuNP-loaded oncolytic viruses have also been shown to render apoptosis in breast cancer cells [283]. Recently, a study developed an oncolytic virus-like nanoplatform for targeting antitumor immunity in a tumor model system. Administration of nanoparticles could induce immunogenicity and render the recruitment of CTLs, leading to the liberation of INF-γ and apoptosis of cancer cells [284]. Further experiments need to explore the capacity of novel developed nanoparticles loaded with oncolytic viruses for targeting TME.
Adoptive cell therapy (ACT)
ACT is another encouraging approach for remodeling cold tumors. ACT is the transfer of engineered immune cells into patients to enhance their antitumor immune response [285]. ACT can be performed using NK cells, DCs, and chimeric antigen receptor (CAR) T cell therapy [286]. ACT has revealed encouraging clinical success in treating certain types of cancer, such as melanoma and hematological malignancies [287]. However, there are several challenges associated with these therapies, including the limited availability of tumor-specific T cells, the hostile TME, and the potential for off-target toxicities [288]. One approach to address these challenges is to use nanoparticles as delivery vehicles for immune cells in ACT. By engineering nanoparticles to target specific cells or tissues, their delivery to the TME can be enhanced, resulting in higher concentrations and reduced systemic toxicity [289, 290].
Nanoparticles can be utilized to induce CAR expression in T lymphocytes. For instance, lipid nanoparticles are suitable for the successful delivery of DNA for inducing CAR expression in T lymphocytes [291]. A study utilized plasmid nanoparticles to reshape CTLs in TME. The researchers evaluated the impact of nanoparticles containing iRGD, PI3K inhibitors, and alpha-GalCer on T-cell proliferation, cytotoxicity, and migration toward cancer cells. These treatments have been performed to increase the homing of CAR-T cells and cytotoxicity against tumor cells in mice-bearing breast or head and neck tumors. The findings exhibited that a specific time frame of about 2 weeks between nanoparticle injection and CAR-T cell infusion resulted in maximal therapeutic benefit. Outside this window, there was little to no improvement in tumor growth inhibition. The results demonstrated that nanoparticles promoted T cell expansion via increased expression of IL-2 and IL-15, enhanced T cell trafficking into tumors due to upregulation of CXCL8, and reduced immunosuppressive factors, such as TGF-β [292]. Another experiment showed that liposomal delivery of an A2aR-specific small molecule antagonist can prevent the exhaustion of CAR-T cells in deep layers of TME. These nanoparticles can penetrate tumors and remodel the immunosuppressive environment to facilitate the function of CAR-T cells [293] (Fig. 6).
Disadvantages of the current modalities for shaping cold to hot tumors
The therapeutic application of the mentioned modalities for shaping cold to hot tumors are associated with some challenges. Cytokines and TLR agonists are often marred by their pleiotropic effects and systemic toxicity. For instance, IL-2, despite its efficacy, can lead to vascular leak syndrome and renal dysfunction. Similarly, inflammatory cytokines may cause severe inflammatory reactions in different organs. TLR agonists may inadvertently promote tumor growth by activating NF-κB signaling, which can support cancer cell survival [294]. Furthermore, TLR agonists may cause some side effects such as flu-like symptoms, fatigue, and injection-site reactions [295]. Nanoparticles have been shown to deliver cytokines and TLR agonists into tumor more effectively with lower side effects in normal tissues [296]. However, further clinical trial studies need to confirm the findings in preclinical studies.
Oncolytic viruses face the obstacle of host antiviral defenses, which can neutralize the therapeutic viruses before they exert their effects. Furthermore, there is a risk of non-target effects, where the virus may infect non-tumorous cells, leading to unintended consequences [297]. Although oncolytic viruses have shown lower toxicity compared to cytokines or TLR agonists, some preclinical studies have shown some side effects for this treatment modality [298]. Some experiments have shown that treatment with these viruses may cause systemic effects and the presence of viruses in normal tissues. However, emerging evidence in preclinical and clinical trial studies show good safety for oncolytic viruses [299]. In addition, development of nanocarriers for the delivery of oncolytic viruses have shown that can remove tumor cells more effectively with lower systemic effects [300].
ICIs and ICD inducers are other important immunomodulators that have shown encouraging results for some cold malignancies. ICIs can cause irAEs due to their mechanism of enhancing immune activity. These events can range from mild skin rashes to severe conditions, such as colitis, hepatitis, and pneumonitis [301]. Managing these adverse effects requires careful monitoring and often immunosuppressive treatments, which can counteract the therapeutic benefits of ICIs. A significant proportion of patients do not respond to ICIs [177, 302]. As explained, cold tumors with the absence of pre-existing antitumor immune responses, low tumor mutational burden, and the presence of immunosuppressive cells can reduce tumor response to ICIs. Consequently, identifying biomarkers predictive of response is critical but remains challenging [303]. Both primary and acquired resistance to ICIs pose significant hurdles. Tumors can adapt to ICIs by upregulating alternative immune checkpoints, modifying antigen presentation pathways, or creating an immunosuppressive TME [304]. In these conditions, the delivery of ICIs using nanoparticles isn’t enough for shifting cold to hot tumors and needs combination therapy modalities for remodeling antitumor immunity in the tumor. However, combination modalities such as using hypofractionated radiotherapy or immunogenic drugs and adjuvants may be effective [128, 305,306,307,308]. There are some challenges for ICD inducers too. Not all chemotherapeutic agents or radiotherapy techniques can induce ICD. In addition, these drugs may cause severe inflammatory responses in normal tissues [309]. The variability in ICD induction across different cancers and treatment regimens complicates the standardization of ICD-based therapies [310]. Moreover, the identification of reliable ICD biomarkers is still in its infancy [311]. These challenges are also existed for other immunomodulator agents such as small molecules for inhibiting immunosuppressive cells [312]. The safety, efficacy, and biocompatibility of different agents are explained in Table 2.
Challenges and future directions
In addition to the mentioned advantages, the delivery of the suggested molecules or cells using nanoparticles also faces challenges that should to be noted. One challenge is the optimization of nanoparticle design to achieve efficient encapsulation, delivery, and controlled release of these agents. The size, shape, surface charge, and composition of nanoparticles can influence their interactions with immune cells, tumor cells, and TME [319]. Therefore, careful design and engineering of nanoparticles are necessary to ensure optimal delivery and therapeutic efficacy. Another challenge is the comprehending of the interactions between nanoparticles and the immune system. Nanoparticles may induce immune responses, which may impact the efficacy and safety of tumor vaccines, small molecules, cytokines, and others [320]. It is important to study the immunological effects of nanoparticles, including their potential to activate or suppress immune cells, induce inflammation, or cause adverse reactions [321]. By gaining a better understanding of these interactions, researchers can optimize nanoparticle design and minimize any potential negative effects. The safety and biocompatibility of nanoparticles need to be thoroughly evaluated. Nanoparticles can accumulate in various organs and tissues, causing toxicity or long-term adverse effects. It is crucial to assess the biodistribution, pharmacokinetics, and biodegradability of nanoparticles to ensure their safe use in clinical applications [322]. In addition, the potential for immune recognition and clearance of nanoparticles by the immune system should be considered to avoid unwanted immune responses [323].
The safety and efficacy of nanoparticles for the delivery of some treatment modalities such as ACT, TLR agonists, ICIs, and some other adjuvants are in the first way and need to be more investigated in preclinical studies. The incorporation of multiple agents and cells or combination therapies into nanoparticle-based therapy may enhance their efficacy by targeting multiple pathways involved in tumor growth and immune evasion. Moreover, the use of advanced imaging techniques can provide valuable insights into the biodistribution and fate of nanoparticles in vivo. Techniques such as positron emission tomography (PET), magnetic resonance imaging (MRI), or fluorescence imaging can help track the accumulation and release of nanoparticles, as well as monitor their interactions with immune cells and tumor cells [324, 325]. This information can guide the optimization of nanoparticle design and improve our understanding of their therapeutic mechanisms.
