Abstract
Breast cancer had been the first non-hematologic malignancy where sub-types based on molecular characterization had entered clinical practice. HER2 over-expression, due to either gene amplification or protein up-regulation, defines one of these sub-types and is clinically exploited by addition of HER2-targeted treatments to the regimens of treatment. Nevertheless, in many occasions HER2-positive cancers are resistant or become refractory to these therapies. Several mechanisms, such as activation of alternative pathways or loss of expression of the receptor in cancer cells, have been proposed as the cause of these therapeutic failures. Cancer stem cells (CSCs, alternatively called tumor-initiating cells) comprise a small percentage of the tumor cells, but are capable of reconstituting and propagating tumors due to their superior intrinsic capacity for regeneration, survival and resistance to therapies. CSCs possess circuits enabling epigenetic plasticity which endow them with the ability to alternate between epithelial and mesenchymal states. This paper will discuss the expression and regulation of HER2 in CSCs of the different sub-types of breast cancer and relationships of the receptor with both the circuits of stemness and epithelial–mesenchymal plasticity. Therapeutic repercussions of the relationship of HER2-initiated signaling with stemness networks will also be proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased expression or amplification of HER2 protein (transcribed from the ERBB2 gene at human chromosome 17q) is present and targeted in a subset of clinically and molecularly characterized breast cancers [1]. The HER2-positive subset constituting 15–20% of all breast cancers is associated with an aggressive biology but responds well, although not uniformly, to targeted treatments inhibiting the receptor [2]. This may imply that, in most tumors belonging to this breast cancer sub-type, tumor cells are addicted to HER2 and thus switching off signaling from it is deleterious for both tumor establishment and maintenance. In terms of the cancer stem cell theory, addiction to a certain molecular lesion may be translated to expression and dependence of both cancer stem and non-stem cell populations to the lesion of interest. Targeted treatments against HER2, such as trastuzumab, pertuzumab, trastuzumab emtansine, lapatinib or neratinib, are able, as a result, to inhibit cancer stem cells (CSCs) that control tumor propagation. In addition these therapies are able to inhibit non-stem cells which are the source of bulk tumor growth. Non-stem cells can eventually acquire stem cell characteristics due to genetic instability and accumulation of random mutations, some of which may happen to confer stemness properties and are positively selected [3]. Clinical success of HER2 targeting treatments notwithstanding, new data from the International Cancer Genome Consortium (ICGC) suggest that HER2-positive breast cancers are a heterogeneous group that encompasses cancers across the breast cancer spectrum, from luminal A to basal-like [4]. This refinement of the initial genomic classification of breast cancers [5] implies that HER2 molecular lesions are not a defining property of any subset of breast cancers, despite their key role in therapeutics. In fact, presence of variable sub-groups within HER2-positive cancers could influence the variable response to HER2-targeting therapies and could be related to lower expression of HER2 receptor in subsets (e.g., CSCs) of cancer cells within sub-groups of HER2-positive breast cancers.
The role of HER2 in other subsets of breast cancer is even less well defined. There are data supporting a role and expression of the receptor in CSCs of luminal types due to post-translational dysregulation of the protein production and turnover rather than genetic lesions, in a manner similar to clinically HER2-positive, HER2-overexpressing but not amplified cancers [6]. In these luminal cancers as well as in triple-negative types, the bulk of the tumor cells do not over-express HER2 and thus they typically are not considered candidates for anti-HER2 treatments. Nevertheless, given the expression of HER2 in stem cells of some cancers that would not fulfill the current clinical criteria for HER2 positivity and the benefit of anti-HER2 therapy in some of these cases in previous clinical trials [7], a new trial (NSABP B-47) has been launched to better characterize HER2 therapy benefit in HER2-negative cancers in the adjuvant setting. Results of this trial presented so far in an abstract form showed that there is no benefit from the addition of trastuzumab in the adjuvant setting for patients with clinically negative HER2 breast cancers, suggesting that HER2 is not expressed in CSC of these cancers or, if expressed, it is not a driving force and, thus, is not a useful target [8].
Based on these considerations, this paper will investigate the role of HER2 as a marker of CSCs and its role in the plasticity that characterize the stem cell state associated with epithelial to mesenchymal and mesenchymal to epithelial transitions (EMT and MET) as well as intermediate states. Transitions between these states, sometimes referred to collectively as epithelial to mesenchymal plasticity (EMP), are associated with stem cell properties [9,10,11].
HER2 signaling and the core stemness and EMT circuits
EGFR family receptor tyrosine kinases and in fact several other receptor tyrosine kinases signal through a hardware of intracellular pathways that include the Ras/Raf/MEK/ERK pathway, the PI3K/Akt pathway and the JAK/STAT pathway [12]. Signals culminate in proliferation, apoptosis inhibition and increased motility, and play a role in acquisition of virtually all cancer characteristics.
HER2 intracellular signaling has multiple connections with signaling pathways involved in the establishment of the pluripotency network [13]. Growth factor receptors of the EGFR family and other receptor tyrosine kinases such as IGF-1R, PDGFR, RET (receptor for GDNF, Glial Cell Derived Neurotrophic Factor) and c-MET (Hepatocyte Growth Factor Receptor), as well as non-tyrosine kinase receptors such as Wnt, Notch and Hedgehog (HH) are initiators of signaling pathways that contribute to establishment of the pluripotency network [14,15,16,17,18]. This network characterized by activation of core transcription factors (TFs), such as OCT4, SOX2, Nanog, c-myc and KLF4, and supported by a panel of additional TFs such as STAT3 and other proteins, such as LIN28 and Musashi, also operates during normal development. Various combinations of the pluripotency network factors have been used in “cocktails” for the reprogramming of differentiated cells to pluripotent cells (termed induced pluripotent stem cells) [19]. These observations suggest that there are multiple pathways to pluripotency which match the number of ways that the permissive epigenetic state allowing for multiple cell fates, characterizing stemness, may be obtained. Similar to pluripotency, the process of EMT is established through induction of another set of transcription regulators that include Snail and Slug, ZEB1 and ZEB2 and TWIST, and receive cues from an overlapping complement of transduction pathways, among which is the EGFR family-initiated signaling [20]. Pathways initiated by engagement of receptor tyrosine kinases and other surface receptors serve to communicate messages from the cellular environment and lead to the activation of intracellular effectors contributing to execution of normal cellular functions. In cancer, induction of the pluripotency state may result from the co-operation of these signals with underlying genetic lesions that determine cell fate and identity. Defects of signaling in virtually all of these transduction pathways have been identified in cancer and are involved in acquisition of the hallmarks of cancer including, among others, invasion and metastatic ability which is closely coupled to EMT and stemness/plasticity states [21].
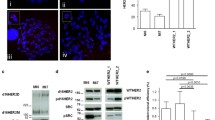
The specific role of HER2 signaling as part of the EGFR pathway in breast cancer has been extensively studied and has led to the successful therapeutic targeting in HER2-positive breast cancers [22]. An association of HER2 signaling with stem cell pathways and markers has been established in breast cancer. HER2 expression has been documented to co-exist with the presence of stem cell markers/phenotypes CD44+/CD24−/low and ALDH [23, 24]. HER2 over-expression in cell lines increases the ALDH-expressing population and xenograft-forming capacity [25]. HER2 transfection induces members of the Notch and HH/Gli pathways as well as transcription factor OCT4 compared to control cells transfected with an empty vector (Fig. 1). An even more robust induction of these proteins is observed in the ALDH-positive subset of HER2-expressing cells [25]. In addition, the ALDH-positive HER2-expressing population shows increased xenograft-forming capability, while the ALDH-negative subset remains unable to form xenografts despite HER2 expression (Fig. 1, right). These experiments argue that HER2 is a facilitator, but not an inducer of stemness.
Left: transfection of HER2 in ALDH-positive CSCs promotes pluripotency and EMT plasticity by up-regulating several pathways such as Notch, HH and TGF-β, as well as pluripotency TF Oct4. In contrast (right), in non-stem cells (ALDH-negative), HER2 transfection by itself is not able to establish stemness. Solid arrows denote activating signaling and dashed arrows denote movement of proteins in different cellular locations. HH hedgehog, TGFβ transforming growth factor β
Notch is a stemness-related pathway with several connections to EGFR/HER2 [26]. Notch signaling, in co-operation with EGFR, is involved in the communication of ER-positive non-stem cells with adjacent ER-negative CSCs [27]. HER2 gene promoter possesses a binding site for CBF1 (also called RBPJ), the transcriptional partner/co-activator of NICD (Notch Intracellular Domain) and thus HER2 may become induced through Notch signaling [26, 28]. HER2 induction in stem cells through this mechanism of Notch-mediated paracrine communication from non-stem hormone receptor-positive cells would not be dependent on gene amplification and would promote stem cell survival through survivin stabilization [29]. Consistent with this, the mammosphere-forming subset of an HER2-positive cell line displayed higher HER2 expression and this expression was down-regulated by pharmacologic treatment with a γ-secretase inhibitor or a Notch1 small interfering RNA [30]. Moreover, trastuzumab treatment of CSCs decreased their ability to form xenografts in mice. In parallel, Notch signaling induces EMT through up-regulation of EMT core TFs Snail and Slug [31,32,33]. On the other hand, HER2 activity suppresses Notch signaling and thus may promote an intermediate phenotype across the EMT/MET spectrum [29, 34]. This may be dependent on the specific cellular environment, given that HER2 up-regulates Notch in some cells as mentioned above [25]. In addition, Notch signaling up-regulation following HER2 inhibition may contribute to dormancy of a residual stem cell population that has the potential to fuel recurrence [35].
