Abstract
Bacterioruberin (BR) is a fat-soluble, dipolar, reddish pigment predominantly found in halophilic archaea. BR is a rare C50 carotenoid from the xanthophyll family, and it has been extensively studied for its potent antioxidant properties, such as its ability to protect cells from oxidative stress. In addition, several studies have shown that BR-rich extracts and its derivatives exhibit significant antiviral, antidiabetic, antibacterial, and anti-inflammatory effects, making them ideal candidates for the development of novel therapeutic interventions against various diseases. Although it possesses remarkable biological properties, studies related to the regulatory aspects of biosynthesis, in vitro and in vivo studies of purified BR have been rare. However, investigations are needed to explore the potential application of BR in various industries. Additionally, optimization of the culture conditions of BR-producing haloarchaea could pave the way for their sustainable production and utilization. The current review provides comprehensive information on BR, which includes the sources of this compound and its bioproduction, extraction, stability, toxicity, and biological activities in relation to its commercial applications. This review also discusses the potential challenges and limitations associated with BR bioproduction and its utilization in various industries. In addition, this treatise highlights the need for further research to optimize production and extraction methods and explore avenues for novel applications of BR in various sectors, such as pharmaceuticals, food, and cosmetics.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are an important class of plant-based pigments that occur ubiquitously in nature [1]. As of now, > 1200 naturally occurring carotenoids have been identified and are distributed into two fragments: oxygenated carotenoids, known as xanthophylls and nonoxygenated carotenoids, known as carotenes. All these compounds have been isolated from bacteria (especially Cyanobacteria), eubacteria, archaebacteria, fungi (yeast), algae (both micro- and macroalgae) and higher plants [1,2,3,4,5,6]. Carotenoids are known to be involved in the reduction of free radicals, which helps to promote animal health by boosting the immune system and strengthening the endocrine system. However, since animals are unable to produce carotenoids, they need to obtain these compounds solely from their diet [5, 7]. Currently, the Carotenoids Database provides information on 1204 carotenoids, the majority of which have a C40 hydrocarbon skeleton (1121 carotenoids). However, there are fewer C30 (37 carotenoids), C35 (5 carotenoids), C45 (13 carotenoids), and C50 hydrocarbon skeletons (33 molecules), and the number of these compounds is continuing to increase as researchers discover new forms of carotenoids [6, 8]. Those carotenoids with a C45 or C50 hydrocarbon skeleton are called higher carotenoids [4]. Decaprenoxanthin, a C50 carotenoid, was first isolated from Flavobacterium dehydrogenans in 1966 and is the first carotenoid with more than 40 carbon atoms [1]. Since then, > 40 different kinds of higher carotenoids have been reported [4]. These higher carotenoid contents are mainly found in moderately to extremely halophilic archaea (halobacteria) [9]. Higher carotenoids are considered to be rare on the basis of their distribution among different taxa, and some of these carotenoids are C45 carotenoids, e.g., nonaflavuxanthin [10]; C50 carotenoids, e.g., bacterioruberin [11]; flavuxanthin [10, 12]; sarcinaxanthin [13, 14]; and decaprenoxanthin [12].
Bacterioruberin
Bacterioruberin (BR) is known to be a rare C50 carotenoid that is mainly found in halophilic archaea. BR is slightly or poorly soluble in water. It is a highly lipophilic molecule and can be dissolved in organic solvents and oils. It is a red‒orange xanthophyll pigment responsible for the coloration observed in halophilic organisms [15, 16]. This carotenoid has 50 carbon atoms (C50) and possesses a longer system of conjugated double bonds than the C40 carotenoids often found in other organisms, such as plants, microalgae, fungi, and bacteria. Haloarchaea also contain C40 carotenoids, such as phytoene, lycopene, and beta-carotene, but in low quantities; these compounds are proposed to be the metabolic intermediates in the biosynthesis of C50 carotenoids [17]. The BR is known to serve as a highly diagnostic biomarker for halobacteria. It has been observed that some high-molecular-weight biomarkers could not be detected since they pose great challenges to analysing them by employing routinely used GC–MS techniques [18]. Carotenoids are known to play a crucial role in photosynthesis by absorbing light energy and protecting cells from harmful free radicals. On the other hand, BR not only provide antioxidant and sunlight protection activity but also aid in maintaining the structural integrity of bio-membranes when exposed to extreme salt concentrations. This unique adaptation allows halophilic archaea to survive in high-salinity environments [18]. It has been shown that C50 carotenoids, comparable to BR, are crucial for enhancing the stability of bio-membranes in psychrophiles. Due to this cellular level adaptability, these organisms are adapted to survive at extremely cold temperatures and continue to perform cellular functions normally. Furthermore, the rigidity of the biomembrane afforded by C50 carotenoids may also guard against damage caused by unfavourable freezing and thawing conditions in the environment [19, 20]. In addition, BR is known to confer resistance to gamma irradiation, intense light, and DNA damage caused by ultraviolet (UV) irradiation, radiography, and H2O2 exposure [21]. The potent free radical scavenging properties of BR make it a suitable candidate for use as a feed supplement in the aquaculture industry [22]. The present review offers a comprehensive overview of BR, with a focus on its diverse sources, biosynthesis, extraction methods, storage conditions, stability profiles, potential toxicity, bioproduction processes, varied biological properties, and its applications. This review provides a depth of information into every aspect of BR and sets the groundwork for understanding and harnessing the potential of this unique compound. To the best of our knowledge, this is the first review of C50-carotenoid bacterioruberin.
History of Bacterioruberin
Helena Franciska Maria Petter, a microbiologist, significantly contributed to our understanding of halophiles through her doctoral thesis on halophilic microorganisms. She carried out her research at the University of Utrecht, Utrecht, Netherlands, in the 1930s. Her thesis is titled "Over roode en andere bacterieën van gezouten visch" (On red and other bacteria of salted fish) [11, 23]. Her research focused on studying various species of halophilic prokaryotes, primarily red pigment-producing members of the Halobacteriaceae population, which were isolated from salted fish and Trapani salt collected from a cannery in Bergen, Norway. Her isolates included rod-shaped bacteria as well as coccoid and sarcina-shaped bacteria. Her research included descriptions of "Bacterium trapanicum” and Bacterium halobium", which are currently known as Halobacterium trapanicum and Halobacterium salinarum, respectively. In 1932, she isolated two crystalline carotenoids, α-BR and β-BR, from a bacterium named Bacterium halobium [24]. Helena Petter was the first to isolate and name the pink-colored carotenoid of Halobacteriaceae as bacterioruberin [25].