Conclusion
In this review, we overviewed the potential of nanoparticles for the delivery of different immunomodulatory agents that can reshape cold tumors and induce inflammatory responses in TME. Different shapes and types of nanocarriers can be utilized for this aim depending on their cargo and treatment modality. Inorganic nanoparticles such as metal and ceramic nanocarriers are useful for rendering ICD during radiotherapy, photodynamic, and photothermal therapy. However, organic nanoparticles can deliver chemotherapy drugs or phytochemicals to enhance their bioavailability. Nanoparticles have shown the ability to deliver TLR agonists, vaccines, antigens, oncolytic viruses, and ICIs into tumors. The delivery of these agents by nanoparticles has shown an enhancement in tumor targeting and preventing severe reactions in normal tissues. In addition, nanoparticles can deliver DNA into tumors to activate CAR-T cells in deep layers of tumors. The ability of nanoparticles to penetrate deep layers of tumors can remodel immunosuppressive TME to augment the infiltration of NK cells and CTLs and reshape a cold environment into a hot environment for the function of the immune system against malignancies. The utilization of nanoparticles for remodeling TME is in the first way and needs several future experiments to examine the safety, efficacy, and potency of each type of nanocarriers for the delivery of different drugs and adjuvants into tumors and reshaping the response of cancer to therapy.
Data and/or Code availability
No new data generated.
References
O’Neill RE, Cao X. Co-stimulatory and co-inhibitory pathways in cancer immunotherapy. Adv Cancer Res. 2019;143:145–94.
Zhang J, Shi Z, Xu X, Yu Z, Mi J. The influence of microenvironment on tumor immunotherapy. FEBS J. 2019;286(21):4160–75.
Zhang J, Huang D, Saw PE, Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 2022;43(7):523–45.
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168.
Wang M, Wang S, Desai J, Trapani JA, Neeson PJ. Therapeutic strategies to remodel immunologically cold tumors. Clin Transl Immunol. 2020;9(12):e1226.
Banu SPNS, Narayan S. Biomaterial based nanocarriers for delivering immunomodulatory agents. Nanomed Res J. 2021;6(3):195–217.
Park W, Heo Y-J, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:1–10.
Fontana F, Liu D, Hirvonen J, Santos HA. Delivery of therapeutics with nanoparticles: what’s new in cancer immunotherapy? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1):e1421.
Lee MS, Dees EC, Wang AZ. Nanoparticle-delivered chemotherapy: old drugs in new packages. Oncology (Williston Park). 2017;31(3):198–208.
Chidambaram M, Manavalan R, Kathiresan K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci. 2011;14(1):67–77.
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761.
Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27(11):863–75.
Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14(3):155–67.
Chow MT, Möller A, Smyth MJ, editors (2012) Inflammation and immune surveillance in cancer. Semin Cancer Biol: Elsevier.
Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2(3):187–93.
Denton AE, Roberts EW, Fearon DT. Stromal cells in the tumor microenvironment. Stromal Immunol. 2018;1060:99–114.
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277–89.
Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
Fu C, Jiang A. Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol. 2018;9:3059.
Hadrup S, Donia M, Thor SP. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6:123–33.
Xie Q, Ding J, Chen Y. Role of CD8+ T lymphocyte cells: Interplay with stromal cells in tumor microenvironment. Acta Pharmaceutica Sinica B. 2021;11(6):1365–78.
Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest. 2006;35(3–4):459–83.
Wicherska-Pawłowska K, Wróbel T, Rybka J. Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int J Mol Sci. 2021;22(24):13397.
Liu Z, Han C, Fu Y-X. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol Immunol. 2020;17(1):13–26.
Verneau J, Sautés-Fridman C, Sun C-M. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol. 2020;48:101410.
Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44(6):1582–92.
Myers JA, Schirm D, Bendzick L, Hopps R, Selleck C, Hinderlie P, et al. Balanced engagement of activating and inhibitory receptors mitigates human NK cell exhaustion. JCI insight. 2022;7(15):e150079.
Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, et al. Human NK cells selective targeting of colon cancer–initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190(5):2381–90.
Wörmann S, Diakopoulos K, Lesina M, Algül H. The immune network in pancreatic cancer development and progression. Oncogene. 2014;33(23):2956–67.
Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest. 2012;41(6–7):738–64. https://doi.org/10.3109/08820139.2012.676122.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18.
Giannotta C, Autino F, Massaia M. The immune suppressive tumor microenvironment in multiple myeloma: the contribution of myeloid-derived suppressor cells. Front Immunol. 2023;13:1102471.
Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8:125–58.
Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15(1):1–14.
Keeley T, Costanzo-Garvey DL, Cook LM. Unmasking the many faces of tumor-associated neutrophils and macrophages: considerations for targeting innate immune cells in cancer. Trends in cancer. 2019;5(12):789–98.
Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–26.
Muz B, de la Puente P, Azab F, Kareem AA. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92.
Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143(4):512–9.
Taylor S, Spugnini EP, Assaraf YG, Azzarito T, Rauch C, Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updates. 2015;23:69–78.
Tomaszewski W, Sanchez-Perez L, Gajewski TF, Sampson JH. Brain tumor microenvironment and host state: implications for immunotherapy. Clin Cancer Res. 2019;25(14):4202–10.
Zhang S, Kohli K, Black RG, Yao L, Spadinger SM, He Q, et al. Systemic interferon-γ increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol Res. 2019;7(8):1237–43.
Mirlekar B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022;10:20503121211069012.
Zhang Y, Guan X-Y, Jiang P. Cytokine and chemokine signals of T-cell exclusion in tumors. Front Immunol. 2020;11:594609.
Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Investig. 2018;128(8):3209–18.
Mortezaee K. Immune escape: a critical hallmark in solid tumors. Life Sci. 2020;258:118110.
Mu Q, Najafi M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int Immunopharmacol. 2021;98:107895.
Pelly VS, Moeini A, Roelofsen LM, Bonavita E, Bell CR, Hutton C, et al. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discov. 2021;11(10):2602–19.
Tekpli X, Lien T, Røssevold AH, Nebdal D, Borgen E, Ohnstad HO, et al. An independent poor-prognosis subtype of breast cancer defined by a distinct tumor immune microenvironment. Nat Commun. 2019;10(1):5499.
Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299–309.
Rezaei M, Danilova ND, Soltani M, Savvateeva LV, Tarasov VV, Ganjalikhani-Hakemi M, et al. Cancer vaccine in cold tumors: clinical landscape, challenges, and opportunities. Curr Cancer Drug Targets. 2022;22(6):437–53.
Runcie KD, Dallos MC. Prostate cancer immunotherapy—finally in from the cold? Curr Oncol Rep. 2021;23(8):88.
De Nunzio C, Andriole GL, Thompson IM Jr, Freedland SJ. Smoking and prostate cancer: a systematic review. Eur Urol Focus. 2015;1(1):28–38.
Tian M, Ma W, Chen Y, Yu Y, Zhu D, Shi J, Zhang Y. Impact of gender on the survival of patients with glioblastoma. Biosci Rep. 2018;38(6):BSR20180752.
McFarlane T, Zajac JD, Cheung AS. Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals—a systematic review. Clin Endocrinol (Oxf). 2018;89(6):700–11.
Allegra A, Caserta S, Genovese S, Pioggia G, Gangemi S. Gender differences in oxidative stress in relation to cancer susceptibility and survival. Antioxidants. 2023;12(6):1255.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218.
Kather JN, Suarez-Carmona M, Charoentong P, Weis C-A, Hirsch D, Bankhead P, et al. Topography of cancer-associated immune cells in human solid tumors. Elife. 2018;7:e36967.
Bernatchez C, Haymaker C, Tannir NM, Kluger H, Tetzlaff M, Bentebibel SE, et al. A CD122-biased agonist increases CD8+ T Cells and natural killer cells in the tumor microenvironment; making cold tumors hot with NKTR-214. Cough. 2016;5(1):3.
Wargo JA, Reddy SM, Reuben A, Sharma P. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol. 2016;41:23–31.
Burkholder B, Huang R-Y, Burgess R, Luo S, Jones VS, Zhang W, et al. (2014) Tumor-induced perturbations of cytokines and immune cell networks. Biochimica et Biophysica Acta (BBA)-Revi Cancer. 1845;2:182–201.
De Guillebon E, Dardenne A, Saldmann A, Séguier S, Tran T, Paolini L, et al. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int J Cancer. 2020;147(6):1509–18.
Fan H, Shi Y, Wang H, Li Y, Mei J, Xu J, Liu C. GBP5 identifies immuno-hot tumors and predicts the therapeutic response to immunotherapy in NSCLC. Int J General Med. 2023;16:1757–69.
Li X, Luo L, Jiang M, Zhu C, Shi Y, Zhang J, et al. Cocktail strategy for ‘cold’tumors therapy via active recruitment of CD8+ T cells and enhancing their function. J Control Release. 2021;334:413–26.
Zhao Z, Liu H, Zhou X, Fang D, Ou X, Ye J, et al. Necroptosis-related lncRNAs: predicting prognosis and the distinction between the cold and hot tumors in gastric cancer. J Oncol. 2021;2021:6718443.