The canonical Wnt pathway culminating in β-catenin activation is another stem cell-promoting signal transduction pathway with associations with HER2 (Fig. 2). HER2 signaling can activate β-catenin through inhibition of kinase GSK3 by kinase Akt [36, 37]. Reciprocally, EMT induction by Wnt pathway may up-regulate metalloproteinases which cleave the extracellular domain of HER2 and result in both increased activity and trastuzumab resistance [38,39,40]. Wnt up-regulation and pathway activation promote EMT and resistance of HER2-positive cell lines to trastuzumab [41]. Notably, Wnt activation leading to β-catenin nuclear localization also promotes EMT features in triple-negative breast cancer cells, suggesting that the pathway is independent of HER2 in these cells [42]. However, EGFR signaling is often active in triple-negative breast cancer cells and may substitute for HER2 absence by activating the same downstream effectors, including PI3K/Akt [43]. PI3K/Akt was found to be activated also in HER2-positive cells that had become resistant to lapatinib and the pan-HER inhibitor AZD8931 by gradually increasing the concentration of these drugs in culture [44]. Resistant cells displayed EMT characteristics and showed activation of kinases Src and Axl that allowed for PI3K/Akt activation without HER2 input.
Signaling from EGFR/HER2 activates β-catenin by an alternative to Wnt signaling route. Other receptors such as Axl and CXCR1 participate in establishment of a network promoting stemness with multiple feedforward loops. Transcription factors NF-κB and STAT3 are prominent in these networks by up-regulating LIN28 and survivin, respectively, but also proteins of the network such as IL-8, CXCR1 and HER2 itself. Solid arrows denote activating signaling and dashed arrows denote movement of proteins in different cellular locations
Cytokine interleukin-8 (IL-8) receptor CXCR1 co-operates with HER2 in promoting stemness [45] (Fig. 2). A positive circuit is established through activation of transcription factor NF-κB by HER2-induced Akt signaling, which promotes transcription of IL-8 that then activates receptor CXCR1 leading to downstream activation of HER2 signaling through kinase Src [45]. In addition, CXCR1 signaling, through Src and STAT3 activation, reinforces NF-κB activity and promotes positive feedback loops through induction of cytokines [46, 47]. An additional positive feedback loop is created by the activation of stemness regulator LIN28 by NF-κB [48]. LIN28 up-regulates HER2 mRNA in breast cancer cells [49]. HER2-positive cell lines have decreased expression of microRNA let-7, an LIN28 mRNA translation inhibitor, compared with HER2-negative cell lines [50]. Inhibition of MEK signaling with pharmacologic inhibitors downstream of HER2 restored let-7 levels in HER2-positive cells. Conversely, forced expression of HER2 in MCF7 HER2-negative cells led to a decrease in let-7 expression. Thus, a positive feedback loop for LIN28 up-regulation/let-7 down-regulation is favored in cells with operational HER2 signaling, promoting stemness. HER2 may also directly activate STAT3 in HER2-positive breast cancer cells, leading to up-regulation of STAT3 target gene survivin, an apoptosis inhibitor [51]. Activation of this pathway promotes resistance to radiotherapy, while inhibition of HER2 by lapatinib or RNA interference reverses this resistance by abrogating phosphorylation of STAT3 at tyrosine 705 and inhibiting survivin transcription [51]. In another study, phosphorylation of STAT3 in HER2-overexpressing breast cancer cells was associated with expression of stem cell transcription factors OCT4 and SOX2 as well as CD44 and was reversed by a combination of trastuzumab and the STAT3 inhibitor Stattic [52]. Additionally, HER2 promoter possesses a STAT3 binding sequence and may be directly up-regulated by STAT3 [53]. STAT3 was also involved in acquisition of lapatinib resistance of HER2-overexpressing cells associated with another cytokine, IL-6 [54]. Resistant cells contained a higher percentage of CD44high/CD24low stem cells. IL-6 is a cytokine associated with stemness and EMT promotion [55,56,57]. A high affinity antibody targeting IL-6 called MEDI5117 was shown to inhibit trastuzumab-resistant HER2-positive breast cancer cells [58]. IL-6 blockade by MEDI5117 decreased phosphorylation of STAT3 and the CD44+/CD24− stem cell population frequency. Besides autocrine and paracrine production and action, IL-6 may also be among the cytokines enriched in the tumor microenvironment from incoming platelets, mediating the adverse effect of thrombocytosis observed in breast cancer [59].
Besides the let-7 miR mentioned above, other miRs are regulators of stem cell and EMT circuits associated with HER2 signaling. miR-124 is down-regulated in HER2-positive cell lines. miR-124 normally targets STAT3 and, thus, its down-regulation contributes to the preservation of STAT3 activity downstream of HER2 signaling [60]. miR-205-5p targets directly HER2 and has been proposed to have a role in resistance of HER2-positive breast CSCs to lapatinib, through down-regulation of HER2 and indirect down-regulation of EGFR [61].
Growth factor receptors including the EGFR family are also involved in EMT induction. Extensive overlap of EMT plasticity and stemness pathways exist, as mentioned above in the paragraphs of Wnt and Notch signaling. TGFβ is a pathway that, besides playing a key role in EMT, is up-regulated in HER2 over-expressing cells [62]. HER2-negative MCF7 and MDA-MB-231 cells transduced with HER2 up-regulate TGFβ and downstream SMAD molecules. In addition, HER2-transduced cells become more invasive and morphologically attain mesenchymal characteristics associated with induction of core EMT TFs Snail, Slug and ZEB1. These effects are partially reversed when HER2 is knocked down with a shRNA [62].
EMT TFs Snail and Twist up-regulate transcription factor FRA-1 (Fos Related Antigen 1), a transcriptional regulator of the AP-1 family, which becomes available for activation by PDGFR/PKCα signaling in cells that have undergone an EMT [63] (Fig. 3). A switch from EGFR signaling, which is predominant in epithelial cells and leads to activation of another member of the AP-1 family, c-FOS, to PDGFR signaling has been observed in these cells when they undergo EMT [63]. On the other hand, PKCα phosphorylates HER2 for recycling to cell surface instead of degradation and it has been proposed as the cause of HER2 over-expression without amplification in HER2-positive (2 + or 3 + by IHC, but not amplified by FISH) breast cancers [64]. Subsequently, HER2 activates PKCα through Src kinase and promotes invasion [65]. Thus, there exist subtleties between cell surface signaling receptors that use the same intracellular transduction systems leading to different outcomes based on the specific cell state. Moreover, over-expression of FRA-1 could induce CD44 in MCF-7 ER-positive cells, but not in ER-negative MDA-MB-231 cells, again confirming the importance of cellular microenvironment [66]. In the same vein, PTEN deficiency in HER2-positive cells treated with trastuzumab leads to EMT and treatment resistance [67]. In this case, EMT is associated with HER2 down-regulation transforming cells to triple negative. Interestingly, during the transition, cells displayed a reversal of their CSC phenotype content from ALDH+ predominance in HER2-positive cells to CD44+/CD24−/low predominance in triple-negative cells. Homeobox transcription factor MEOX1 (Mesenchyme cells homeobox 1) up-regulation was found to be associated with PTEN loss-mediated EMT. In addition, MEOX1 nuclear staining was associated with worse prognosis in a series of breast cancer patients [67]. In triple-negative breast cancer cells, where HER2 is not operative, AP-1 signaling from c-JUN/FRA-1 dimers leads to ZEB2 up-regulation and EMT promotion [68]. FRA-1 was significantly over-expressed in tumor samples from triple-negative breast cancer patients compared to luminal breast cancer patients [69]. Moreover, transfection of FRA-1 in mammary epithelial cells led to ZEB1 and TGFβ up-regulation and increase in their metastatic potential [70].
Signaling from EGFR/HER2 promotes recycling of HER2 to the membrane by activating kinase Src, which then activates PKCα that phosphorylates endocytosed HER2, averting its ubiquitination for proteasomal degradation. EGFR/HER2 and other receptor kinases, such as PDGFR, signaling as well as availability of intracellular mediators such as c-Fos and FRA-1 affect stemness and EMT-related proteins expression through transcription regulation of AP-1 promoters. Solid arrows denote activating signaling and dashed arrows denote movement of proteins in different cellular locations
Overall, it appears that HER2 activity augments stem cell circuits, promotes plasticity of cells with stem cell characteristics and favors phenotypic transitions between epithelial and mesenchymal characteristics of these stem cells depending on the specific cellular context (Fig. 4). c-FOS transcription factor overrides FRA-1 in HER2-positive cells and favors the epithelial side of the EMT spectrum, while FRA-1 signaling is promoted in the absence of HER2 or upon suppression of HER2 signaling and reinforces EMT TFs expression and metastasis.