Sources of Bacterioruberin
The natural sources of BR are archaea, such as haloarchaea or halophilic archaea, and a few other extremophiles, such as psychrophiles (Arthrobacter, Micrococcus), Azospirillum sp., and radioresistant bacteria (Rubrobacter) [26]. BRs are produced predominantly by most of the members of Halobacteriaceae and Haloferacaceae (Table 1). It is abundant in halobacteria, and these bacteria are ubiquitous in salty habitats such as salt lakes and evaporating seawater pools. Thus, BR could be valuable biomarker for identifying and studying these unique microbial communities [18]. Halobacteriaceae are easy to distinguish by Raman spectroscopy due to the presence of distinctive carotenoid pigments (BR and its derivatives) [27,28,29]. Using different analytical techniques, researchers have reported the presence of BR and its derivatives in diverse microorganisms. The various microbial sources of BR and its derivatives are listed in Table 1.
Deposition of Bacterioruberin in Animals
Flamingos and pelicans glow in vibrant pink or reddish colors due to pigments that they cannot synthesize de novo. The primary source of these colours is carotenoids, which are found in food substances such as microalgae (Dunaliella) and small shrimp (Artemia) that are high in carotenoids. Recent research has indicated that microorganisms such as Haloarchaea inhabiting salt lakes and ponds where these birds nest may significantly contribute to the pink‒reddish coloration of flamingos' feathers. Interestingly, it has been discovered that the feathers of flamingos contain live cells of Haloarchaea belonging to the genera Halococcus and Halogeometricum. In addition, pigment analysis of the feathers of these birds revealed the presence of BR and its derivatives [89, 90]. However, further research is needed to determine whether Haloarchaea may play a role in regulating environmental factors that affect bird plumage coloration or perhaps in shielding feather microstructures from UV radiation [89]. As a result, BR is now considered to be a new pigment that needs to be investigated in relation to animal coloration in marine environments.
Biosynthesis of Bacterioruberin
Until 2015, only three biosynthetic pathways of C50 carotenoids, the ε-cyclic C50 carotenoid decaprenoxanthin in Corynebacterium glutamicum [12, 91, 92], the γ-cyclic C50 carotenoid sarcinaxanthin in Micrococcus luteus NCTC2665 [93] and the β-cyclic C50 carotenoid 2,2′-bis-(4-hydroxy-3-methybut-2-enyl)-β,β-carotene in the Dietzia sp. strain CQ4 [94], have been described based on their chemical structures. In 2015, Ying Yang and his coworkers at the Tokyo Institute of Technology, Yokohama, Japan, first elucidated the complete biosynthetic pathway of the C50-carotenoid-BR in Haloarcula japonica, an extremely halophilic archaeon. Their research showed that a gene cluster comprising three genes, C0505, C0506, and C0507, encodes the C50 carotenoid 2′′, 3′′-hydratase (CruF), a bifunctional lycopene elongase and 1,2-hydratase (LyeJ), and the carotenoid 3,4-desaturase (CrtD), respectively. In H. japonica, a series of chemical reactions converting lycopene into BR are catalyzed by the three carotenoid biosynthetic enzymes mentioned above [95]. The discovery of this biosynthesis pathway has provided valuable insights into the production of BR and a conceptual basis for investigating the intricacies of carotenoid biosynthesis pathways in other halophilic archaea.
The biosynthesis pathway of BR involves a series of enzymatic reactions that occur within the cell (Fig. 1). These reactions are responsible for the bioproduction of a set of precursors that eventually form BR. All carotenoids are synthesized from common precursors, namely, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are synthesized via either the well-known mevalonate (MVA) pathway or the recently discovered non-mevalonate pathway (MEP) [96]. Several enzymatic steps are involved in the initiation of carotenoid biosynthesis, including the condensation of IPP and DMAPP to produce geranyl pyrophosphate (GPP), which is catalyzed by geranyl pyrophosphate synthase (dimethylallyl transferase, or GPPS) (IspA). Subsequently, farnesyl pyrophosphate synthase (FPPS) (IspA) catalyzes the condensation of GPP with another molecule of IPP to produce farnesyl pyrophosphate (FPP). The enzyme GGPP synthase (CrtE) converts FPP into the main carotenoid precursor geranylgeranyl pyrophosphate (GGPP). The enzyme phytoene synthase (CrtB) converts GGPP, a key precursor in the biosynthesis of carotenoids, into phytoene. The first reaction specific to the carotenoid branch of isoprenoid metabolism is the formation of phytoene, a compound found in all carotenogenic organisms [97]. The enzyme phytoene desaturase (CrtI) converts phytoene to lycopene. These enzymes introduce double bonds and rearrange the carbon skeleton of phytoene to form lycopene. Lycopene is an important key molecule in global carotenogenesis since it is the precursor for several carotenogenic branches. Thus, lycopene has been unequivocally established to be a precursor for the synthesis of relevant carotenoids in nature, such as lutein and its precursors and derivatives, neurosporaxanthin, and the C50 carotenoid BR [95,96,97]. LyeJ, CrtD, and CruF are the three key enzymes responsible for the conversion of lycopene to BR [8, 96].