O’Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21(2):249–57.
Wang L, Geng H, Liu Y, Liu L, Chen Y, Wu F, et al. Hot and cold tumors: immunological features and the therapeutic strategies. MedComm. 2023;4(5):e343.
Nassar KW, Tan AC. The mutational landscape of mucosal melanoma. Semin Cancer Biol. 2020;61:139–48.
O’Brien SM, Klampatsa A, Thompson JC, Martinez MC, Hwang W-T, Rao AS, et al. Function of human tumor-infiltrating lymphocytes in early-stage non–small cell lung cancer. Cancer Immunol Res. 2019;7(6):896–909.
Lazdun Y, Si H, Creasy T, Ranade K, Higgs BW, Streicher K, Durham NM. A new pipeline to predict and confirm tumor neoantigens predict better response to immune checkpoint blockade. Mol Cancer Res. 2021;19(3):498–506.
Gameiro SF, Evans AM, Mymryk JS. The tumor immune microenvironments of HPV+ and HPV−head and neck cancers. WIREs Mechanisms of Disease. 2022;14(2):e1539.
Kanavy HE, Gerstenblith MR. Ultraviolet radiation and melanoma. Semin Cutan Med Surg. 2011;30:222–8.
Ernst SM, Mankor JM, van Riet J, von der Thüsen JH, Dubbink HJ, Aerts JG, et al. Tobacco smoking-related mutational signatures in classifying smoking-associated and nonsmoking-associated NSCLC. J Thorac Oncol. 2023;18(4):487–98.
Farling KB. Bladder cancer: risk factors, diagnosis, and management. Nurse Pract. 2017;42(3):26–33.
Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. Br Dent J. 2022;233(9):780–6.
Schwartz MR, Luo L, Berwick M. Sex differences in melanoma. Current Epidemiol Rep. 2019;6:112–8.
Frega S, Ferro A, Bonanno L, Guarneri V, Conte P, Pasello G. Sex-based heterogeneity in non-small cell lung cancer (NSCLC) and response to immune checkpoint inhibitors (ICIs): a narrative review. Precision Cancer Medi. 2021;4:100251.
Gul ZG, Liaw CW, Mehrazin R. Gender differences in incidence, diagnosis, treatments, and outcomes in clinically localized bladder and renal cancer. Urology. 2021;151:176–81. https://doi.org/10.1016/j.urology.2020.05.067.
Park J-O, Nam I-C, Kim C-S, Park S-J, Lee D-H, Kim H-B, et al. Sex Differences in the prevalence of head and neck cancers: a 10-year follow-up study of 10 million healthy people. Cancers (Basel). 2022;14(10):2521.
Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. 2021;12:731798.
Toor SM, Nair VS, Decock J, Elkord E. Immune checkpoints in the tumor microenvironment. Semin Cancer Biol. 2020;65:1–12.
Giannone G, Ghisoni E, Genta S, Scotto G, Tuninetti V, Turinetto M, Valabrega G. Immuno-metabolism and microenvironment in cancer: key players for immunotherapy. Int J Mol Sci. 2020;21(12):4414.
Chyuan I-T, Chu C-L, Hsu P-N. Targeting the tumor microenvironment for improving therapeutic effectiveness in cancer immunotherapy: focusing on immune checkpoint inhibitors and combination therapies. Cancers. 2021;13(6):1188.
Ma Y, Conforti R, Aymeric L, Locher C, Kepp O, Kroemer G, Zitvogel L. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 2011;30:71–82.
Galassi C, Klapp V, Yamazaki T, Galluzzi L. Molecular determinants of immunogenic cell death elicited by radiation therapy. Immunol Rev. 2023;321:20–32.
Wang Q, Ju X, Wang J, Fan Y, Ren M, Zhang H. Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies. Cancer Lett. 2018;438:17–23.
Fabian KP, Kowalczyk JT, Reynolds ST, Hodge JW. Dying of stress: chemotherapy, radiotherapy, and small-molecule inhibitors in immunogenic cell death and immunogenic modulation. Cells. 2022;11(23):3826.
Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clin Cancer Res. 2012;18(17):4522–5.
Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280(1):126–48.
Nkune NW, Simelane NWN, Montaseri H, Abrahamse H. Photodynamic therapy-mediated immune responses in three-dimensional tumor models. Int J Mol Sci. 2021;22(23):12618.
Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10(1):3349.
Panzarini E, Inguscio V, Dini L. Immunogenic cell death: can it be exploited in photodynamic therapy for cancer? BioMed Res Int. 2013. https://doi.org/10.1155/2013/482160.
Wang S-B, Zhang C, Ye J-J, Zou M-Z, Liu C-J, Zhang X-Z. Near-infrared light responsive nanoreactor for simultaneous tumor photothermal therapy and carbon monoxide-mediated anti-inflammation. ACS Cent Sci. 2020;6(4):555–65.
Lima-Sousa R, Melo BL, Alves CG, Moreira AF, Mendonça AG, Correia IJ, de Melo-Diogo D. Combining photothermal-photodynamic therapy mediated by nanomaterials with immune checkpoint blockade for metastatic cancer treatment and creation of immune memory. Adv Func Mater. 2021;31(29):2010777.
Kong C, Chen X. Combined photodynamic and photothermal therapy and immunotherapy for cancer treatment: a review. Int J Nanomed. 2022;17:6427.
Jurj A, Braicu C, Pop L-A, Tomuleasa C, Gherman CD, Berindan-Neagoe I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des Devel Ther. 2017;11:2871–90.
Paul W, Sharma CP. Inorganic nanoparticles for targeted drug delivery. Biointegr Med Implant Mater. 2020. https://doi.org/10.1016/B978-0-08-102680-9.00013-5.
Zhao J, Lee P, Wallace J, M, P Melancon M,. Gold nanoparticles in cancer therapy: efficacy, biodistribution, and toxicity. Curr Pharm Des. 2015;21(29):4240–51.
Goddard ZR, Marín MJ, Russell DA, Searcey M. Active targeting of gold nanoparticles as cancer therapeutics. Chem Soc Rev. 2020;49(23):8774–89.
Beik J, Khateri M, Khosravi Z, Kamrava SK, Kooranifar S, Ghaznavi H, Shakeri-Zadeh A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord Chem Rev. 2019;387:299–324.
Fu Q, Zhang X, Song J, Yang H. Plasmonic gold nanoagents for cancer imaging and therapy. View. 2021;2(5):20200149.
Zhao S, Yu X, Qian Y, Chen W, Shen J. Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics. Theranostics. 2020;10(14):6278.
Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano. 2016;10(2):2436–46.
Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–84.
Lappas CM. The immunomodulatory effects of titanium dioxide and silver nanoparticles. Food Chem Toxicol. 2015;85:78–83.
Alyassin Y, Sayed EG, Mehta P, Ruparelia K, Arshad MS, Rasekh M, et al. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov Today. 2020;25(8):1513–20.
Barkat A, Beg S, Panda SK, Alharbi KS, Rahman M, Ahmed FJ. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin Cancer Biol. 2021;69:365–75.
Sabio RM, Meneguin AB, Ribeiro TC, Silva RR, Chorilli M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int J Pharm. 2019;564:379–409.
Rao PV, Nallappan D, Madhavi K, Rahman S, Jun Wei L, Gan SH. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid Med Cell Longev. 2016. https://doi.org/10.1155/2016/3685671.
Tu X, Ma Y, Cao Y, Huang J, Zhang M, Zhang Z. PEGylated carbon nanoparticles for efficient in vitro photothermal cancer therapy. J Mater Chem B. 2014;2(15):2184–92.
Xu G, Liu S, Niu H, Lv W. Functionalized mesoporous carbon nanoparticles for targeted chemo-photothermal therapy of cancer cells under near-infrared irradiation. RSC Adv. 2014;4(64):33986–97.
Sadeghi MS, Sangrizeh FH, Jahani N, Abedin MS, Chaleshgari S, Ardakan AK, et al. Graphene oxide nanoarchitectures in cancer therapy: drug and gene delivery, phototherapy, immunotherapy, and vaccine development. Environ Res. 2023;237:117027.
Li K, Liu B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem Soc Rev. 2014;43(18):6570–97.
Lu Y, Yue Z, Xie J, Wang W, Zhu H, Zhang E, Cao Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat Biomed Eng. 2018;2(5):318–25.