Schematic representation of stemness to differentiation continuum (y axis) versus the epithelial to mesenchymal continuum (x axis) and relationships to HER2. Stemness state is associated with the ability to move along the x axis to various epithelial, mesenchymal and in-between states, which is lost as cells move up the differentiation axis where they become fixed to the epithelial state. HER2 signaling favors movement toward the epithelial part of the EMT axis and promotes stemness
A caveat of the information discussed in this section is that a significant proportion of data on the subject of HER2, stemness and plasticity of breast cancer is derived from studies with cancer cell lines, either in vitro or in vivo in mice xenografts. This is in general true for mechanistic studies in many areas of molecular and cellular biology of cancer and it has to be taken into consideration when evaluating these data and eventually attempting to translate to clinical therapeutics.
Breast cancer stem cells and expression of HER2 in stem cells
Identification of HER2 expression in clinical HER2-positive breast cancers implies that the receptor is amplified or over-expressed in the majority of cells which are non-stem cells. It is not excluded, though, that the receptor is not expressed in subsets of CSCs of HER2-positive cancers. Inversely, in other breast cancer sub-types, luminal and triple-negative cancers, the HER2 receptor is not over-expressed in bulk tumor cells and thus it does not satisfy the criteria of positivity in clinical pathology samples. But what is the expression status of the HER2 receptor in stem cells of these different sub-types? A related question, given the plasticity inherent to the stem cells nature, is whether the putative expression of HER2 in these cells is comparatively stable and embedded in the circuits that maintain stemness or is post-translationally up-regulated and thus potentially lost in progeny, a fact that explains HER2 negativity in the pathologic evaluation of these cancers. For therapeutic purposes, even in the latter case, HER2 may provide an opportunity for stem cells targeting, but combination with treatments addressing non-stem cells may be required. This section will discuss the available data on HER2 expression in clinical samples of different sub-types of breast cancer and compare it to non-stem populations. An important consideration during investigating the above questions of HER2 expression in stem cells of breast cancer sub-types is how stem cells have been defined in different studies and specifically the distinction between studies that include functional or only phenotypic definitions. The former requires establishment of tumors as xenografts in mice, while the latter relies on positivity to certain surface markers which, for breast cancer stem cells, include the CD44+/CD24−/low phenotype and ALDH positivity [71]. Phenotype-based characterization is practical in studies employing clinical samples but, on the other hand, it may be less accurate, given that phenotypic positivity for these markers only partially overlaps with functional stem cells. In fact, the overlap of expression of the CD44+/CD24−/low phenotype with ALDH positivity is very low and observed in about 1% of cells [72]. Moreover, it has been noted that there are subsets of breast cancer stem cells that express CD44 or ALDH and these are not functionally equivalent [73,74,75]. These distinct stem cell subsets reflect distinct normal luminal and basal stem cell populations of the breast [76]. Critically, as shown in xenograft experiments, the population of breast cancer cells with a combined CD44+/CD24− phenotype and ALDH positivity displayed the highest xenograft-forming capacity, while the ALDH-positive cells without CD44+/CD24− phenotype had a lower xenograft-forming capacity and cells with the CD44+/CD24−/ALDH− phenotype had no xenograft-forming capacity [77]. However, this may not necessarily imply that CD44+/CD24− cells with ALDH negativity have no stem cell potential, but may instead be due to a requirement for paracrine signals from ALDH-positive cells or alternatively may be due to their quiescence which does not allow for xenograft establishment within the experimental time frame. This is consistent with data from a p53 knockdown model which suggests dependence of tumor-initiating cell populations on other supportive cell subsets [78].
HER2 expression in stem cells of HER2-positive breast cancers
Cells with the CD44+/CD24−/low phenotype and ALDH positivity are expressed with a variable frequency across the spectrum of both invasive and DCIS breast cancer sub-types, including cases of HER2+ breast cancers [75]. A small study of clinical breast cancer samples that included all sub-types reported a range of 0.1–21.2% for the CD44+/CD24−/low population (average of 6.1%). The great majority of cases (91%) contained cells with this phenotype, when examined by an immuno-fluorescence and confocal microscopy method [79]. Expression of the stem cell phenotypes CD44+/CD24−/low and ALDH+ was observed in about 30% and 10% of patients with HER2-positive cancers, respectively [80]. This study considered positive cases to be those with 10% or more of the tumor cells staining positive. Another study that used also 10% of tumor cells as the cutoff for positivity found a higher percentage of CD44+/CD24−/low-positive cases (63%) among clinically HER2+ carcinomas [72]. ALDH positivity was observed in about 14% of HER2+ cases, in the same study with a cutoff of 1% in this case [72]. Co-expression of HER2 with stem cell markers was not specifically examined in these studies, but the fact that examined cases were HER2-positive as per clinical criteria implies that HER2 may be expressed in both stem and non-stem cells of HER2-positive breast cancer. The cutoff of 10% used in some studies for stem cell marker positivity does not exclude that the rest of cases had lower numbers of cells with the stem cell phenotype and those may co-express HER2. Importantly, one of the studies showed that the two positive stem cell populations had a positive correlation with each other, implying that they are inter-connected and probably part of a stem cell spectrum [80]. Cases positive for the CD44+/CD24−/low population had a stronger association with ER negativity and the basal-like phenotype (defined as CD5/6 or EGFR1 positivity by immunohistochemistry), while ALDH+ cases had a less strong and statistically borderline association with these phenotypes. CD44 expression may confer resistance of HER2+ cells to trastuzumab [81]. Despite that, the two proteins, CD44 and HER2, display significant network relatedness in gene set enrichment analysis (GSEA), a fact that indicates overlapping downstream signaling of the two molecules that could allow for maintenance of signals in CD44+ cells in the absence of HER2. CD44 isoforms may also act as co-activators of all three ERBB family members, besides HER2 (EGFR1, HER3 and HER4), that have ligand-binding capacity [82].
In a study of HER2+ breast cancer patients treated with chemotherapy and trastuzumab, co-expression of the basal phenotype as defined by positivity for CK5/6 or EGFR was associated with worse survival outcomes [83]. Although basal phenotype may be associated with an increase in stemness characteristics, this was not specifically addressed in the clinical samples. In a correlative cell line study, the investigators showed that HER2+ cell lines with basal characteristics were resistant to trastuzumab, whereas non-basal HER2+ cell lines were sensitive and the combination of paclitaxel and trastuzumab had additive cytotoxic effects on them. In addition, basal HER2+ cell lines were enriched in stem cell characteristics as defined by a significantly higher ability for mammosphere formation than non-basal HER2+ cell lines. Trastuzumab resistance was traced to kinase Akt activity, given that phosphorylation of Akt was reversed by trastuzumab in sensitive, but not resistant cell lines and an Akt inhibitor reduced cell proliferation of trastuzumab-resistant cell lines [83]. The prognostic importance of significant levels (> 10% of cells) of co-expression of CK5/6 in HER2+ carcinomas was confirmed in another study, which showed that tumors having more than 10% CK5/6-positive cells had a worse survival [84]. In this study CK5/6 positivity was associated with expression of EMT TFs, Twist and Slug (Snail2). Slug association with HER2-positive cells’ resistance to trastuzumab was confirmed in another study and knockdown of Slug restored trastuzumab sensitivity of these cells in xenograft experiments [85]. Slug expression was associated with the stem cell phenotype CD44+/CD24−/low, while Slug knockdown up-regulated CD24. Given the relationship of EMT and stemness networks, these data corroborate the above discussed evidence from breast cancer cell lines.
A model was proposed, attempting to revisit all data of stem cells diversity and relationship to different subsets of HER2-positive cancers, encompassing pure HER2 positive, HER2 positive with ER positivity (luminal-like) and HER2 positive with basal-like or claudin low-like features [74, 86]. The model hypothesizes that each of these sub-types within the clinical HER2-positive cancer spectrum has a different percentage of CSCs. A variable number of these CSCs in each case are epithelial-like expressing ALDH and show trastuzumab sensitivity, or mesenchymal-like (CD44+/CD24−/low) and trastuzumab resistant. Pure HER2+ cancers display the highest number of CSCs which are mostly of the epithelial-like type. HER2-positive, luminal-like sub-types contain a lower number of CSCs that are also essentially epithelial-like, while the basal and claudin low-like types have an intermediate number of CSCs which are in their majority mesenchymal-like and do not express high levels of HER2 or they are HER negative.
The above model does not explicitly take into consideration the underlying cause of HER2 over-expression, and specifically whether HER2 positivity is due to gene amplification or protein up-regulation. The underlying causative lesion would be in the latter case an up-regulation or increase function of the network regulating HER2 gene expression instead of a genetic lesion (amplification) in HER2 gene per se, which is observed in the former case. Up-regulation of alternative promoters could also play a role in the production of the alternative HER2 form p95HER2, which is associated with trastuzumab resistance [87,88,89] and may also be the result of up-regulation from an alternative network of TFs compared to the ones regulating the full protein, p185 expression. These differences in HER2-producing molecular lesions could explain the observation of different sub-types within the HER2-positive cancers and is worth further investigation, regarding, among other questions, the underlying upstream networks regulating HER2 expression and their relationship with stemness.