The conversion of lycopene to dihydro-isopentenyl-dehydro-rhodopin (DH-IDR) is one of the crucial intermediate steps in BR biosynthesis. This reaction is catalyzed by the bifunctional lycopene elongase and 1,2-hydratase enzyme (LyeJ), which also plays a key role in converting isopentenyl-dehydro-rhodopin (IDR) to dihydro-bis-anhydro-BR (DH-BABR) and DH-IDR to tetrahydro-bis-anhydro-BR (TH-BABR). Carotenoid 3,4-desaturase (CrtD) is known to play important roles in the conversion of dihydro-isopentenyl-dehydro-rhodopin (DH-IDR) to isopentenyl-dehydro-rhodopin (IDR) and dihydro-bis-anhydro-BR (DH-BABR) to bisanhydro-BR (BABR). In addition, it is also known to catalyze the conversion of TH-BABR to DH-BABR. This enzyme facilitates the removal of hydrogen atoms from specific positions within the molecule, leading to the formation of important carotenoids. The enzyme C50 carotenoid 2'',3''-hydratase (CruF) is responsible for the final 2 steps of BR biosynthesis. This enzyme plays a crucial role in the conversion of bis-anhydro-BR (BABR) to BR through two hydration reactions. The first step involves the hydration of bis-anhydro-BR (BABR) to form mono-anhydro-BR (MABR), followed by the second step where mono-anhydro-BR (MABR) is further hydrated to yield BR [95]. The enzymes involved in the biosynthesis of different forms of carotenoids are considered important biocatalysts because they are involved in multiple steps in their biosynthesis. It is also pertinent to mention that enzymes involved in BR biosynthesis are not exempt from their catalytic role in this bioproduction process.
The regulation of BR biosynthesis in haloarchaea is a relatively unexplored field of study. However, recent studies show that the LonB protease (membrane protease), found in the cell membrane of haloarchaea, plays a crucial role in controlling this process. One study clearly demonstrates that LonB deficiency correlates with elevated levels of BR, strongly suggesting the direct involvement of LonB in regulating BR biosynthesis [98]. Furthermore, another study reveals that LonB deficiency induces cellular over pigmentation, indicating alterations in carotenoid production, including BR [99]. Further investigation revealed that LonB protease targets phytoene synthase (PSY), a key enzyme in carotenoid biosynthesis, including BR. The rapid degradation of PSY upon LonB induction underscores the protease's role in modulating BR biosynthesis through targeted degradation of key enzymes like PSY [100]. Moreover, additional research provides compelling evidence suggesting that LonB may selectively recognize specific sequences, such as the C-terminal region of PSY, facilitating its degradation and thereby influencing BR production [101]. Collectively, these findings propose a mechanistic model wherein LonB protease finely tunes cellular BR levels by targeting key enzymes like PSY for degradation, thus intricately regulating BR biosynthesis in response to environmental stimuli.
Chemical Structure of Bacterioruberin and its Derivatives
BR is a member of the xanthophyll family because it contains not only carbon and hydrogen but also oxygen atoms (Fig. 2a). The molecular formula of BR is C50H76O4, and the International Union of Pure and Applied Chemistry (IUPAC) name for BR is (2S,2′S)-2,2′-bis(3-hydroxy-3-methylbutyl)-3,4,3′,4′-tetradehydro-1,2,1′,2′-tetrahydro-y,y-carotene-1,1′-diol. BR was found to be a C50 carotenoid with a unique molecular structure compared to other carotenoids. It comprises a primary conjugated isoprenoid chain length of 13 C=C units. Moreover, it has no subsidiary conjugation arising from terminal groups, which contain only four hydroxyl groups [102]. BR is a tertiary alcohol and a tetrol (a polyhydric alcohol with four hydroxyl groups; Fig. 2b) [88]. It is a fat-soluble pigment [15, 103]. BR has a molecular weight of 741.1 g mol−1. The melting point of BR is 225 °C. BR exhibits a series of geometrical isomers (Cis/E or Trans/Z). These isomers arise due to the difference in the positions of the double bonds within the carbon chain (Table 2). The variation in the double bond position gives rise to distinct spatial arrangements, resulting in different physical and chemical properties for each of the isomers. These isomers exhibit a wide range of colours, ranging from deep red to orange. All these isomers are found in natural sources (halophilic archaea). The geometric isomers of BR are all-trans-BR, 5-cis-BR, 9-cis-BR, 13-cis-BR, 15-cis-BR, 5-cis-9-cis-BR, 5-cis-26-cis-BR, 9-cis-9-cis-BR, and 9-cis-26-cis-BR [53]. In addition, different BR derivatives (Table 3) have been identified in various organisms, such as halophilic archaea and bacteria, but have not been studied for their biological activities. All these derivatives are rare and novel compounds that exhibit variations in their chemical structures and possess unique characteristics. Some of these derivatives include mono-, di-, tri-, and tetra-anhydro-BR. In addition, BR has glycosylated derivatives, including mono-, di-, and tetra-glycoside-BR. The varied chemical structures of these derivatives make them promising candidates for further research on their biological activities and therapeutic uses. However, there are no reports available suggesting that BR derivatives possess significant biological activity. Therefore, additional studies are needed to explore the potential benefits and limitations of these derivatives in different biological systems.