Chen L, Zang F, Wu H, Li J, Xie J, Ma M, et al. Using PEGylated magnetic nanoparticles to describe the EPR effect in tumor for predicting therapeutic efficacy of micelle drugs. Nanoscale. 2018;10(4):1788–97.
Khalid M, El-Sawy HS. Polymeric nanoparticles: promising platform for drug delivery. Int J Pharm. 2017;528(1–2):675–91.
Wang Y, Lin Y-X, Qiao S-L, An H-W, Ma Y, Qiao Z-Y, et al. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–63.
Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, et al. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10(45):26777–91.
Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571.
Souto EB, Baldim I, Oliveira WP, Rao R, Yadav N, Gama FM, Mahant S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin Drug Deliv. 2020;17(3):357–77.
Baek J-S, Cho C-W. Controlled release and reversal of multidrug resistance by co-encapsulation of paclitaxel and verapamil in solid lipid nanoparticles. Int J Pharm. 2015;478(2):617–24.
Wilson RJ, Li Y, Yang G, Zhao C-X. Nanoemulsions for drug delivery. Particuology. 2022;64:85–97.
Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol. 2018;28(8):R435–44.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405.
Kibria G, Ramos EK, Wan Y, Gius DR, Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol Pharm. 2018;15(9):3625–33.
Kaur D, Jain K, Mehra NK, Kesharwani P, Jain NK. A review on comparative study of PPI and PAMAM dendrimers. J Nanopart Res. 2016;18:1–14.
Chauhan AS. Dendrimers for drug delivery. Molecules. 2018;23(4):938.
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23(8):4854–65.
Ashrafizadeh M, Farhood B, Musa AE, Taeb S, Najafi M. The interactions and communications in tumor resistance to radiotherapy: therapy perspectives. Int Immunopharmacol. 2020;87:106807.
Zhu M, Yang M, Zhang J, Yin Y, Fan X, Zhang Y, et al. Immunogenic cell death induction by ionizing radiation. Front Immunol. 2021;12:705361.
Khodamoradi E, Hoseini-Ghahfarokhi M, Amini P, Motevaseli E, Shabeeb D, Musa AE, et al. Targets for protection and mitigation of radiation injury. Cell Mol Life Sci. 2020;77:3129–59.
Liu P, Zhao L, Zitvogel L, Kepp O, Kroemer G. Immunogenic cell death (ICD) enhancers—Drugs that enhance the perception of ICD by dendritic cells. Immunol Rev. 2023;32:7–19.
Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala A, Shen S, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91.
Yahyapour R, Salajegheh A, Safari A, Amini P, Rezaeyan A, Amraee A, Najafi M. Radiation-induced non-targeted effect and carcinogenesis; implications in clinical radiotherapy. J Biomed Phys Eng. 2018;8(4):435.
Moloudi K, Khani A, Najafi M, Azmoonfar R, Azizi M, Nekounam H, et al. Critical parameters to translate gold nanoparticles as radiosensitizing agents into the clinic. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15:e1886.
Maggiorella L, Barouch G, Devaux C, Pottier A, Deutsch E, Bourhis J, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8(9):1167–81.
Zhang P, Darmon A, Marill J, Mohamed Anesary N, Paris S. Radiotherapy-activated hafnium oxide nanoparticles produce abscopal effect in a mouse colorectal cancer model. Int J Nanomed. 2020;15:3843–50.
Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82–90.
Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40(1):10–24.
Bonvalot S, Le Pechoux C, De Baere T, Kantor G, Buy X, Stoeckle E, et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin Cancer Res. 2017;23(4):908–17. https://doi.org/10.1158/1078-0432.Ccr-16-1297.
Bonvalot S, Rutkowski PL, Thariat J, Carrère S, Ducassou A, Sunyach M-P, et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (ActInSarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20(8):1148–59. https://doi.org/10.1016/S1470-2045(19)30326-2.
Vanmeerbeek I, Sprooten J, De Ruysscher D, Tejpar S, Vandenberghe P, Fucikova J, et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9(1):1703449.
Liu Z, Xu X, Liu K, Zhang J, Ding D, Fu R. Immunogenic cell death in hematological malignancy therapy. Adv Sci. 2023;10(13):2207475.
Kim D-Y, Pyo A, Yun M, Thangam R, You S-H, Zhang Y, et al. Imaging calreticulin for early detection of immunogenic cell death during anticancer treatment. J Nucl Med. 2021;62(7):956–60.
Zhao C-Y, Cheng R, Yang Z, Tian Z-M. Nanotechnology for cancer therapy based on chemotherapy. Molecules. 2018;23(4):826.
Fu L, Ma X, Liu Y, Xu Z, Sun Z. Applying nanotechnology to boost cancer immunotherapy by promoting immunogenic cell death. Chin Chem Lett. 2022;33(4):1718–28.
Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019;58(3):670–80.
Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–80. https://doi.org/10.1002/anie.201804882.
Guo J, Yu Z, Sun D, Zou Y, Liu Y, Huang L. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol Cancer. 2021;20(1):10. https://doi.org/10.1186/s12943-020-01297-0.
Liu J, Li Z, Zhao D, Feng X, Wang C, Li D, Ding J. Immunogenic cell death-inducing chemotherapeutic nanoformulations potentiate combination chemoimmunotherapy. Mater Des. 2021;202:109465.
Wang Y, Wang Z, Chen B, Yin Q, Pan M, Xia H, et al. Cooperative self-assembled nanoparticle induces sequential immunogenic cell death and toll-like receptor activation for synergistic chemo-immunotherapy. Nano Lett. 2021;21(10):4371–80. https://doi.org/10.1021/acs.nanolett.1c00977.
Overchuk M, Weersink RA, Wilson BC, Zheng G. Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano. 2023;17(9):7979–8003.
Kadkhoda J, Tarighatnia A, Barar J, Aghanejad A, Davaran S. Recent advances and trends in nanoparticles based photothermal and photodynamic therapy. Photodiagnosis Photodyn Ther. 2022;37:102697.
Bucharskaya A, Maslyakova G, Terentyuk G, Yakunin A, Avetisyan Y, Bibikova O, et al. Towards effective photothermal/photodynamic treatment using plasmonic gold nanoparticles. Int J Mol Sci. 2016;17(8):1295.
Zhang H, Zhou F, Yang Q, Huang M. Targeting the oral tumor microenvironment by nanoparticles: a review of progresses. J Drug Deliv Sci Technol. 2023;91:105248.
Qin L, Wu J. Targeting anticancer immunity in oral cancer: drugs, products, and nanoparticles. Environ Res. 2023;239:116751.
Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioactive Mater. 2021;6(7):1973–87.
Lu H. TLR agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Front Immunol. 2014;5:83.
Chakraborty S, Ye J, Wang H, Sun M, Zhang Y, Sang X, Zhuang Z. Application of toll-like receptors (TLRs) and their agonists in cancer vaccines and immunotherapy. Front Immunol. 2023;14:1227833.
Ma F, Zhang J, Zhang J, Zhang C. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cell Mol Immunol. 2010;7(5):381–8.
Keshavarz A, Pourbagheri-Sigaroodi A, Zafari P, Bagheri N, Ghaffari SH, Bashash D. Toll-like receptors (TLRs) in cancer; with an extensive focus on TLR agonists and antagonists. IUBMB Life. 2021;73(1):10–25.
Chen X, Zhang Y, Fu Y. The critical role of Toll-like receptor-mediated signaling in cancer immunotherapy. Med Drug Discov. 2022;14:100122.
Sultan H, Salazar AM, Celis E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin Immunol. 2020;49:101414.
Zhang Y, Yuan T, Li Z, Luo C, Wu Y, Zhang J, et al. Hyaluronate-based self-stabilized nanoparticles for immunosuppression reversion and immunochemotherapy in osteosarcoma treatment. ACS Biomater Sci Eng. 2021;7(4):1515–25. https://doi.org/10.1021/acsbiomaterials.1c00081.
Huang Y, Nahar S, Alam MDM, Hu S, McVicar DW, Yang D. Reactive oxygen species-sensitive biodegradable mesoporous silica nanoparticles harboring theravac elicit tumor-specific immunity for colon tumor treatment. ACS Nano. 2023;17(20):19740–52. https://doi.org/10.1021/acsnano.3c03195.
Tambunlertchai S, Geary SM, Naguib YW, Salem AK. Investigating silver nanoparticles and resiquimod as a local melanoma treatment. Eur J Pharm Biopharm. 2023;183:1–12.
Kim H, Niu L, Larson P, Kucaba TA, Murphy KA, James BR, et al. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53. https://doi.org/10.1016/j.biomaterials.2018.02.034.