HER2 expression in stem cells of HER2-negative breast cancer sub-types
CSCs have been associated with therapy resistance in breast and other cancers. In luminal breast cancers that acquire resistance to hormonal therapies, receptor tyrosine kinase-activated pathways have been implicated in this resistance, either by direct phosphorylation of the ER that becomes able to signal without estrogen ligation (ligand-independent signaling) or by bypassing ER signaling altogether [90]. One of the main tyrosine kinase families implicated in these actions is EGFR family, including HER2. These data suggest that CSCs may have an active EGFR family signaling contributing to their inherent resistance which becomes clinically evident under endocrine treatment pressure. A preclinical study specifically addressing the role of HER2 in CSCs of ER+ , aromatase inhibitor (AI)-resistant cell lines showed that ALDH+ stem cells co-expressed higher levels of pluripotency factors OCT4 and Nanog, EMT-associated factor Twist and drug resistance protein ABCG2 (also known as BCRP) [91]. In addition, they exhibited higher colony forming capacity and a slight increase in HER2 expression than the population of the same AI-resistant cell line with the lower expression of ALDH (non-stem cell population). Overall, the AI-resistant cell line had a higher stem cell percentage than a sensitive cell line and a higher HER2 expression. Another study confirmed that the side population of ER-positive cells, which express BRCP and have tumor-initiating properties, co-express HER2 [92]. These results may argue for a role of HER2 in stem cell maintenance and hormone therapy resistance of ER+ breast cancer. Other in vitro studies in MCF7 HER2-negative cell line observed a low expression of HER2 in the CD44+/CD24− stem cell population of these cells, and the percentage of cells with expression of stem cell markers was decreased by exposure to trastuzumab under 3D culture conditions [93]. In another study of MCF7 cells growing in mammosphere conditions, cells were sorted according to their HER2 expression [94]. Cells with a lower 10% expression of HER2 had equal expression of stem cell core circuit TFs, OCT4, SOX2 and NANOG, compared with cells with the 10% highest HER2 expression after culture for 2 days, but the low HER2-expressing cells had significantly higher stem cell TFs expression after 7 days of culture compared with the high HER2-expressing subsets. SOX2 expression was reduced by treatment of cells with the stem-targeting agent salinomycin, but not by trastuzumab. The combination of the two drugs had a synergistic effect in killing mammosphere cells, suggesting that targeting of stem and non-stem cells by salinomycin and trastuzumab, respectively, produces synergism [94]. Given that the mammosphere conditions favor the growth of cells with stem cell characteristics, non-stem cells, i.e., cells with lower stemness TFs expression, in these conditions may correspond to progenitors with pluripotency capacity and higher proliferation rate, possibly cells with ALDH positivity, as opposed to CD44+/CD24− phenotype. This hypothesis would agree with the data from HER2-amplified cancers where pure HER2-positive cases had the higher ALDH-positive progenitor percentage as detailed above [74].
HER2 expression in CSCs of ER-positive breast cancers led to the hypothesis that the receptor could underline the development of resistance to treatment characteristic of these cells and could favor therapeutic escape from endocrine agents. Preclinical models with luminal breast cancer cells xenografted in mice favored the hypothesis that cancer-initiating cells, established in the bone microenvironment, show up-regulation of HER2 without amplification through RANKL (Receptor Activator of NF-κB Ligand)/RANK signaling [95]. A randomized trial designed to test the value of blocking HER2 with trastuzumab in clinically negative for HER2 breast cancers in the adjuvant setting, inspired by retrospective data for a benefit of trastuzumab and the putative HER2 expression in CSCs of HER2-negative breast cancers, was recently reported to be negative, as mentioned above [8]. This unanticipated, at least for stem cell theorists, result could be explained by two factors that could reconcile it with the data for CSC HER2 positivity in some luminal cancers. First, the CSC found positive for HER2 may be only part of the total CSC population, and another part, and possibly more significant for dormancy and subsequent clinical resistance development, could be HER2 negative or expressing low levels of HER2. Second, and perhaps related to the first reason, HER2 may not be an integral part of the stemness–plasticity/EMT circuitry in HER2-negative cancer CSCs and thus the pressure to down-regulate it exerted by targeted therapy is high, given that it would promote tumor cell survival (by neutralizing trastuzumab effect) without significant untoward effects for important stemness cell networks. Beyond HER2, these negative results serve as a reminder that mere expression of a protein in subsets of cancer cells (be it CSCs or bulk cancer cells) does not guarantee its therapeutic targeting value in a certain cancer setting even if the same protein has proof of targeting value in another setting.
EGFR expression is a hallmark of some triple-negative (ER-, PR- and HER2-negative) breast cancers. The triple-negative subset of breast cancer is characterized by an expanded CD44+/CD24−/low stem cell population. Autocrine signaling from EGFR in triple-negative cells results in further expansion of this CD44+/CD24−/low stem cell population [96]. However HER2 may not be part of this signal cascade. In fact, forced expression of HER2 in triple-negative breast cancer cells promotes expression of CD24 [97]. Interestingly, triple-negative (as well as luminal A) cancers with higher expression of CD24 were reported to have a worse prognosis [98]. This may suggest that CD24+ cell population retain stem cell potential or may revert to CD24 negativity associated with stem cell properties. In triple-negative cancers, EGFR signaling cascades may use alternative members such as HER3 to co-operate with EGFR for signal transduction, a mechanism that has been observed also in HER2-positive breast cancers that become less dependent on HER2 and HER2-targeting treatment refractory [99, 100].
HER2 expression in tumor buds, circulating tumor cells (CTCs), disseminated tumor cells (DTCs) and established metastases
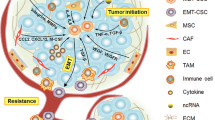
Stem cells are associated with tumor initiation and propagation and, in addition, stemness and pluripotency present a close relationship with EMT and MET [9, 10]. The process of EMT has been proposed to provide epithelial cells with the motility and invasiveness properties required for entering the circulation and eventually seeding metastatic sites through a reverse process, MET [101]. The two reciprocal processes represent the two extremes of a spectrum that encompasses also intermediate states of incomplete epithelial or mesenchymal status and thus a unifying term, epithelial–mesenchymal plasticity, has been proposed and evokes directly the pluripotency-associated plasticity [102]. Given the interconnections of the two plasticity features, CTCs (Circulating Tumor Cells) and DTCs (Disseminated Tumor Cells) would be predicted to be enriched in CSCs and, thus, present a window of opportunity for studying genes and proteins expressed in the stem cell state. This opportunity may be lost once stem cells establish themselves in the metastatic site, undergo MET and start producing non-stem cell progeny, establishing a macroscopic metastasis, detected, for example, by radiologic studies [103] (Fig. 5). This section will discuss data on expression of HER2 in metastatic cells starting to dissociate from the tumor bulk (tumor buds), in transit (CTCs) or in remote organs (DTCs or established macroscopic metastases) and compare them with expressions in respective primary tumors. Such data could inform the question of HER2 expression not only in CSCs in general, but also in those subsets of CSCs actively participating in the metastatic process. They could, thus, provide or refute support for the presence of HER2 in cells with the putative highest functional importance in tumor metastasis.
Different stages of the metastatic cascade discussed. Mobile cells in the edge of tumors are seen in histologic sections as small cell aggregates called tumor buds. Bud cells enter the circulation as isolated cells or small aggregates and arrive to remote organs, where they may extravasate and be observed as isolated cells among cells of the host organ, termed disseminated tumor cells (DTCs). The model proposes that metastatic cells, from the bud stage to the DTCs stage, contain a higher percentage of CSCs with increased plasticity and thus variable HER2 expression and signaling. In these stages, several of these cells have undergone an EMT, while when they arrive in the metastatic site they revert, in their majority, back to the epithelial state through a MET
Tumor buds
Before entering the circulation, metastasizing tumor cells detach from the rest of the tumor and are seen in histologic sections as the so-called tumor buds, defined as independent aggregates of up to four to five cells not connected to the bulk of the tumor [104]. Cells forming these buds have already demonstrated an ability of detachment and movement, and, thus, they may be cells with EMT features related to plasticity and stemness. IHC studies in histologic sections from breast cancer patients have indeed confirmed that bud cells display higher vimentin expression and lower E-cadherin expression than the center of the tumors, implying that they have undergone an EMT [105]. However, unfortunately, few data are available for the expression of HER2 in tumor bud cells of either HER2-positive or HER2-negative cancers. It appears that expression of HER2, as well as hormone receptors, are mostly concordant between the main tumor mass and tumor buds in most tumors [106]. Isolated tumor cells at the invasive front of ER-positive, HER2-negative luminal cancers have been shown to co-express HER2 and ALDH [95].