Biochemistry of Bacterioruberin
Conjugated systems with < 8 conjugated double bonds appear colourless to the human eye and absorb only in the UV region. With each double bond added, the excitation wavelength increases, requiring less energy to be excited, and the color we observe can range from yellow to red [104]. Therefore, the red color of BR is due to the 13 conjugated double bonds at the centre of the compound (Fig. 2c). The conjugated C = C chain of BR is found in the hydrophobic core of lipid bilayers, with glucose moieties anchored in the hydrophilic region and branched fatty acid moieties curved back into the hydrophobic region (Fig. 3a), thereby reinforcing the cell membrane of halophilic archaea [105]. Variations in the orientation of carotenoids can significantly impact membrane properties. Carotenoids like zeaxanthin, with two polar end groups spanning the membrane, can act as structural "rivets," enhancing membrane rigidity and mechanical strength. Building on this, it was proposed that BR, with its four hydroxyl substituents, could act as a similar "rivet" in haloarchaeal cell membranes. Despite their similar lengths, BR integrates more effectively into lipid vesicles than zeaxanthin or decapreno-zeaxanthin. Therefore, the integration of BR into the haloarchaeal lipid vesicles has some effect on membrane fluidity, acts as a barrier to water, allows permeability to oxygen and other molecules, and increases the rigidity of bilayers (Fig. 3b); thus, strains can survive in hypersaline or low-temperature conditions [62, 106, 107]. In addition, BR has been proven to present greater antioxidant activity than other commercially available carotenoids, such as beta-carotene, ascorbic acid, butylated hydroxytoluene (BHT), lycopene, astaxanthin, alpha-tocopherol, and trolox (a water-soluble derivative of vitamin E) [33, 46, 53, 56], because it can traverse the cell membrane from the inside to the outside (Fig. 3a). Molecular dynamics simulations revealed that the thickness of the archaeal tetraether monolayer is 39 Å. The length of the BR is 38 Å, which indicates that the BR can connect both leaflets of the phospholipid bilayer in specific regions of the cell membrane and easily interact with transmembrane proteins [108]. BRs are not occurred free in cells but appear to be rather firmly bound to proteins. When the cells are lysed by exposing them to low salt concentrations, an almost clear red solution is obtained from which the pigment cannot be extracted by nonpolar solvents. This also applied to cell extracts made through sonic disintegration. When such cell extracts are heated, the pigment remains attached to the precipitated protein. However, the pigment-protein complex splits when the protein is precipitated by polar organic solvents [109]. Microbial rhodopsins (MR) are a class of photoreceptors found in halophilic archaea and bacteria. They are retinal-binding proteins that share a seven-transmembrane structure and a light-sensitive retinal molecule (a primary chromophore) that is covalently bound to a lysine residue on helix G through a protonated Schiff base linkage [110]. MR is also an integral membrane protein that provides light-dependent ion transport, which captures and utilizes sunlight for the synthesis of ATP [111] and sensory functions in halophilic cells. Additionally, it has been found to play a role in an array of biological processes, such as phototaxis. Certain proteins contain not only the retinal chromophore but also a noncovalently bound pigmented carotenoid molecule as the second chromophore to enable their function (e.g., BR, Fig. 3a) [110]. The C50 carotenoid “bacterioruberin” was identified as a second chromophore in some MRs and is thought to protect against photobleaching. Research via crystallographic studies demonstrated that BR is tightly aligned in the crevices between the adjacent protein subunits within the trimer of archaerhodopsins [111,112,113], cruxrhodopsins [114], deltarhodopsins [115], and halorhodopsins [103, 116].
Bioproduction of Bacterioruberin and its Current Status of Commercialization
Only a few studies have attempted to enhance the bioproduction of BR in halobacteria. Several recent studies on the production of BR from haloarchaea have reported that the production of this rare C50 carotenoid may be readily enhanced by modifying culture conditions, such as pH, oxygen availability, salt concentrations, light incidence, and temperature [117]. Additionally, the biosynthesis of BR has been shown to be induced by the presence of different compounds, such as aniline [118]. One finding suggested that the response surface methodology (RSM) approach is highly useful for determining the optimal conditions of cell culture, such as temperature, pH, and salinity, for large-scale production of BR by haloarchaea [52]. On the other hand, Noby et al. reported that cheese whey-based medium has been proven to be a potent and nutritious supplement for producing BR from Arthrobacter agilis NP20. This newly developed, cost-effective medium highlights the great potential for large-scale bioproduction of BR. Furthermore, the results of the study suggested that the use of this rare C50 carotenoid in the food, cosmetics, and pharmaceutical industries could be achieved through the low-cost production of BR from whey-based media [15]. In addition, a few other researchers have worked on the optimization of culture conditions to augment the BR yield and biomass of halobacteria, with a focus on commercial applications [54]. For instance, the yield of BR from H. volanii increased 1.7-fold under low-salt conditions but decreased cell growth under osmotic stress [119]. To address this, a 2-step cultivation of H. mediterranei was tested in a 20-L jar fermenter, in which the biomass was first produced under optimal growth conditions and subsequently transferred to a hypoosmotic medium optimized for BR production. This process increased production 6.4-fold in fermented broth [62]. However, this process increases the number of cultivation steps and work needed for production. Furthermore, single-step cultivation of H. mediterranei at relatively low salt concentrations under optimized conditions was shown to increase both the BR yield and biomass concentration. At a salt concentration of 230 g/L, this species yielded 125 mg/L total carotenoids and a maximum cell density of 7.7 × 109 cells/mL. This remarkable increase in productivity represents the highest production ever reported for a wild-type strain. This increase corresponds to a 4.4-fold increase in yield and a 20% increase in biomass [120]. Additionally, hyperpigmented mutants, known as HVLON3, exhibited even more impressive performance, producing BR at an astonishing rate of 3.14 mg/g CDM, which is approximately 15 times higher than that of the wild type (0.2 mg/g CDM). This unprecedented achievement resulted in the highest yield ever observed for haloarchaea [98, 121].
As mentioned before, only a few studies on haloarchaea carotenoid accumulation support the idea that these microorganisms might be good carotenoid producers, specifically for BR and other C50 carotenoid pigments. All these studies reported that accelerating the rapid growth of halophilic archaea requires high salt concentrations (from 20 to 25% w/v) in the culture medium. However, it has been observed that promoting higher level of carotenoid accumulation in halophilic bacteria requires a relatively lower concentration of NaCl, which is < 16% w/v. It was also observed that a lower salt concentration of NaCl leads to slower growth rates or even cell lysis. Therefore, carotenoid accumulation and the growth of halophilic archaea are often inversely related. In addition to the salinity of the culture medium, other physicochemical factors, such as pH and temperature, have been shown to significantly affect the accumulation of carotenoids and the growth rate of halophilic microorganisms [102].
In the current scenario, the only commercially available products from archaea were extracted from halophilic archaea: C50-BR and C30-Squalene (two nonpolar archaeolipids), Bacteriorhodopsin (a membrane-bound protein), and Di- or tetraether lipids. These products do not qualify for “Biotechnological Readiness Level 3” (BTRL 3), which means that even though they are commercialized, none of them are manufactured on an industrial scale. Instead, their demand is fulfilled by selling very small quantities of these products at very expensive prices [22]. Currently, only two companies have commercialized cosmetic products in micro quantities that contain BR as an active ingredient or a BR-rich extract. It is predicted that more companies may enter the market in the near future.