Bahmani B, Gong H, Luk BT, Haushalter KJ, DeTeresa E, Previti M, et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12(1):1999.
Widmer J, Thauvin C, Mottas I, Nguyen VN, Delie F, Allémann E, Bourquin C. Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int J Pharm. 2018;535(1):444–51. https://doi.org/10.1016/j.ijpharm.2017.11.031.
Yin W, Qian S. Delivery of cisplatin and resiquimod in nanomicelles for the chemoimmunotherapy of ovarian cancer. Cancer Nanotechnology. 2022;13(1):8. https://doi.org/10.1186/s12645-021-00094-8.
Zhang H, Tang WL, Kheirolomoom A, Fite BZ, Wu B, Lau K, et al. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J Control Release. 2021;330:1080–94. https://doi.org/10.1016/j.jconrel.2020.11.013.
Kakwere H, Zhang H, Ingham ES, Nura-Raie M, Tumbale SK, Allen R, et al. Systemic immunotherapy with micellar resiquimod-polymer conjugates triggers a robust antitumor response in a breast cancer model. Adv Healthc Mater. 2021;10(10):e2100008. https://doi.org/10.1002/adhm.202100008.
Singh B, Maharjan S, Pan DC, Zhao Z, Gao Y, Zhang YS, Mitragotri S. Imiquimod-gemcitabine nanoparticles harness immune cells to suppress breast cancer. Biomaterials. 2022;280:121302. https://doi.org/10.1016/j.biomaterials.2021.121302.
Gondan AIB, Ruiz-de-Angulo A, Zabaleta A, Blanco NG, Cobaleda-Siles BM, García-Granda MJ, et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials. 2018;170:95–115.
Yan W, Li Y, Zou Y, Zhu R, Wu T, Yuan W, et al. Co-delivering irinotecan and imiquimod by pH-responsive micelle amplifies anti-tumor immunity against colorectal cancer. Int J Pharm. 2023;648:123583. https://doi.org/10.1016/j.ijpharm.2023.123583.
Wen YH, Hsieh PI, Chiu HC, Chiang CW, Lo CL, Chiang YT. Precise delivery of doxorubicin and imiquimod through pH-responsive tumor microenvironment-active targeting micelles for chemo- and immunotherapy. Mater Today Bio. 2022;17:100482. https://doi.org/10.1016/j.mtbio.2022.100482.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Marei HE, Hasan A, Pozzoli G, Cenciarelli C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023;23(1):64.
Nagasaki J, Ishino T, Togashi Y. Mechanisms of resistance to immune checkpoint inhibitors. Cancer Sci. 2022;113(10):3303.
Shi C, Wang Y, Xue J, Zhou X. Immunotherapy for EGFR-mutant advanced non-small-cell lung cancer: current status, possible mechanisms and application prospects. Front Immunol. 2022;13:940288.
Zhao Y, Yang W, Huang Y, Cui R, Li X, Li B. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol Biochem. 2018;47(2):721–34.
Chauvin J-M, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2):000957.
Chu X, Tian W, Wang Z, Zhang J, Zhou R. Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: mechanisms and clinical trials. Mol Cancer. 2023;22(1):1–31.
Dong M, Yu T, Zhang Z, Zhang J, Wang R, Tse G, et al. ICIs-related cardiotoxicity in different types of cancer. J Cardiovasc Develop Dis. 2022;9(7):203.
Boone CE, Wang L, Gautam A, Newton IG, Steinmetz NF. Combining nanomedicine and immune checkpoint therapy for cancer immunotherapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(1):e1739.
Badiee P, Maritz MF, Dmochowska N, Cheah E, Thierry B. Intratumoral Anti-PD-1 nanoformulation improves its biodistribution. ACS Appl Mater Interface. 2022;14(14):15881–93. https://doi.org/10.1021/acsami.1c22479.
Ordikhani F, Uehara M, Kasinath V, Dai L, Eskandari SK, Bahmani B, et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.122700.
Wu Y, Gu W, Li L, Chen C, Xu ZP. Enhancing PD-1 gene silence in T lymphocytes by comparing the delivery performance of two inorganic nanoparticle platforms. Nanomaterials. 2019;9(2):159.
Wu Y, Gu W, Li J, Chen C, Xu ZP. Silencing PD-1 and PD-L1 with nanoparticle-delivered small interfering RNA increases cytotoxicity of tumor-infiltrating lymphocytes. Nanomedicine. 2019;14(8):955–67.
Jeong W-j, Bu J, Han Y, Drelich AJ, Nair A, Král P, Hong S. Nanoparticle Conjugation stabilizes and multimerizes β-hairpin peptides to effectively target PD-1/PD-L1 β-sheet-rich interfaces. J Am Chem Soc. 2020;142(4):1832–7.
Zhang Z, Wang Q, Liu Q, Zheng Y, Zheng C, Yi K, et al. Dual-locking nanoparticles disrupt the PD-1/PD-L1 pathway for efficient cancer immunotherapy. Adv Mater. 2019;31(51):1905751. https://doi.org/10.1002/adma.201905751.
Zhang B, Wang Y, Wang S, Tang Y, Li Z, Lin L, et al. Precise RNA editing: cascade self-uncloaking dual-prodrug nanoassemblies based on CRISPR/Cas13a for pleiotropic immunotherapy of PD-L1-resistant colorectal cancer. Adv Func Mater. 2023;33(46):2305630. https://doi.org/10.1002/adfm.202305630.
Lan X, Zhu W, Huang X, Yu Y, Xiao H, Jin L, et al. Microneedles loaded with anti-PD-1–cisplatin nanoparticles for synergistic cancer immuno-chemotherapy. Nanoscale. 2020;12(36):18885–98.
Xu S, Cui F, Huang D, Zhang D, Zhu A, Sun X, et al. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int J Nanomed. 2019;14:17–32.
Mu X, Zhang M, Wei A, Yin F, Wang Y, Hu K, Jiang J. Doxorubicin and PD-L1 siRNA co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of PCa bone metastases. Nanoscale. 2021;13(19):8998–9008.
Zhu W, Bai Y, Zhang N, Yan J, Chen J, He Z, et al. A tumor extracellular pH-sensitive PD-L1 binding peptide nanoparticle for chemo-immunotherapy of cancer. J Mater Chem B. 2021;9(20):4201–10.
Cai S, Chen Z, Wang Y, Wang M, Wu J, Tong Y, et al. Reducing PD-L1 expression with a self-assembled nanodrug: an alternative to PD-L1 antibody for enhanced chemo-immunotherapy. Theranostics. 2021;11(4):1970–81. https://doi.org/10.7150/thno.45777.
Zou M-Z, Liu W-L, Li C-X, Zheng D-W, Zeng J-Y, Gao F, et al. A Multifunctional biomimetic nanoplatform for relieving hypoxia to enhance chemotherapy and inhibit the PD-1/PD-L1 Axis. Small. 2018;14(28):1801120. https://doi.org/10.1002/smll.201801120.
Liang J, Wang H, Ding W, Huang J, Zhou X, Wang H, et al. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci Adv. 2020;6(35):eabc646.
Sun Z, Zhang Y, Cao D, Wang X, Yan X, Li H, et al. PD-1/PD-L1 pathway and angiogenesis dual recognizable nanoparticles for enhancing chemotherapy of malignant cancer. Drug Deliv. 2018;25(1):1746–55. https://doi.org/10.1080/10717544.2018.1509907.
Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21(5):298–312.
Gupta RG, Li F, Roszik J, Lizée G. Exploiting tumor neoantigens to target cancer evolution: current challenges and promising therapeutic approaches. Cancer Discov. 2021;11(5):1024–39.
Accolla R, Lombardo L, Abdallah R, Raval G, Forlani G, Tosi G. Boosting the MHC class II-restricted tumor antigen presentation to CD4+ T helper cells: a critical issue for triggering protective immunity and re-orienting the tumor microenvironment toward an anti-tumor state. Front Oncol. 2014;4:79407.
Sánchez-Paulete A, Teijeira A, Cueto FJ, Garasa S, Pérez-Gracia JL, Sánchez-Arráez A, et al. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Annal Oncol. 2017;28:xii44–55.
Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of CpG oligonucleotides. Pharm Res. 2008;25:551–62.
Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35(2):814–24. https://doi.org/10.1016/j.biomaterials.2013.10.003.
Lin AY, Almeida JP, Bear A, Liu N, Luo L, Foster AE, Drezek RA. Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS ONE. 2013;8(5):e63550. https://doi.org/10.1371/journal.pone.0063550.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74.