CTCs
An extensive literature exists regarding CTCs in patients with breast cancer. Currently, isolation of these cells in the peripheral blood of breast cancer patients is based on an enrichment step relying on epithelial markers and, as a result, the process may miss CTCs that have undergone EMT and thus have lost expression of epithelial surface proteins, replacing them with mesenchymal markers [107]. However, alternative methods based on enrichment by filtration and characterization by In situ hybridization confirmed that most breast cancer patients with CTCs have both epithelial and mesenchymal circulating cells, suggesting that CSCs are among the circulating population [108, 109]. This contrasts with established metastasis, where histologic sections most invariably show epithelial morphology similar to the primary breast tumor.
HER2 status has been examined in CTCs of breast cancer patients and compared with the status of primary tumors. An increase in HER2 expression frequency in CTCs compared to primary tumors could imply that the receptor is preferentially expressed in stem cells which may be enriched among CTCs. However, it has to be taken into consideration that studies have in general considered percentages of HER2 positivity in primary tumors versus CTCs rather than frequency of expression of HER2 in individual cases. This means that most studies consider, for example, cases with CTCs expressing the receptor in a certain percentage of cells above a threshold as concordant with the positive HER2 primary, without taking into account the exact percentage or intensity of expression. HER2 may change between primary tumors, CTCs (as identified by the CellSearch system) and metastases [110]. In a series of 107 patients with metastatic breast cancer and CTCs in their blood, concordance for HER2 status between primary tumors and CTCs (defined as the sum of the percentage of both primary tumors and CTCs being HER2 positive or both being negative) was 69% [96] (Table 1). Concordance between CTCs and metastatic sites was 74% and between primary tumors and metastases 83%. Six of 16 (38%) patients with primary HER2-positive tumors had HER2-negative CTCs, while 27 of 91 (30%) patients with HER2-negative primary tumors had HER2-positive CTCs (Table 1). Eight of 40 (20%) patients with HER2 negativity in their metastases had HER2-positive CTCs, while 4 of 6 (66%) patients with metastases that were HER2 positive had CTCs that were HER2 negative [110].
In another study of advanced breast cancers that used the CellSearch platform for CTC enrichment and then IF or FISH for HER2 identification showed that CTCs were present in 40 of 66 (61%) of patients [111]. HER2 concordance between HER2 primary tumors and CTCs was 68%. Eight of 28 patients (32%) with HER2-negative primary tumors had HER2-positive CTCs, while 5 of 12 (42%) with HER2-positive primary tumors had HER2-negative CTCs.
A study of metastatic breast cancer patients identified CTCs in 43% of cases (36 of 84) by AdnaTest and in 53% of cases (42 of 79) by the CellSearch kit [112]. HER2-positive CTCs were present in 50% (18/36) of cases. Concordance between primary tumors and CTCs for HER2 in this study was 59%, while the same concordance was 39% and 44%, respectively, for ER and PR. Concordance between CTCs and metastases for HER2 was 67%, while it was 43% and 46% for ER and PR, respectively.
A similar evaluation of CTCs in patients with metastatic breast cancer disclosed a frequency of CTCs in 39% of cases (90 of 229) by AdnaTest and 50% of cases (122 of 245) by the CellSearch test [113]. The two tests showed a prevalence of HER2-positive CTCs in 47% (42 of 90 with AdnaTest) and 41% (50 of 122 with CellSearch) of cases, respectively. Primary HER2-negative tumors had HER2-positive CTCs in 32% (25/78) of cases by CellCerch, and in 49% (28/57) of cases by AdnaTest BreastCancer [113].
Another study in the setting of HER2-positive early breast cancer disclosed the presence of CTCs in 258 of 642 (40.2%) cases by CellSearch, using a cutoff of one cell per 30 ml of peripheral blood. HER2-positive CTCs were present in 149 of these 258 cases (57.8%) [114].
A series compared expressions of HER2 between primary sites and CTCs in 95 breast cancer patients across stages (Table 1). It also compared HER2 expressions between primary sites and DTCs in 78 patients [115]. One of nine patients (11.1%) with HER2-positive primary tumors displayed HER2-positive CTCs. Three of 11 (27.3%) patients with an HER2-positive primary had HER2-positive DTCs. In patients with an HER2-negative primary tumor, HER2-positive CTCs were observed in 5 of 79 (6.3%) and HER2-positive DTCs were observed in 14 of 67 (20.8%) of the cases. Thus, the discordance for HER2 status between primary sites and CTCs was 15% and between primary sites and DTCs 28.2% in this study [115]. A small study that included metastatic triple-negative breast cancer patients found that six of ten such patients had HER2-positive CTCs [116].
HER2 expression is observed in sub-populations of CTCs from ER-positive HER2-negative breast cancer patients who have received multiple therapies [117]. HER2-positive sub-populations were more proliferative than HER2-negative counterparts. The two populations of CTCs could inter-convert and both had tumor-initiating capacities. HER2-positive cells were not sensitive to HER2 inhibition, but were sensitive to chemotherapy. In contrast, the HER2-negative sub-population of CTCs was resistant to chemotherapy, but sensitive to a Notch inhibitor. The combination of chemotherapy with the Notch inhibitor had a complete suppressive effect of tumorigenesis in a CTC xenograft model [117].
None of the above studies examined specifically CSCs markers such as CD44 or ALDH in CTCs concomitantly with the HER2 status and thus associations discovered rely on the fact that CSC and EMT characteristics have been suggested to be present in breast CTCs as discussed above [96]. With this assumption in mind and in the absence of actual data for co-expression of HER2 with stem cell markers in CTCs, it appears that a significant percentage of patients (30% to 40% in most cases) present with discordant HER2 status between primary tumors and CTCs and in most studies change in status is observed in both directions (positive to negative and negative to positive) with similar frequency. In contrast, the concordance for HER2 status between primary and established metastatic sites is above 80% [112, 118]. In one of the above-mentioned studies, concordance between primary and metastatic sites was higher for all three receptors, specifically 84% for HER2, 90% for ER and 83% for PR, than concordance between primary sites and CTCs [112]. Similarly in another study comparing primary and recurrent tumors, 11 of 75 (14.7%) of patients had HER2-negative primaries and became HER2 positive when they recurred, while three of 22 (13.6%) of patients with HER2-positive primaries developed HER2-negative recurrences [118]. Thus, the overall concordance rate between primary sites and recurrent metastatic sites was 85.6%. In an additional study that compared HER2 status in primary versus metastatic tumors the rate of discordance was about 7–10% [119].
These observations would argue for an involvement of HER2 signaling only in some stem cells without an absolute prerequisite for such signaling for maintenance of stemness circuits which appear to be fluctuating in regard to their HER2 status.
DTCs
DTCs are present as bone marrow micrometastases in about 30% of breast cancer patients with localized stage disease and portend poor prognosis [120]. DTCs in bone marrow of breast cancer patients have an increased prevalence of the putative CSC phenotype CD44+/CD24−/low (mean 72% of cells, median 67% with a range of 33–100% across 50 cases) [121]. This study did not report the sub-types of the examined cases.
A series examining receptor expressions in DTCs included 42 ER-positive and 12 HER2-positive patients in the primary site. Immunocytochemistry with triple fluorescence (cytokeratins, ER, HER2) of bone marrow aspirates was used [122]. Of the 42 ER-positive patients, 34 (81%) had at least one ER-positive DTC and 39 of 42 patients (93%) had at least one ER-negative DTC. Concordance for ER status between primary sites and DTCs was 74%. 22 of 48 (46%) patients with HER2-negative primary tumors had at least one HER2-positive DTC. All six patients with HER2-positive primary tumors had at least one HER2-negative DTC. HER2 concordance between primary sites and DTCs was 52%.
In another series of 569 early breast cancer patients 151 (27%) had DTCs (examined by cytokeratin staining) in BM aspirates. Among 124 primary HER2-negative tumors, 61 (49%) had HER2-negative DTCs and 63 (51%) had HER2-positive DTCs. Among the 27 HER2-positive primary tumors, 11 (41%) had HER2-negative DTCs and 16 (59%) had HER2-positive DTCs. Thus, concordance between primary sites and DTCs for HER2 was 51% [123].
A study of 105 early breast cancer patients examined HER2 status by Hercept score in the primary tumors and compared them with DTCs. In patients with primary tumors with a Hercept score of 0 or 1+, DTCs were HER2 positive in 10 of 79 cases (12%). In patients with primary tumors having Hercept score of 2 or 3+, HER2-positive DTCs were observed in 12 of 26 cases (46%) [124].
Comparisons regarding HER2 expression status between primary tumors and paired metastatic sites show a higher rate of concordance, as mentioned above. The totality of these data suggest that the discordance for HER2 is significantly greater between primary tumors and CTCs or DTCs than between primary tumors and established metastases, arguing for the cells in transit possessing significant plasticity, the hallmark of stemness, and EMT/MET states and thus expressing HER2 in different rates from the primaries. Once established in metastatic sites, CSCs produce bulk tumor cells with a phenotype more similar to the primary bulk cells, possibly with less fluctuation potential for HER2. This appears to be the case, although at different overall levels, for both clinically positive and negative HER2 breast cancers.