Storage, Stability and Toxicity of Bacterioruberin
The stability of BR under various environmental conditions is not well understood. Investigating the effects of temperature, pH, and light exposure on the stability of BR could provide valuable insights into its potential applications. Mongkol Yachai's PhD thesis titled "Carotenoid Production by Halophilic Archaea and Its Applications" [63] indicated that BR extracted from H. salinarum HM3 exhibited good stability under high-temperature conditions and light exposure in soybean oil and surimi paste (a paste made from bigeye snapper fish). No color bleeding was observed in white surimi gel containing BR under steaming. During refrigerated storage, the color of the surimi gels supplemented with BR slightly changed. Moreover, BR is known to prevent lipid oxidation in surimi gels during storage and does not alter the taste or texture of the gel. BR was found to be stable at 70–90 °C in soybean oil, with 78%-87% retention of the BR content [63]. In addition, this study further investigated the acute oral toxicity of BR from H. salinarum HM3 in Wistar rats (50 rats). During the course of the study, fifty rats were divided into 5 groups (5 from each sex) and fed BR in soybean oil at different dosages (0, 125, 250, 500, 1000 mg/kg/day). At 24 h intervals, the Wistar rats were carefully examined for symptoms of toxicity. Both male and female rats fed BR exhibited a slight decrease in body weight gain during the first week. In the second week, no significant differences were observed in male rats, but female rats showed decreases in body weight gain at 1000 mg/kg/day BR. No deaths were observed in any group during the 14-day study, and all surviving rats did not exhibit any major pathological lesions [63]. Therefore, the stability and nontoxicity of BR at high temperatures make it a valuable ingredient for food, pharmaceutical, and nutraceutical applications. Microbial pigments are widely used in textile and paper printing as a long-term alternative to synthetic dyes [122]. According to this, BR's vibrant red color and stability make it an excellent candidate for the future development of environmentally friendly and safe natural colorants for various industries. Additionally, the growing consumer demand for clean label products has further fuelled interest in natural pigments like BR. As a result, it is inferred that BR can be effectively used in the production of heat-resistant items, food, and textile coloring agents.
Extraction and Analysis of Bacterioruberin
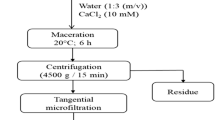
BR is present in the claret membrane of haloarchaeal cells, is slightly soluble in water and is a highly lipophilic molecule that can be dissolved in organic solvents. Extracting BR pigments involves several steps, including cell lysis, centrifugation, solvent extraction, and chromatography. Cell bleaching by cell lysis involves breaking the haloarchaeal cell membrane through sonication [95, 123], freeze‒thawing [53], lyophilization [46], or homogenization [124]. Once the haloarchaea cells have been lysed, centrifugation is used to separate the cell components from the BR-containing supernatant. The supernatant was subsequently subjected to solvent extraction, in which a suitable solvent was used to dissolve and extract BR pigments from other cellular components. Methanol [53, 124], acetone [51], or a methanol/acetone mixture [55, 56, 121] are the most widely used solvents for extracting BR. Chromatography techniques, such as liquid chromatography‒mass spectrometry (LC‒MS), thin-layer chromatography, gas chromatography‒mass spectrometry (GC‒MS), and high-performance liquid chromatography (HPLC), are being extensively employed to analyse, quantify, purify, and separate BR from other cellular components. BR is characterized by analysing their molecular structure and chemical properties through various techniques. These methods include UV‒visible Spectroscopy [15], Ultrahigh-Performance Liquid Chromatography (UHPLC) [125], and Mass Spectrometry (MS) [63] to determine the absorption spectrum (430–530 nm), retention time, and molecular weight (741.1 g mol−1), respectively. Additionally, the functional groups present in BR and its chemical structure were determined by employing Fourier Transform Infrared Spectroscopy (FTIR) [43], Raman Spectroscopy [46], and Nuclear Magnetic Resonance (NMR) spectroscopy [56]. These techniques provide information about the composition and chemical properties of BR. Some of the extraction methods and analysis techniques for BR are listed in Table 4. Most of the studies listed in Table 4 extracted the total carotenoids from archaea and estimated the percentage of BR as a function of total carotenoid content (TCC). It could be inferred that only a few studies have extracted and purified BR to homogeneity.
Bioavailability of Bacterioruberin
The term "bioavailability" describes how much carotenoids are absorbed through circulation and made available for both physiological processes and storage in the human body. Factors such as digestion, absorption, movement, and storage influence carotenoid availability. Sometimes, crystallization of carotenoids can decrease their bioavailability, with only five percent being absorbed in the intestine. However, several investigations have shown that thermal treatment increases carotenoid availability by disrupting cell walls and loosening bonds [5]. The ability of BR to be absorbed and utilized by the human body is referred to as its bioavailability. The potential health benefits of BR, including its antioxidant activity and several other biological properties, have been studied. Understanding its bioavailability is important for determining its effectiveness as a therapeutic agent or nutritional supplement. Due to its chemical lability, poor water solubility, and low bioavailability, the application potential of this compound has significantly decreased, especially for therapeutic uses [127]. Additionally, factors such as dosage, formulation, and individual variations may also influence bioavailability. Until now, there has been no clear evidence to suggest that its bioavailability is limited. However, further research is needed to fully understand how BR is metabolized and distributed in the body. It can be inferred that Lipinski’s rule of five methodology might be useful in identifying bioavailability and pharmacokinetic drug properties by employing computational methodologies. According to Lipinski’s rule, an active compound or drug (orally active) with good permeability has the following criteria: molecular weight (MW) < 450 g mol−1, log P ≤ 5, hydrogen bond acceptor (HBA) < 4, hydrogen bond donor (HBD) < 7, and polar surface (PSA) < 90 Å2 [128]. According to Lipinski’s rule and based on the information available from both the PubChem and carotenoid databases [6], BR has various drug properties, as listed in Table 5, which show almost reasonable drug likeness criteria.