Bobisse S, Foukas PG, Coukos G, Harari A. Neoantigen-based cancer immunotherapy. Ann Transl Med. 2016;4(14):262.
Wirth TC, Kühnel F. Neoantigen targeting—dawn of a new era in cancer immunotherapy? Front Immunol. 2017;8:1848.
Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Polymer nanoparticles for immunotherapy from encapsulated tumor-associated antigens and whole tumor cells. Mol Pharm. 2007;4(1):47–57. https://doi.org/10.1021/mp060107e.
Tan S, Sasada T, Bershteyn A, Yang K, Ioji T, Zhang Z. Combinational delivery of lipid-enveloped polymeric nanoparticles carrying different peptides for anti-tumor immunotherapy. Nanomedicine (Lond). 2014;9(5):635–47. https://doi.org/10.2217/nnm.13.67.
Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8(4):3636–45. https://doi.org/10.1021/nn500216y.
Arbelaez CA, Estrada J, Gessner MA, Glaus C, Morales AB, Mohn D, et al. A nanoparticle vaccine that targets neoantigen peptides to lymphoid tissues elicits robust antitumor T cell responses. NPJ Vaccin. 2020;5(1):106. https://doi.org/10.1038/s41541-020-00253-9.
Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci Adv. 2020;6(12):6eaaw6071.
Zhu J, Ji Z, Wang J, Sun R, Zhang X, Gao Y, et al. Tumor-inhibitory effect and immunomodulatory activity of fullerol C60(OH)x. Small. 2008;4(8):1168–75. https://doi.org/10.1002/smll.200701219.
Liu Y, Jiao F, Qiu Y, Li W, Qu Y, Tian C, et al. Immunostimulatory properties and enhanced TNF- alpha mediated cellular immunity for tumor therapy by C60(OH)20 nanoparticles. Nanotechnology. 2009;20(41):415102. https://doi.org/10.1088/0957-4484/20/41/415102.
Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36(3):171–98. https://doi.org/10.1007/s10585-019-09966-1.
Yoo J, Seo BK, Park EK, Kwon M, Jeong H, Cho KR, et al. Tumor stiffness measured by shear wave elastography correlates with tumor hypoxia as well as histologic biomarkers in breast cancer. Cancer Imaging. 2020;20(1):1–10.
Salavati H, Debbaut C, Pullens P, Ceelen W. Interstitial fluid pressure as an emerging biomarker in solid tumors. Biochimica et Biophysica Acta (BBA) Rev Cancer. 2022;1877:188792.
Zhang T, Jia Y, Yu Y, Zhang B, Xu F, Guo H. Targeting the tumor biophysical microenvironment to reduce resistance to immunotherapy. Adv Drug Del Rev. 2022;186:114319.
Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A, et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8(10):9874–83.
Kanapathipillai M, Mammoto A, Mammoto T, Kang JH, Jiang E, Ghosh K, et al. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano Lett. 2012;12(6):3213–7. https://doi.org/10.1021/nl301206p.
Zhang B, Shen S, Liao Z, Shi W, Wang Y, Zhao J, et al. Targeting fibronectins of glioma extracellular matrix by CLT1 peptide-conjugated nanoparticles. Biomaterials. 2014;35(13):4088–98.
Zhang B, Jiang T, Shen S, She X, Tuo Y, Hu Y, et al. Cyclopamine disrupts tumor extracellular matrix and improves the distribution and efficacy of nanotherapeutics in pancreatic cancer. Biomaterials. 2016;103:12–21. https://doi.org/10.1016/j.biomaterials.2016.06.048.
Duan S, Sun F, Qiao P, Zhu Z, Geng M, Gong X, et al. Detachable dual-targeting nanoparticles for improving the antitumor effect by extracellular matrix depletion. ACS Biomater Sci Eng. 2023;9(3):1437–49. https://doi.org/10.1021/acsbiomaterials.2c01179.
Lee S, Han H, Koo H, Na JH, Yoon HY, Lee KE, et al. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J Control Release. 2017;263:68–78. https://doi.org/10.1016/j.jconrel.2017.02.035.
Wang L, Dou J, Jiang W, Wang Q, Liu Y, Liu H, Wang Y. Enhanced intracellular transcytosis of nanoparticles by degrading extracellular matrix for deep tissue radiotherapy of pancreatic adenocarcinoma. Nano Lett. 2022;22(17):6877–87. https://doi.org/10.1021/acs.nanolett.2c01005.
Fang T, Zhang J, Zuo T, Wu G, Xu Y, Yang Y, et al. Chemo-photothermal combination cancer therapy with ROS scavenging, extracellular matrix depletion, and tumor immune activation by telmisartan and diselenide-paclitaxel prodrug loaded nanoparticles. ACS Appl Mater Interfaces. 2020;12(28):31292–308.
Xiao M, He J, Yin L, Chen X, Zu X, Shen Y. Tumor-associated macrophages: critical players in drug resistance of breast cancer. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.799428.
Lafta HA, AbdulHussein AH, Al-Shalah SA, Alnassar YS, Mohammed NM, Akram SM, et al. Tumor-associated macrophages (TAMs) in cancer resistance; modulation by natural products. Curr Top Med Chem. 2023;23(12):1104–22.
Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines. 2016;4(3):28. https://doi.org/10.3390/vaccines4030028.
Zhang Y, Hughes KR, Raghani RM, Ma J, Orbach S, Jeruss JS, Shea LD. Cargo-free immunomodulatory nanoparticles combined with anti-PD-1 antibody for treating metastatic breast cancer. Biomaterials. 2021;269:120666. https://doi.org/10.1016/j.biomaterials.2021.120666.
Sun M, Gu P, Yang Y, Yu L, Jiang Z, Li J, et al. Mesoporous silica nanoparticles inflame tumors to overcome anti-PD-1 resistance through TLR4-NFκB axis. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2021-002508.
Guo X-Y, Zhang J-Y, Shi X-Z, Wang Q, Shen W-L, Zhu W-W, Liu L-K. Upregulation of CSF-1 is correlated with elevated TAM infiltration and poor prognosis in oral squamous cell carcinoma. Am J Transl Res. 2020;12(10):6235.
Li M, Li M, Yang Y, Liu Y, Xie H, Yu Q, et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release. 2020;321:23–35.
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–59.
Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. 2017;32(5):654-68.e5.
Almahariq MF, Quinn TJ, Kesarwani P, Kant S, Miller CR, Chinnaiyan P. Inhibition of colony-stimulating factor-1 receptor enhances the efficacy of radiotherapy and reduces immune suppression in glioblastoma. In Vivo. 2021;35(1):119–29.
Ramesh A, Brouillard A, Kumar S, Nandi D, Kulkarni A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials. 2020;227:119559.
Alhudaithi SS, Almuqbil RM, Zhang H, Bielski ER, Du W, Sunbul FS, et al. Local targeting of lung-tumor-associated macrophages with pulmonary delivery of a CSF-1R inhibitor for the treatment of breast cancer lung metastases. Mol Pharm. 2020;17(12):4691–703.
Sun Y, Cronin MF, Mendonca MC, Guo J, O’Driscoll CM. Sialic acid-targeted cyclodextrin-based nanoparticles deliver CSF-1R siRNA and reprogram tumour-associated macrophages for immunotherapy of prostate cancer. Eur J Pharm Sci. 2023;185:106427.
Tu MM, Abdel-Hafiz HA, Jones RT, Jean A, Hoff KJ, Duex JE, et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun Biol. 2020;3(1):720.
Liu Y, Tiruthani K, Wang M, Zhou X, Qiu N, Xiong Y, et al. Tumor-targeted gene therapy with lipid nanoparticles inhibits tumor-associated adipocytes and remodels the immunosuppressive tumor microenvironment in triple-negative breast cancer. Nanoscale Horizons. 2021;6(4):319–29.
Wang Y, Tiruthani K, Li S, Hu M, Zhong G, Tang Y, et al. mRNA delivery of a bispecific single-domain antibody to polarize tumor-associated macrophages and synergize immunotherapy against liver malignancies. Adv Mater. 2021;33(23):2007603.
Gu X, Gao Y, Wang P, Wang L, Peng H, He Y, et al. Nano-delivery systems focused on tumor microenvironment regulation and biomimetic strategies for treatment of breast cancer metastasis. J Control Release. 2021;333:374–90.