Conclusions and therapeutic perspective of HER2 signaling in stem cells to combat resistance in breast cancer
The discussed data pinpoint to the conclusion that HER2 appears to be often expressed in CSCs populations that give up quiescence and acquire proliferative potential to propagate tumors. In contrast, it is less or not expressed in the smaller quiescent stem cell populations. Populations of cancer cells in transit, containing variable subsets of CSCs and being in variable states across the EMP axis, contain HER2-expressing cells. Resistance is associated with down-regulation or bypassing HER2 in the proliferative stem cell population that can give rise to the bulk tumor cells.
Important HER2 signal-initiated pathways that promote concomitantly stemness and EMP have been outlined in the above discussion. PI3K and Akt kinases figure prominently as a hub of signal transduction downstream of HER2 signaling as well as a mediator of resistance to HER2 treatments when activated by alternative routes. PTEN inactivation, for example, is a common lesion in human breast cancer and has been found to promote Akt-mediated mammary stem cell enrichment and hyperplastic lesion generation in mice [125]. Targeting Akt with the inhibitor perifosine decreases specifically the stem/tumor-initiating population. In the clinic, targeting kinase mTOR, downstream of Akt by everolimus synergizes with HER2 inhibition to prolong progression-free survival in trastuzumab-resistant HER2-positive cancers [126]. These cancers may be enriched in stem cell populations, given that such populations are associated with therapy resistance. However, mTOR is only one of the multiple targets of kinase Akt that contributes to resistance, and a more complete inhibition by targeting Akt itself in the future may become a more effective therapeutic option. Unfortunately, early trials with a pan-Akt inhibitor, AZD5363, did not show activity in PI3K mutated breast cancers, despite preclinical data to the contrary [127]. An alternative strategy, that of targeting the upstream kinase of Akt, PI3K, has also been investigated. PI3K inhibition was more effective than Akt inhibition in preclinical in vitro and in vivo models of HER2-positive cells and xenografts, and this was found to be due to concomitant inhibition of wild-type Ras that is activated downstream of PI3K [128]. The pan-PI3K inhibitor buparlisib has been shown to have activity in combination with trastuzumab in trastuzumab-refractory patients in a preliminary phase Ib study [129]. However, liver toxicity was a significant limiting factor when the two drugs were combined with paclitaxel in the neo-adjuvant setting [130]. Thus, it appears that despite the significance of the pathway, clinical development of inhibitors would be more complicated than initially thought and would require more detailed biomarker profile knowledge for appropriate patient selection. This, on the other hand, would make trial logistics more demanding.
Additional key components of the HER2 network with stemness connections, the LIN28/let-7 loop and STAT3 signaling, are candidates for therapeutic targeting. One of the mRNAs down-regulated by let-7 is that of the beta2 adrenergic receptor (β2-AR). Thus in HER2-positive cells, where let-7 is suppressed, β2-AR is up-regulated and participates in signaling through STAT3. STAT3, in turn, binds the HER2 promoter and up-regulates HER2 expression [131, 132]. Several components of these loops are targeted by drugs that are available in the clinic or are in development, including trastuzumab, pertuzumab and lapatinib for HER2, adrenergic blockers for β2-AR, currently used in cardiovascular diseases, and napabucasin, a STAT3 inhibitor currently in development. These could be repurposed to inhibit HER2-positive cancer cells where the loop is active.
The subset of HER2-positive breast cancers with the CD44+/CD24−/low phenotype that is resistant to trastuzumab treatment has been found to be sensitive to metformin, a biguanide drug used for many years in diabetes therapeutics [133]. Combination treatment of metformin with trastuzumab had synergistic effect in a mouse xenograft model of human trastuzumab-resistant HER2-positive breast cancer. Metformin is currently studied in an adjuvant breast cancer trial in ER-positive cancers (both HER2-positive and -negative) and results are awaited. Persistence of CD44 variant form CD44v in CTCs following neo-adjuvant treatment of HER2+ breast cancer patients with trastuzumab and lapatinib may be a marker of resistance to these treatments [134] and could serve as a trigger for addition of metformin in the design of future trials.
These are only a selected few stem/plasticity pathways that are actively investigated to overcome resistance in HER2-positive breast cancers and several other therapeutic opportunities exist and are pursued. Although HER2 is not part of the core stem cell circuit, its proven validity as a therapeutic target and its expression in subsets of stem cells suggest that combination treatments based on an understanding of resistance-associated stem cell states will provide avenues for combating this resistance and for further improving outcomes of HER2-expressing breast cancers.
References
Ahmed S, Sami A, Xiang J. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer. 2015;22:101–16.
Prat A, Pineda E, Adamo B, Galván P, Fernádez A, Gaba L, et al. Clinical implications of the intrinsic subtypes of breast cancer. Breast. 2015;24:S26–35.
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci. 2011;108:7950–5.
Ferrari A, Vincent-Salomon A, Pivot X, Sertier AS, Thomas E, Tonon L, et al. A whole-genome sequence and transcriptome perspective on HER2-positive breast cancers. Nat Commun. 2016;7:12222.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Korkaya H, Wicha MS. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013;73:3489–93.
Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–11.
Fehrenbacher L, Cecchini RS, Geyer CE, Rastogi P, Costantino JP, Atkins JN et al. NSABP B-47 (NRG oncology): Phase III randomized trial comparing adjuvant chemotherapy with adriamycin (A) and cyclophosphamide (C) → weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-Low IBC). Cancer Res. 2018;78(4 Suppl):Abstract nr GS1-02.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS One. 2008;3:e2888.
Voutsadakis IA. The network of pluripotency, epithelial mesenchymal transition and prognosis of breast cancer. Breast Cancer Targets Therap. 2015;7:303–19.
Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303.
Nami B, Wang Z. HER2 in breast cancer stemness: A negative feedback loop towards trastuzumab resistance. Cancer. 2017;9:40.
Meng F, Speyer CL, Zhang B, Zhao Y, Chen W, Gorski DH, et al. PDGFRα and β play critical roles in mediating Foxq1-driven breast cancer stemness and chemoresistance. Cancer Res. 2015;75:584–93.
Nozaki Y, Tamori S, Inada M, Katayama R, Nakane H, Minamishima O, et al. Correlation between c-Met and ALDH1 contributes to the survival and tumor-sphere formation of ALDH1 positive breast cancer stem cells and predicts poor clinical outcome in breast cancer. Gene Cancer. 2017;8:628–39.
Zhao D, Mo Y, Li MT, Li MT, Zou SW, Cheng ZL, et al. NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells. J Clin Invest. 2014;124:5453–65.
Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One. 2013;8:e67811.
Tanaka H, Nakamura M, Kameda C, Kubo M, Sato N, Kuroki S, et al. The Hedgehog pathway plays an essential role in maintaining the CD44+CD24−/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29:2147–57.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Voutsadakis IA. The ubiquitin-proteasome system and signal transduction pathways regulating epithelial mesenchymal transition of cancer. J Biomed Sci. 2012;19:67.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2009;365:1273–83.
Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2 negative breast cancer cells. Clin Cancer Res. 2012;18:6634–47.
Oliveras-Ferraros C, Vazquez-Martin A, Martin-Castillo B, Cufí S, Del Barco S, Lopez-Bonet E, et al. Dynamic emergence of the mesenchymal CD44posCD24neg/low phenotype in HER2-gene amplified breast cancer cells with de novo resistance to trastuzumab (Herceptin). Biochem Biophys Res Commun. 2010;397:27–33.
Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30.
Baker AT, Zlobin A, Osipo C. Notch-EGFR/HER2 bidirectional crosstalk in breast cancer. Front Oncol. 2014;4:1–15.
Harrison H, Simões BM, Rogerson L, Howell SJ, Landberg G, Clarke RB. Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Res. 2013;15:R21.
Chen Y, Fischer WH, Gill GN. Regulation of the ERBB2 promoter by RBPJκ and NOTCH. J Biol Chem. 1997;272:14110–4.
Ju JH, Yang W, Oh S, Nam K, Lee KM, Noh DY, et al. HER2 stabilizes survivin while concomitantly down-regulating survivin gene transcription by suppressing Notch cleavage. Biochem J. 2013;451:123–34.
Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–21.
Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, et al. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Gene Dev. 2004;18:99–115.
Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, et al. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–48.
Shao S, Zhao X, Zhang X, Luo M, Zuo X, Huang S, et al. Notch1 signaling regulates the epithelial–mesenchymal transition and invasion of breast cancer in a Slug-dependent manner. Mol Cancer. 2015;14:1–17.
Osipo C, Patel P, Rizzo P, Clementz AG, Hao L, Golde TE, et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene. 2008;27:5019–32.
Abravanel DL, Belka GK, Pan TC, Pant DK, Collins MA, Sterner CJ, et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J Clin Invest. 2015;125:2484–96.
Voutsadakis IA. Pathogenesis of colorectal carcinoma and therapeutic implications: the roles of the ubiquitin-proteasome system and Cox-2. J Cell Mol Med. 2007;11:252–85.