Biological Properties of Bacterioruberin
The carotenoids produced by halophilic archaea exhibit a stronger antioxidant capacity than the carotenoids produced by other microbes (whether they are extremophilic or not). BR, a fat-soluble, bright red carotenoid pigment produced by halophilic archaea, has potent and superior antioxidant activity [15]. According to antioxidant studies on carotenoids so far, the capacity for oxygen-reactive species (ROS) scavenging depends on the concentration of carotenoids. This means that higher concentrations of carotenoids generally result in greater antioxidant activity. Therefore, increasing the concentration of carotenoids in a system can potentially enhance its overall antioxidant capacity. Carotenoids with longer carbon chains and more pairs of conjugated double bonds tend to have greater antioxidant capacities [56, 129]. Additionally, the functional groups and their positions within the carotenoid molecule [129, 130], as well as oxygen-containing substituents [56], can also affect its antioxidant activity. BR contains 13 carbon double bonds, which are more than the nine carbon double bonds of beta-carotene. Therefore, BR is a more effective radical scavenger than beta-carotene is [56, 131]. Furthermore, studies have shown that increasing the concentration of BR can increase its overall antioxidant activity, making it an effective natural antioxidant. On the other hand, carotenoid extracts from halophilic archaea, which are rich in BR, exhibit antimicrobial, anti-haemolytic [51], anticancer [26], and antiviral activities [132]; enhance sperm cell viability during freezing and thawing [121]; and inhibit cholinesterase [42]. The potential of BR extract to repair UV-induced damage to human DNA strands has led to research into its potential for preventing skin cancer [68]. The various biological properties of the BR and BR-rich carotenoid extracts are listed in Table 6. Most of the studies so far have used BR-rich total carotenoid pigment extracts and evaluated the biological properties of total carotenoid pigments. However, further investigations are needed to determine the individual contributions of BR and its potential applications in various fields. Currently, there is limited research on the specific effects of BR. Most related studies have evaluated its anti-inflammatory and antioxidant effects (Table 6). Therefore, there is still a lack of comprehensive understanding of its other potential benefits. Further research is needed to explore the potential therapeutic applications of BR beyond its antioxidant and anti-inflammatory properties. Investigating its impact on immune function, cellular signalling pathways, and disease prevention could provide valuable insights into its biological properties. Additionally, studying the safety profile and potential side effects of BR is crucial for its future use in clinical settings.
Current Applications of Bacterioruberin
Animal Uses
Aquaculture industry: BR is considered a potential feed additive in aquaculture. Metazoans that thrive in salt get their food from halophilic archaea. For instance, Artemia may thrive by consuming nutrients from a mono-diet that is based on halophilic archaea [140]. In one study, researchers reported that the feed containing Haloferax volcani improved Artemia nauplii biomass production and antioxidant content, with BR being the major contributor [141]. In another study, Wei et al. applied carotenoids containing Archaea Halorubrum to aquaculture for the first time. The Halorubrum strain used in their study is a high-BR-producing halobacteria. The Halorubrum strain was fermented in a culture medium, and the cells were fed to Litopenaeus vanammei post-larvae through A. nauplii enrichment. The results showed that Halorubrum-enriched A. nauplii improved L. vanamme survival and growth. The study discovered that dietary supplementation with Halorubrum had a beneficial impact on L. vanammei's ability to tolerate osmotic stress and ammonia stress. This could be linked to the antioxidative capacity of BR, which exists in the archaea. The results suggest that the red halophilic archaea Halorubrum could be a useful feed supplement in shrimp larviculture [7].
Human Uses
Cosmetic industry: HALOTEK, a Germany-based company, has recently launched a product called Halorubin, which is a skincare product made from a natural haloarchaeal ingredient, BR [142]. Another company, ADEKA, a Japanese company, uses halorubin (a BR-rich source) as an active ingredient in more than 15 COSMOS-approved cosmetic products. Some of the ADEKA products that have halorubin as an active ingredient are Pure Serum Retinal, Pure Serum Retinal Jellified, Perfect Eye Contour, Regenerating Retinal Face Cream, Transparent Pectin Gel Lotion, Crystal Make-Up Remover, The Oléo Cleansing Balm, Clear Night Scalp Serum, Natural Concrete Perfume, Crystalline Hand Gel Care, Silky Body Spray, Purified Anti-Acne, Blue Light Power Mask, and Refresh Transparent Cleansing Oil [143]. These halorubin-based products are found to provide several healthcare benefits for the skin. Its unique formula combines the power of BR with a variety of advanced skincare solutions. These products offer a comprehensive barrier against environmental stressors due to their ability to protect against ultraviolet and the consequent DNA damage. Furthermore, its radical scavenging activity helps to combat signs of aging and promote skin rejuvenation, making it an ideal choice for those looking for effective anti-pollution, sun care, and regenerative face cosmetics [143].
While BR currently finds applications in aquaculture and cosmetics, its potential biological properties suggest it may have future applications in other industries, including food and pharmaceuticals. Research and development in this area may uncover additional uses for BR in the future.