Mardani R, Hamblin MR, Taghizadeh M, Banafshe HR, Nejati M, Mokhtari M, et al. Nanomicellar-curcumin exerts its therapeutic effects via affecting angiogenesis, apoptosis, and T cells in a mouse model of melanoma lung metastasis. Pathol Res Pract. 2020;216(9):153082.
Zheng Y, Jia R, Li J, Tian X, Qian Y. Curcumin-and resveratrol-co-loaded nanoparticles in synergistic treatment of hepatocellular carcinoma. J Nanobiotechnol. 2022;20(1):339.
Lin M, Yao W, Xiao Y, Dong Z, Huang W, Zhang F, et al. Resveratrol-modified mesoporous silica nanoparticle for tumor-targeted therapy of gastric cancer. Bioengineered. 2021;12(1):6343–53.
Li C, Xu Y, Zhang J, Zhang Y, He W, Ju J, et al. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci Rep. 2023;13(1):13278.
Kuo I-M, Lee J-J, Wang Y-S, Chiang H-C, Huang C-C, Hsieh P-J, et al. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro-hyperthermia. BMC Cancer. 2020;20:1–13.
Ashkbar A, Rezaei F, Attari F, Ashkevarian S. Treatment of breast cancer in vivo by dual photodynamic and photothermal approaches with the aid of curcumin photosensitizer and magnetic nanoparticles. Sci Rep. 2020;10(1):21206.
Xiao Z, Su Z, Han S, Huang J, Lin L, Shuai X. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy. Sci Adv. 2020;6(6):7785.
Ghoreyshi N, Ghahremanloo A, Javid H, Homayouni Tabrizi M, Hashemy SI. Effect of folic acid-linked chitosan-coated PLGA-based curcumin nanoparticles on the redox system of glioblastoma cancer cells. Phytochem Anal. 2023;34(8):950–8.
Xue D, Hsu E, Fu Y-X, Peng H. Next-generation cytokines for cancer immunotherapy. Antibody Therap. 2021;4(2):123–33.
Mirlekar B, Pylayeva-Gupta Y. IL-12 family cytokines in cancer and immunotherapy. Cancers (Basel). 2021;13(2):167.
Todorović-Raković N. The role of cytokines in the evolution of cancer: IFN-γ paradigm. Cytokine. 2022;151:155442.
Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. 2017;6(3):e1277306. https://doi.org/10.1080/2162402x.2016.1277306.
Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–35. https://doi.org/10.1016/j.ejca.2010.07.033.
Saif A, Rossi AJ, Sarnaik A, Hernandez JM, Zager JS. Efficacy of neoadjuvant intratumoral darleukin/fibromun (L19IL2 + L19TNF) in patients with clinical stage IIIB/C melanoma (Neo-DREAM). Ann Surg Oncol. 2022;29(6):3377–8. https://doi.org/10.1245/s10434-022-11447-x.
Barberio AE, Smith SG, Correa S, Nguyen C, Nhan B, Melo M, et al. Cancer cell coating nanoparticles for optimal tumor-specific cytokine delivery. ACS Nano. 2020;14(9):11238–53.
Liu X, Gao X, Zheng S, Wang B, Li Y, Zhao C, et al. Modified nanoparticle mediated IL-12 immunogene therapy for colon cancer. Nanomed Nanotechnol Biol Med. 2017;13(6):1993–2004.
Shin H, Kang S, Won C, Min D-H. Enhanced local delivery of engineered IL-2 mRNA by porous silica nanoparticles to promote effective antitumor immunity. ACS Nano. 2023;17(17):17554–67. https://doi.org/10.1021/acsnano.3c06733.
Kim J, Kang S, Kim KW, Heo M-G, Park D-I, Lee J-H, et al. Nanoparticle delivery of recombinant IL-2 (BALLkine-2) achieves durable tumor control with less systemic adverse effects in cancer immunotherapy. Biomaterials. 2022;280:121257. https://doi.org/10.1016/j.biomaterials.2021.121257.
Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, et al. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367(2):330–5. https://doi.org/10.1016/j.bbrc.2007.12.112.
Barberio AE, Smith SG, Pires IS, Iyer S, Reinhardt F, Melo MB, et al. Layer-by-layer interleukin-12 nanoparticles drive a safe and effective response in ovarian tumors. Bioeng Transl Med. 2023;8(2):e10453.
Li Y, Su Z, Zhao W, Zhang X, Momin N, Zhang C, et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat Cancer. 2020;1(9):882–93. https://doi.org/10.1038/s43018-020-0095-6.
Liu J-Q, Zhang C, Zhang X, Yan J, Zeng C, Talebian F, et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J Control Release. 2022;345:306–13.
Lai I, Swaminathan S, Baylot V, Mosley A, Dhanasekaran R, Gabay M, Felsher DW. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J Immunother Cancer. 2018;6(1):125. https://doi.org/10.1186/s40425-018-0431-x.
Zhao Y, Song Q, Yin Y, Wu T, Hu X, Gao X, et al. Immunochemotherapy mediated by thermosponge nanoparticles for synergistic anti-tumor effects. J Control Release. 2018;269:322–36. https://doi.org/10.1016/j.jconrel.2017.11.037.
Zhao P, Tian Y, Lu Y, Zhang J, Tao A, Xiang G, Liu Y. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. J Nanobiotechnol. 2022;20(1):525. https://doi.org/10.1186/s12951-022-01731-z.
Thaker PH, Brady WE, Lankes HA, Odunsi K, Bradley WH, Moore KN, et al. A phase I trial of intraperitoneal GEN-1, an IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer, administered with pegylated liposomal doxorubicin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancers: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017;147(2):283–90. https://doi.org/10.1016/j.ygyno.2017.08.001.
Shaw AR, Suzuki M. Recent advances in oncolytic adenovirus therapies for cancer. Curr Opin Virol. 2016;21:9–15.
Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74.
Li F, Sheng Y, Hou W, Sampath P, Byrd D, Thorne S, Zhang Y. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2019-000131.
Wang X, Zhong L, Zhao Y. Oncolytic adenovirus: a tool for reversing the tumor microenvironment and promoting cancer treatment. Oncol Rep. 2021;45(4):1–9.
Zhao Y, Liu Z, Li L, Wu J, Zhang H, Zhang H, et al. Oncolytic adenovirus: prospects for cancer immunotherapy. Front Microbiol. 2021;12:707290.
Kalus P, De Munck J, Vanbellingen S, Carreer L, Laeremans T, Broos K, et al. Oncolytic herpes simplex virus type 1 induces immunogenic cell death resulting in maturation of BDCA-1+ myeloid dendritic cells. Int J Mol Sci. 2022;23(9):4865.
Gebremeskel S, Nelson A, Walker B, Oliphant T, Lobert L, Mahoney D, Johnston B. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2020-002096.
Martini V, D’Avanzo F, Maggiora PM, Varughese FM, Sica A, Gennari A. Oncolytic virotherapy: new weapon for breast cancer treatment. Ecancermedicalscience. 2020;14:1149.
Niavarani S-R, Lawson C, Boudaud M, Simard C, Tai L-H. Oncolytic vesicular stomatitis virus-based cellular vaccine improves triple-negative breast cancer outcome by enhancing natural killer and CD8+ T-cell functionality. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2019-000465.
Fournier P, Bian H, Szeberényi J, Schirrmacher V. Analysis of three properties of Newcastle disease virus for fighting cancer: tumor-selective replication, antitumor cytotoxicity, and immunostimulation. Methods Mol Biol. 2012;797:177–204. https://doi.org/10.1007/978-1-61779-340-0_13.
Ma F, Cao Y, Yan J, Lu Z, Sun L, Hussain Z, et al. Multifunctional hybrid oncolytic virus-mimicking nanoparticles for targeted induce of tumor-specific pyroptosis and enhanced anti-tumor immune response in melanoma. Nano Today. 2024;54:102063. https://doi.org/10.1016/j.nantod.2023.102063.
Jabir MS, Al-Shammari AM, Ali ZO, Albukhaty S, Sulaiman GM, Jawad SF, et al. Combined oncolytic virotherapy gold nanoparticles as synergistic immunotherapy agent in breast cancer control. Sci Rep. 2023;13(1):16843. https://doi.org/10.1038/s41598-023-42299-4.
Wu F, Li Y, Meng Y, Cai X, Shi J, Li J, et al. An ion-enhanced oncolytic virus-like nanoparticle for tumor immunotherapy. Angew Chem. 2022;134(45):e202210487.
Chan JD, Lai J, Slaney CY, Kallies A, Beavis PA, Darcy PK. Cellular networks controlling T cell persistence in adoptive cell therapy. Nat Rev Immunol. 2021;21(12):769–84.