Pap M, Cooper GM. Role of glycogen synthase kinas-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–32.
Dey N, Young B, Abramovitz M, Bouzyk M, Barwick B, De P, et al. Differential activation of Wnt-β-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS One. 2013;8:1–17.
Wu Y, Tran T, Dwabe S, Sarkissyan M, Kim J, Nava M, et al. A83-01 inhibits TGF-β-induced upregulation of Wnt3 and epithelial to mesenchymal transition in HER2-overexpressing breast cancer cells. Br Cancer Res Treat. 2017;163:449–60.
Liu X, Fridman JS, Wang Q, Caulder E, Yang G, Covington M, et al. Selective inhibition of ADAM metalloproteases blocks HER-2 extracellular domain (ECD) cleavage and potentiates the anti-tumor effects of trastuzumab. Cancer Biol Ther. 2006;5:648–56.
Wu Y, Ginther C, Kim J, Mosher N, Chung S, Slamon D, et al. Expression of Wnt3 activates Wnt/-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol Cancer Res. 2012;10:1597–606.
Martínez-Revollar G, Garay E, Martin-Tapia D, Nava P, Huerta M, Lopez-Bayghen E, et al. Heterogeneity between triple negative breast cancer cells due to differential activation of Wnt and PI3K/AKT pathways. Exp Cell Res. 2015;339:67–80.
O’Brien NA, McDonald K, Tong L, von Euw E, Kalous O, Conklin D, et al. Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin Cancer Res. 2014;20:3507–20.
Creedon H, Gómez-Cuadrado L, Tarnauskaite Z, Balla J, Canel M, MacLeod KG, et al. Identification of novel pathways linking epithelial-to-mesenchymal transition with resistance to HER2-targeted therapy. Oncotarget. 2016;7:11539–52.
Singh JK, Farnie G, Bundred NJ, Simões BM, Shergill A, Landberg G, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19:643–56.
Aceto N, Duss S, Macdonald G, Meyer DS, Roloff TC, Hynes NE, et al. Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cancer cells via stimulation of interleukin-8 autocrine secretion. Breast Cancer Res. 2012;14:R131.
Korkaya H, Wicha MS. Breast cancer stem cells: we’ve got them surrounded. Clin Cancer Res. 2013;19:511–3.
Iliopoulos D, Hirsch HA, Struhl K. An Epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706.
Feng C, Neumeister V, Ma W, Xu J, Lu L, Bordeaux J, et al. Lin28 regulates HER2 and promotes malignancy through multiple mechanisms. Cell Cycle. 2012;11:2486–94.
Liu D, Deng Q, Sun L, Wang T, Yang Z, Chen H, et al. A Her2-let-7-β2-AR circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer. 2015;15:1–10.
Kim J, Kim H, Seong M, Seol H, Oh JS. STAT3-survivin signaling mediates a poor response to radiotherapy in HER2-positive breast cancers. Oncotarget. 2016;7:1–11.
Chung SS, Giehl N, Wu Y, Vadgama JV. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial–mesenchymal transition and cancer stem cell traits. Int J Oncol. 2014;44:403–11.
Qian L, Chen L, Shi M, Yu M, Jin B, Hu M, et al. A novel cis-acting element in Her2 promoter regulated by Stat3 in mammary cancer cells. Biochem Biophys Res Commun. 2006;345:660–8.
Huang WC, Hung CM, Wei CT, Chen TM, Chien PH, Pan HL, et al. Interleukin-6 expression contributes to lapatinib resistance through maintenance of stemness property in HER2-positive breast cancer cells. Oncotarget. 2016;7:62352–63.
Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375:51–61.
Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25:961–9.
Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, et al. Activation of an IL-6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–84.
Zhong H, Davis A, Ouzounova M, Carrasco RA, Chen C, Breen S, et al. A novel IL6 antibody sensitizes multiple tumor types to chemotherapy including trastuzumab-resistant tumors. Cancer Res. 2016;76:480–90.
Stravodimou A, Voutsadakis IA. Pre-treatment thrombocytosis as a prognostic marker in metastatic breast cancer. Int J Breast Cancer 2013;2013:Article ID 289563.
Fu Y, Xiong J. MicroRNA-124 enhances response to radiotherapy in human epidermal growth factor receptor 2-positive breast cancer cells by targeting signal transducer and activator of transcription 3. Croat Med J. 2016;57:457–64.
De Cola A, Volpe S, Budani MC, Ferracin M, Lattanzio R, Turdo A, et al. MIR-205-5p-mediated downregulation of ERBB/HER receptors in breast cancer stem cells results in targeted therapy resistance. Cell Death Dis. 2015;6:e1823.
Gupta P, Srivastava SK. HER2 mediated de novo production of TGFβ leads to SNAIL driven epithelial-to-mesenchymal transition and metastasis of breast cancer. Mol Oncol. 2014;8:1532–47.
Tam WL, Lu H, Buikhuisen J, Soh BS, Lim E, Reinhardt F, et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24:347–64.
Magnifico A, Albano L, Campaner S, Campiglio M, Pilotti S, Ménard S, et al. Protein kinase Cα determines HER2 fate in breast carcinoma cells with HER2 protein overexpression without gene amplification. Cancer Res. 2007;67:5308–17.
Tan M, Li P, Sun M, Yin G, Yu D. Upregulation and activation of PKCα by ErbB2 through Src promotes breast cancer cell invasion that can be blocked by combined treatment with PKCα and Src inhibitors. Oncogene. 2006;25:3286–95.
Oliveira-Ferrer L, Kürschner M, Labitzky V, Wicklein D, Müller V, Lüers G, et al. Prognostic impact of transcription factor Fra-1 in ER-positive breast cancer: contribution to a metastatic phenotype through modulation of tumor cell adhesive properties. J Cancer Res Clin Oncol. 2015;141:1715–26.
Sun L, Burnett J, Gasparyan M, Xu F, Jiang H, Lin CC, et al. Novel cancer stem cell targets during epithelial to mesenchymal transition in PTEN-deficient trastuzumab-resistant breast cancer. Oncotarget. 2016;7:51408–22.
Zhao C, Qiao Y, Jonsson P, Wang J, Xu L, Rouhi P, et al. Genome-wide profiling of AP-1-regulated transcription provides insights into the invasiveness of triple-negative breast cancer. Cancer Res. 2014;74:3983–94.
Kharman-Biz A, Gao H, Ghiasvald R, Zhao C, Zendehdel K, Dahlman-Wright K. Expression of activator protein-1 (AP-1) family members in breast cancer. BMC Cancer. 2013;13:1–10.
Bakiri L, Macho-Maschler S, Custic I, Niemiec J, Guío-Carrión A, Hasenfuss SC, et al. Fra-1/AP-1 induces EMT in mammary epithelial cells by modulating Zeb1/2 and TGFβ expression. Cell Death Differ. 2015;22:336–50.
Lin L, Hutzen B, Lee HF, Peng Z, Wang W, Zhao C, et al. Evaluation of STAT3 signaling in ALDH+ and ALDH+/CD44+/CD24− subpopulations of breast cancer cells. PLoS One. 2013;8:e82821.
Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–44.
Da Paula AC, Lopes C. Implications of different cancer stem cell phenotypes in breast cancer. Anticancer Res. 2017;37:2173–83.
Martin-Castillo B, Lopez-Bonet E, Cuyàs E, Viñas G, Pernas S, Dorca J, et al. Cancer stem cell-driven efficacy of trastuzumab (Herceptin): towards a reclassification of clinically HER2-positive breast carcinomas. Oncotarget. 2015;6:32317–38.
Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res. 2010;16:876–87.
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2:78–91.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Zhang M, Tsimelzon A, Chang CH, Fan C, Wolff A, Perou CM, et al. Intratumoral heterogeneity in a Trp53-null mouse model of human breast cancer. Cancer Discov. 2015;5:520–33.
Perrone G, Gaeta LM, Zagami M, Nasorri F, Coppola R, Borzomati D, et al. In situ identification of CD44+/CD24− cancer cells in primary human breast carcinomas. PLoS One. 2012;7:e43110.
Seo AN, Lee HJ, Kim EJ, Jang MH, Kim YJ, Kim JH, et al. Expression of breast cancer stem cell markers as predictors of prognosis and response to trastuzumab in HER2-positive breast cancer. Br J Cancer. 2016;114:1109–16.
Boulbes DR, Chauhan GB, Jin Q, Bartholomeusz C, Esteva FJ. CD44 expression contributes to trastuzumab resistance in HER2-positive breast cancer cells. Breast Cancer Res Treat. 2015;151:501–13.
Morath I, Jung C, Lévêque R, Linfeng C, Toillon RA, Warth A, et al. Differential recruitment of CD44 isoforms by ErbB ligands reveals an involvement of CD44 in breast cancer. Oncogene. 2018;37:1472–84.
Chung A, Choi M, Han BC, Bose S, Zhang X, Medina-Kauwe L, et al. Basal protein expression is associated with worse outcome and trastuzumab resistance in HER2+ invasive breast cancer. Clin Breast Cancer. 2015;15:448–57.