Limitations and Conclusion
According to the current literature, BR has various biological properties, including protection against DNA damage; antioxidant, antimicrobial, anti-inflammatory, and anticancer activity; and use as a natural food colorant. Because of these properties, there will be increased demand for BR in the near future. However, the effects of BR on human nutrition, metabolism, and intracellular targets have not yet been unequivocally reported. Until now, BR has not been used in preclinical trials due to constraints related to limited biological sources, optimized cell culture conditions, and a lack of understanding of its potential therapeutic applications. Its unique structure and biological properties and recent advancements in research have sparked interest in exploring the therapeutic applications of BR, opening doors for future clinical research. Accordingly, it is being emphasized that intense research is needed to fully understand the benefits and limitations of BR in preclinical settings before progressing to clinical trials. Furthermore, there is a scarcity of research on the potential health benefits of pure BR. Many of the biological properties discussed in the literature are based on BR-rich extracts. Thus, the existing lacuna in research findings and the consequent speculation related to the projected biological properties of pure BR need to be substantiated with appropriate methodologies and research studies. Hence, there is a need for extensive research to evaluate the possible biological properties of BR. Additionally, it is important to investigate the potential interactions of BR with other compounds commonly found in environmental samples to better understand its stability and potential applications. Further, it is crucial to explore the effect of different storage conditions, such as temperature and light exposure, on BR's longevity, which will provide valuable insights for its practical use in various industries. Overall, a comprehensive understanding of BR's stability and toxicity profile is crucial for its successful integration into biotechnological and biomedical applications. Currently, there is no synthetic source of BR available; it is predominantly present in natural resources such as halophilic archaea. Despite the fact that haloarchaea species have the innate ability to produce BR, especially under optimized culture conditions, the production of BR in a laboratory setting and on large scales remains a challenge, making it difficult to investigate its potential applications and benefits. Only a few studies have been conducted on the optimization of culture conditions for haloarchaea to produce BR under in vitro conditions. Accordingly, further investigations are needed to fully understand and maximize its potential for commercial use. There is growing interest in optimizing culture conditions for high production of BR as well as in searching for alternative production methods due to the limitations in the in vitro production of BR from haloarchaea. Researchers are actively investigating synthetic and biotechnological approaches for increasing the production of BR that involve the utilization of genetic engineering methodologies to augment BR biosynthesis in halobacteria and other hosts. One promising approach involves harnessing the power of CRISPR-Cas9 technology to directly manipulate the genes responsible for BR biosynthesis. This innovative technique holds great potential for significantly boosting BR yields. Moreover, advancements in metabolic engineering offer another avenue for enhancing the metabolic pathways involved in BR bioproduction, thereby leading to increased yields. Furthermore, it is important to explore the feasibility of genetically modifying plants or microbes to serve as a sustainable and cost-effective source of BR production. By employing these cutting-edge approaches, it is hoped that the benefits of BR can be fully assessed and that the demand for BR in sectors such as pharmaceuticals, cosmetics, and food production can be effectively met in the near future. Furthermore, further investigations are needed to understand the intricacies of BR biosynthesis and the regulatory mechanisms of the BR biosynthesis pathway. By delving deeper into BR biosynthesis and its regulation, researchers can uncover the intricate mechanisms underlying BR production and elucidate the factors that influence its production. This knowledge could ultimately pave the way for enhanced production strategies and the utilization of BR in various industries. Additionally, the need for the development of cost-effective and scalable production methods for BR is crucial for its widespread commercialization.
Data Availability Statement
All the data and materials provided in the manuscript are obtained from included references and available upon request.
References
Pfander H (1994) C45- and C50-carotenoids. Pure Appl Chem 66:2369–2374. https://doi.org/10.1351/pac199466102369
Flegler A, Lipski A (2021) The C50 carotenoid bacterioruberin regulates membrane fluidity in pink-pigmented Arthrobacter species. Arch Microbiol 204:70. https://doi.org/10.1007/s00203-021-02719-3
Jain A, Sirisha VL (2020) Algal carotenoids. In: Encyclopedia of marine biotechnology. Wiley, pp 33–64
Maoka T (2020) Carotenoids as natural functional pigments. J Nat Med 74:1–16. https://doi.org/10.1007/s11418-019-01364-x
Shabbir U, Nuzhat H (2017) Natural carotenoids a weapon to fight against life style related disorders. J Food Nutr Popul Health 2:1–6. https://doi.org/10.21767/2577-0586.10036
Yabuzaki J (2017) Carotenoids database: structures, chemical fingerprints and distribution among organisms. Database J Biol Databases Curation. https://doi.org/10.1093/database/bax004
Wei X, Ma Y, Ren B et al (2021) Artemia nauplii enriched with archaea Halorubrum increased survival and challenge tolerance of Litopenaeus vannamei postlarvae. Aquaculture 533:736087. https://doi.org/10.1016/j.aquaculture.2020.736087
Grivard A, Goubet I, Duarte Filho LMS et al (2022) Archaea carotenoids: natural pigments with unexplored innovative potential. Mar Drugs 20:524. https://doi.org/10.3390/md20080524
Henke NA, Frohwitter J, Peters-Wendisch P, Wendisch VF (2018) Carotenoid production by recombinant Corynebacterium glutamicum: strain construction, cultivation, extraction, and quantification of carotenoids and terpenes. Methods Mol Biol Clifton NJ 1852:127–141. https://doi.org/10.1007/978-1-4939-8742-9_8
Martínez-Cámara S, Ibañez A, Rubio S et al (2021) Main carotenoids produced by microorganisms. Encyclopedia 1:1223–1245. https://doi.org/10.3390/encyclopedia1040093
Petter HFM (1932) Over roode en andere bacteriën van gezouten visch. University of Utrecht, Utrecht