Fuentes-Antrás J, Guevara-Hoyer K, Baliu-Piqué M, García-Sáenz JAn, Pérez-Segura P, Pandiella A, Ocaña A,. Adoptive cell therapy in breast cancer: a current perspective of next-generation medicine. Front Oncol. 2020;10:605633.
Haslauer T, Greil R, Zaborsky N, Geisberger R. CAR T-cell therapy in hematological malignancies. Int J Mol Sci. 2021;22(16):8996.
Kirtane K, Elmariah H, Chung CH, Abate-Daga D. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer. 2021;9(7):2723.
Balakrishnan PB, Sweeney EE. Nanoparticles for enhanced adoptive T cell therapies and future perspectives for CNS tumors. Front Immunol. 2021;12:600659.
Zheng C, Zhang J, Chan HF, Hu H, Lv S, Na N, et al. Engineering nano-therapeutics to boost adoptive cell therapy for cancer treatment. Small Methods. 2021;5(5):2001191.
Prazeres PHDM, Ferreira H, Costa PAC, da Silva W, Alves MT, Padilla M, et al. Delivery of plasmid DNA by ionizable lipid nanoparticles to induce CAR expression in T cells. Int J Nanomed. 2023;18:5891–904.
Zhang F, Stephan SB, Ene CI, Smith TT, Holland EC, Stephan MT. Nanoparticles that reshape the tumor milieu create a therapeutic window for effective T-cell therapy in solid malignancies. Cancer Res. 2018;78(13):3718–30. https://doi.org/10.1158/0008-5472.Can-18-0306.
Siriwon N, Kim YJ, Siegler E, Chen X, Rohrs JA, Liu Y, Wang P. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol Res. 2018;6(7):812–24. https://doi.org/10.1158/2326-6066.CIR-17-0502.
Braunstein MJ, Kucharczyk J, Adams S. Targeting toll-like receptors for cancer therapy. Target Oncol. 2018;13(5):583–98.
Qin M, Li Y, Yang X, Wu H. Safety of Toll-like receptor 9 agonists: a systematic review and meta-analysis. Immunopharmacol Immunotoxicol. 2014;36(4):251–60.
Zhou L, Zou M, Xu Y, Lin P, Lei C, Xia X. Nano drug delivery system for tumor immunotherapy: next-generation therapeutics. Front Oncol. 2022. https://doi.org/10.3389/fonc.2022.864301.
Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6:1–13.
Schirrmacher V. Cancer vaccines and oncolytic viruses exert profoundly lower side effects in cancer patients than other systemic therapies: a comparative analysis. Biomedicines. 2020;8(3):61.
Rasa A, Alberts P. Oncolytic virus preclinical toxicology studies. J Appl Toxicol. 2023;43(5):620–48.
Ajam-Hosseini M, Akhoondi F, Doroudian M. Nano based-oncolytic viruses for cancer therapy. Crit Rev Oncol Hematol. 2023;185:103980. https://doi.org/10.1016/j.critrevonc.2023.103980.
Myers G. Immune-related adverse events of immune checkpoint inhibitors: a brief review. Curr Oncol. 2018;25(5):342–7.
Ashrafizadeh M, Zarrabi A, Hushmandi K, Zarrin V, Moghadam ER, Zabolian A, et al. PD-1/PD-L1 axis regulation in cancer therapy: the role of long non-coding RNAs and microRNAs. Life Sci. 2020;256:117899. https://doi.org/10.1016/j.lfs.2020.117899.
Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8(1):34.
Kawashima S, Togashi Y. Resistance to immune checkpoint inhibitors and the tumor microenvironment. Exp Dermatol. 2023;32(3):240–9.
Gupta R, Kadhim MM, Turki Jalil A, Qasim Alasheqi M, Alsaikhan F, Khalimovna Mukhamedova N, et al. The interactions of docetaxel with tumor microenvironment. Int Immunopharmacol. 2023;119:110214. https://doi.org/10.1016/j.intimp.2023.110214.
Yu D-L, Lou Z-P, Ma F-Y, Najafi M. The interactions of paclitaxel with tumour microenvironment. Int Immunopharmacol. 2022;105:108555. https://doi.org/10.1016/j.intimp.2022.108555.
Moslehi M, Moazamiyanfar R, Dakkali MS, Rezaei S, Rastegar-Pouyani N, Jafarzadeh E, et al. Modulation of the immune system by melatonin; implications for cancer therapy. Int Immunopharmacol. 2022;108:108890. https://doi.org/10.1016/j.intimp.2022.108890.
Mu Q, Najafi M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int Immunopharmacol. 2021. https://doi.org/10.1016/j.intimp.2021.107895.
Qin Y, Zhang H, Li Y, Xie T, Yan S, Wang J, et al. Promotion of ICD via nanotechnology. Macromol Biosci. 2023;23(9):2300093.
Hernández Á-P, Juanes-Velasco P, Landeira-Viñuela A, Bareke H, Montalvillo E, Góngora R, Fuentes M. Restoring the immunity in the tumor microenvironment: insights into immunogenic cell death in onco-therapies. Cancers (Basel). 2021;13(11):2821.
Birmpilis AI, Paschalis A, Mourkakis A, Christodoulou P, Kostopoulos IV, Antimissari E, et al. Immunogenic cell death, DAMPs and prothymosin α as a putative anticancer immune response biomarker. Cells. 2022;11(9):1415.
Chen S, Song Z, Zhang A. Small-molecule immuno-oncology therapy: advances, challenges and new directions. Curr Top Med Chem. 2019;19(3):180–5.
Qiu Y, Su M, Liu L, Tang Y, Pan Y, Sun J. Clinical application of cytokines in cancer immunotherapy. Drug Des Devel Ther. 2021;15:2269–87.
Lee SN, Jin SM, Shin HS, Lim YT. Chemical strategies to enhance the therapeutic efficacy of toll-like receptor agonist based cancer immunotherapy. Acc Chem Res. 2020;53(10):2081–93. https://doi.org/10.1021/acs.accounts.0c00337.
Keshavarz M, Miri SM, Behboudi E, Arjeini Y, Dianat-Moghadam H, Ghaemi A. Oncolytic virus delivery modulated immune responses toward cancer therapy: challenges and perspectives. Int Immunopharmacol. 2022;108:108882.
Howard F, Muthana M. Designer nanocarriers for navigating the systemic delivery of oncolytic viruses. Nanomedicine. 2020;15(1):93–110.
Kepp O, Senovilla L, Kroemer G. Immunogenic cell death inducers as anticancer agents. Oncotarget. 2014;5(14):5190.
Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(4):e1008866.
Mazari SA, Ali E, Abro R, Khan FSA, Ahmed I, Ahmed M, et al. Nanomaterials: applications, waste-handling, environmental toxicities, and future challenges—a review. J Environ Chem Eng. 2021;9(2):105028.
Muhammad Q, Jang Y, Kang SH, Moon J, Kim WJ, Park H. Modulation of immune responses with nanoparticles and reduction of their immunotoxicity. Biomater Sci. 2020;8(6):1490–501.
Colaço M, Marques AP, Jesus S, Duarte A, Borges O. Safe-by-design of glucan nanoparticles: size matters when assessing the immunotoxicity. Chem Res Toxicol. 2020;33(4):915–32.
Sonin D, Pochkaeva E, Zhuravskii S, Postnov V, Korolev D, Vasina L, et al. Biological safety and biodistribution of chitosan nanoparticles. Nanomaterials. 2020;10(4):810.
Lee Y, Jeong M, Park J, Jung H, Lee H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp Mol Med. 2023;55(10):2085–96.
Simón M, Jørgensen JT, Norregaard K, Kjaer A. 18F-FDG positron emission tomography and diffusion-weighted magnetic resonance imaging for response evaluation of nanoparticle-mediated photothermal therapy. Sci Rep. 2020;10(1):7595.
Liu X, Wang M, Jiang Y, Zhang X, Shi C, Zeng F, et al. Magnetic resonance imaging nanoprobe quantifies nitric oxide for evaluating M1/M2 macrophage polarization and prognosis of cancer treatments. ACS Nano. 2023;17(24):24854–66.
Acknowledgements
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the small Research Group Project under Grant No. R.G.P.2/44/45.
Author information
Authors and Affiliations
Contributions
All authors contributed in the first draft writing. I A edited and reviewed final version of paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, I., Altameemi, K.K.A., Hani, M.M. et al. Shifting cold to hot tumors by nanoparticle-loaded drugs and products. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03577-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03577-3