Martin-Castillo B, Lopez-Bonet E, Buxó M, Dorca J, Tuca-Rodríguez F, Ruano MA, et al. Cytokeratin 5/6 fingerprinting in HER2-positive tumors identifies a poor prognosis and trastuzumab-resistant basal-HER2 subtype of breast cancer. Oncotarget. 2015;6:7104–22.
Oliveras-Ferraros C, Corominas-Faja B, Cufí S, Vazquez-Martin A, Martin-Castillo B, Iglesias JM, et al. Epithelial-to-mesenchymal transition (EMT) confers primary resistance to trastuzumab (Herceptin). Cell Cycle. 2012;11:4020–32.
Martin-Castillo B, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Moreno JM, Corominas-Faja B, et al. Basal/HER2 breast carcinomas. Cell Cycle. 2013;12:225–45.
Arribas J, Baselga J, Pedersen K, Parra-Palau JL. p95HER2 and breast cancer. Cancer Res. 2011;71:1515–9.
Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, et al. Expression of p95 HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38.
Sperinde J, Jin X, Banerjee J, Penuel E, Saha A, Diedrich G, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–35.
Zhao M, Ramaswamy B. Mechanisms and therapeutic advances in the management of endocrine-resistant breast cancer. World J Clin Oncol. 2014;5:248–62.
Gilani RA, Kazi AA, Shah P, Schech AJ, Chumsri S, Sabnis G, et al. The importance of HER2 signaling in the tumor-initiating cell population in aromatase inhibitor-resistant breast cancer. Breast Cancer Res Treat. 2012;135:681–92.
Nakanishi T, Chumsri S, Khakpour N, Brodie AH, Leyland-Jones B, Hamburger AW, et al. Side-population cells in luminal-type breast cancer have tumour-initiating cell properties, and are regulated by HER2 expression and signalling. Br J Cancer. 2010;102:815–26.
Rodríguez CE, Berardi DE, Abrigo M, Todaro LB, de Bal KJED, Fiszman GL. Breast cancer stem cells are involved in Trastuzumab resistance through the HER2 modulation in 3D culture. J Cell Biochem. 2018;119:1381–91.
Oak PS, Kopp F, Thakur C, Ellwart JW, Rapp UR, Ullrich A, et al. Combinatorial treatment of mammospheres with trastuzumab and salinomycin efficiently targets HER2-positive cancer cells and cancer stem cells. Int J Cancer. 2012;131:2808–19.
Ithimakin A, Day KC, Malik F, Zen Q, Dawsey SJ, Bersano-Begey TF, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of trastuzumab. Cancer Res. 2013;73:1635–46.
Wise R, Zolkiewska A. Metalloprotease-dependent activation of EGFR modulates CD44+/CD24− populations in triple negative breast cancer cells through the MEK/ERK pathway. Breast Cancer Res Treat. 2017;166:421–33.
Hosonaga M, Arima Y, Sugihara E, Kohno N, Saya H. Expression of CD24 is associated with HER2 expression and supports HER2-Akt signaling in HER2-positive breast cancer cells. Cancer Sci. 2014;105:779–87.
Kwon MJ, Han J, Seo JH, Song K, Jeong HM, Choi JS, et al. CD24 overexpression is associated with poor prognosis in luminal A and triple-negative breast cancer. PLoS One. 2015;10:e0139112.
Savage P, Blanchet-Cohen A, Revil T, Badescu D, Saleh SMI, Wang YC, et al. A targetable EGFR-dependent tumor-initiating program in breast cancer. Cell Rep. 2017;21:1140–9.
Xia W, Petricoin EF 3rd, Zhao S, Liu L, Osada T, Chen Q, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15:R85.
Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;2016(166):21–45.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91.
Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–5.
Grigore A, Jolly M, Jia D, Farach-Carson M, Levine H. Tumor budding: the name is EMT. Partial EMT. J Clin Med. 2016;5:51.
Liang F, Cao W, Wang Y, Li L, Zhang G, Wang Z. The prognostic value of tumor budding in invasive breast cancer. Pathol Res Pract. 2013;209:269–75.
Laedrach C, Salhia B, Cihoric N, Zlobec I, Tapia C. Immunophenotypic profile of tumor buds in breast cancer. Pathol Res Pract. 2018;214:25–9.
Krawczyk N, Meier-Stiegen F, Banys M, Neubauer H, Ruckhaeberle E, Fehm T. Expression of stem cell and epithelial–mesenchymal transition markers in circulating tumor cells of breast cancer patients. Biomed Res Int. 2014;2014:415721.
Zhang S, Wu T, Peng X, Liu J, Liu F, Wu S, et al. Mesenchymal phenotype of circulating tumor cells is associated with distant metastasis in breast cancer patients. Cancer Manag Res. 2017;9:691–700.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4.
Wallwiener M, Hartkopf AD, Riethdorf S, Nees J, Sprick MR, Schönfisch B, et al. The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: a retrospective study in 107 patients. BMC Cancer. 2015;15:1–7.
Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, et al. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat. 2009;118:523–30.
Aktas B, Kasimir-Bauer S, Müller V, Janni W, Fehm T, Wallwiener D, et al. Comparison of the HER2, estrogen and progesterone receptor expression profile of primary tumor, metastases and circulating tumor cells in metastatic breast cancer patients. BMC Cancer. 2016;16:1–8.
Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124:403–12.
Jaeger BAS, Neugebauer J, Andergassen U, Melcher C, Schochter F, Mouarrawy D, et al. The HER2 phenotype of circulating tumor cells in HER2-positive early breast cancer: a translational research project of a prospective randomized phase III trial. PLoS One. 2017;12:e0173593.
Krishnamurthy S, Bischoff F, Ann Mayer J, Wong K, Pham T, Kuerer H, et al. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer. Cancer Med. 2013;2:226–33.
Agelaki S, Dragolia M, Markomanolaki H, Alkahtani S, Stournaras C, Georgoulias V, et al. Phenotypic characterization of circulating tumor cells in triple negative breast cancer patients. Oncotarget. 2017;8:5309–22.
Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102–6.
Nishimura R, Osako T, Okumura Y, Tashima R, Toyozumi Y, Arima N. Changes in the ER, PgR, HER2, p53 and Ki-67 biological markers between primary and recurrent breast cancer: discordance rates and prognosis. World J Surg Oncol. 2011;9:1–7.
Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, et al. Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin Breast Cancer. 2015;15:307–12.
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802.
Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21.
Jäger BAS, Finkenzeller C, Bock C, Majunke L, Jueckstock JK, Andergassen U, et al. Estrogen receptor and HER2 status on disseminated tumor cells and primary tumor in patients with early breast cancer. Transl Oncol. 2015;8:509–16.
Hartkopf AD, Banys M, Meier-Stiegen F, Hahn M, Röhm C, Hoffmann J, et al. The HER2 status of disseminated tumor cells in the bone marrow of early breast cancer patients is independent from primary tumor and predicts higher risk of relapse. Breast Cancer Res Treat. 2013;138:509–17.
Becker S, Becker-Pergola G, Fehm T, Wallwiener D, Solomayer EF. Her2 expression on disseminated tumor cells from bone marrow of breast cancer patients. Anticancer Res. 2005;25:2171–5.
Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/β-catenin signaling. PLoS Biol. 2009;7:e1000121.
André F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab-resistant, HER-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–91.
Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, et al. Preclinical pharmacology of AZD5363, an Inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–87.
Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334–48.
Saura C, Bendell J, Jerusalem G, Su S, Ru Q, De Buck S, et al. Phase lb study of buparlisib plus trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on trastuzumab-based therapy. Clin Cancer Res. 2014;20:1935–45.
Loibl S, de la Pena L, Nekljudova V, Zardavas D, Michiels S, Denkert C, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer. 2017;85:133–45.
Wang WCH, Juan AH, Panebra A, Liggett SB. MicroRNA let-7 establishes expression of beta2-adrenergic receptors and dynamically down-regulates agonist-promoted down-regulation. Proc Natl Acad Sci USA. 2011;108:6246–51.
Shi M, Liu D, Duan H, Qian L, Wang L, Niu L, et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351–62.
Cufí S, Corominas-Faja B, Vazquez-Martin A, Oliveras-Ferraros C, Dorca J, Bosch-Barrera J, et al. Metformin-induced preferential killing of breast cancer initiating CD44+ CD24−/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget. 2012;3:395–8.
Yamauchi T, Espinosa Fernandez JR, Imamura CK, Yamauchi H, Jinno H, Takahashi M, et al. Dynamic changes in CD44v-positive cells after preoperative anti-HER2 therapy and its correlation with pathologic complete response in HER2-positive breast cancer. Oncotarget. 2018;9:6872–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflicts of interest regarding this paper.
Ethical approval
This paper involves no human participants or animals.
Informed consent
No informed consent was necessary.
Rights and permissions
About this article
Cite this article
Voutsadakis, I.A. HER2 in stemness and epithelial–mesenchymal plasticity of breast cancer. Clin Transl Oncol 21, 539–555 (2019). https://doi.org/10.1007/s12094-018-1961-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1961-x