Krubasik P, Kobayashi M, Sandmann G (2001) Expression and functional analysis of a gene cluster involved in the synthesis of decaprenoxanthin reveals the mechanisms for C50 carotenoid formation. Eur J Biochem 268:3702–3708. https://doi.org/10.1046/j.1432-1327.2001.02275.x
Osawa A, Ishii Y, Sasamura N et al (2010) Characterization and Antioxidative Activities of Rare C50 Carotenoids-Sarcinaxanthin, Sarcinaxanthin Monoglucoside, and Sarcinaxanthin Diglucoside-Obtained from Micrococcus yunnanensis. J Oleo Sci 59:653–659. https://doi.org/10.5650/jos.59.653
Polyakov NE, Focsan AL, Gao Y, Kispert LD (2023) The endless world of carotenoids—structural, chemical and biological aspects of some rare carotenoids. Int J Mol Sci 24:9885. https://doi.org/10.3390/ijms24129885
Noby N, Khattab SN, Soliman NA (2023) Sustainable production of bacterioruberin carotenoid and its derivatives from Arthrobacter agilis NP20 on whey-based medium: optimization and product characterization. Bioresour Bioprocess 10:46. https://doi.org/10.1186/s40643-023-00662-3
Torregrosa-Crespo J, Montero Z, Fuentes JL et al (2018) Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar Drugs 16:203. https://doi.org/10.3390/md16060203
Gómez-Villegas P, Vigara J, Vila M et al (2020) Antioxidant, antimicrobial, and bioactive potential of two new haloarchaeal strains isolated from Odiel salterns (Southwest Spain). Biology 9:298. https://doi.org/10.3390/biology9090298
Brocks JJ, Summons RE (2014) 10.3 - Sedimentary hydrocarbons, biomarkers for early life. In: Holland HD, Turekian KK (eds) Treatise on geochemistry 2 edn. Elsevier, Oxford, pp 61–103
Flegler A, Lipski A (2022) Engineered CRISPR/Cas9 system for transcriptional gene silencing in Arthrobacter species indicates bacterioruberin is indispensable for growth at low temperatures. Curr Microbiol 79:199. https://doi.org/10.1007/s00284-022-02887-5
Fong N, Burgess M, Barrow K, Glenn D (2001) Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol 56:750–756. https://doi.org/10.1007/s002530100739
Rani A, Saini K, Bast F et al (2021) Microorganisms: a potential source of bioactive molecules for antioxidant applications. Molecules 26:1142. https://doi.org/10.3390/molecules26041142
Morilla MJ, Ghosal K, Romero EL (2023) More than pigments: the potential of Astaxanthin and bacterioruberin-based nanomedicines. Pharmaceutics 15:1828. https://doi.org/10.3390/pharmaceutics15071828
Petter HFM (1931) On bacteria of salted fish. Kon Akad Wet Amst Proc 34:1417–1423. https://dwc.knaw.nl/
Jensen SL, Vesterager E, Clauson-Kaas N, Söderquist R (1960) Bacterial carotenoids. VI. A note on the constitution of bacterioruberine alpha. Acta Chem Scand 14:950–952. https://doi.org/10.3891/acta.chem.scand.14-0950
Baxter BK, Gunde-Cimerman N, Oren A (2014) Salty sisters: The women of halophiles. Front Microbiol 5:192. https://doi.org/10.3389/fmicb.2014.00192
Metwally RA, El-Sersy NA, El Sikaily A et al (2022) Optimization and multiple in vitro activity potentials of carotenoids from marine Kocuria sp. RAM1. Sci Rep 12:18203. https://doi.org/10.1038/s41598-022-22897-4
Jehlička J, Edwards HGM, Oren A (2013) Bacterioruberin and salinixanthin carotenoids of extremely halophilic Archaea and Bacteria: a Raman spectroscopic study. Spectrochim Acta A Mol Biomol Spectrosc 106:99–103. https://doi.org/10.1016/j.saa.2012.12.081
Marshall CP, Leuko S, Coyle CM et al (2007) Carotenoid analysis of halophilic archaea by resonance Raman spectroscopy. Astrobiology 7:631–643. https://doi.org/10.1089/ast.2006.0097
Oren A (2014) Halophilic archaea on Earth and in space: growth and survival under extreme conditions. Philos Trans R Soc Math Phys Eng Sci 372:20140194. https://doi.org/10.1098/rsta.2014.0194
Serino I, Squillaci G, Errichiello S et al (2023) antioxidant capacity of carotenoid extracts from the haloarchaeon Halorhabdus utahensis. Antioxidants 12:1840. https://doi.org/10.3390/antiox12101840
Hassan N, Rafiq M, Haleem A et al (2023) Glaciochemistry and pigment producing ability of bacteria from the roof of the World, the Glaciers of Karakoram, Pakistan. Geomicrobiol J 40:143–151. https://doi.org/10.1080/01490451.2022.2128115
Sharova NYu, Prichepa AO, Sverdlova OP, Printseva AA (2023) Adaptive properties of Arthrobacter agilis strain wb28 isolated from wheat bran. Microbiology 92:666–674. https://doi.org/10.1134/S0026261723600684
Kesbiç FI, Gültepe N (2023) Bioactive components, sun protective properties, and total phenolic contents of halobacterial extracts. Biochem Syst Ecol 108:104647. https://doi.org/10.1016/j.bse.2023.104647
Ávila-Román J, Gómez-Villegas P, de Carvalho CCCR et al (2023) Up-regulation of the Nrf2/HO-1 antioxidant pathway in macrophages by an extract from a new halophilic archaea isolated in Odiel Saltworks. Antioxidants 12:1080. https://doi.org/10.3390/antiox12051080
Delgado-Garcia M, Gómez-Secundino O, Rodríguez JA et al (2023) Identification, antioxidant capacity, and matrix metallopeptidase 9 (MMP-9) In Silico inhibition of haloarchaeal carotenoids from Natronococcus sp. and Halorubrum tebenquichense. Microorganisms 11:2344. https://doi.org/10.3390/microorganisms11092344
Ma Y-C, Gao M-R, Yang H et al (2023) Optimization of C50 carotenoids production by open fermentation of Halorubrum sp. HRM-150. Appl Biochem Biotechnol 195:3628–3640. https://doi.org/10.1007/s12010-023-04319-x
Vasey J (2022) characterisation of pigmentation in a novel isolate of Arthrobacter recovered from soils of the Namib Desert. M.Sc. Thesis, Auckland University of Technology
Giani M, Gervasi L, Loizzo MR, Martínez-Espinosa RM (2022) Carbon source influences antioxidant, antiglycemic, and antilipidemic activities of Haloferax mediterranei carotenoid extracts. Mar Drugs 20:659. https://doi.org/10.3390/md20110659
Hwang CY, Cho E-S, Rhee WJ et al (2022) Genomic and physiological analysis of C50 carotenoid-producing novel Halorubrum ruber sp. nov. J Microbiol 60:1007–1020. https://doi.org/10.1007/s12275-022-2173-1
Kesbiç FI, Gültepe N (2022) C50 carotenoids extracted from Haloterrigena thermotolerans strain K15: antioxidant potential and identification. Folia Microbiol (Praha) 67:71–79. https://doi.org/10.1007/s12223-021-00905-w
Sahli K, Gomri M, Esclapez J et al (2022) Characterization and biological activities of carotenoids produced by three haloarchaeal strains isolated from Algerian salt lakes. Arch Microbiol. https://doi.org/10.1007/s00203-021-02611-0
Lizama C, Romero-Parra J, Andrade D et al (2021) Analysis of carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution UHPLC-Q-Orbitrap-mass spectrometry: antioxidant potential and biological effect on cell viability. Antioxidants 10:1230. https://doi.org/10.3390/antiox10081230
Alvares JJ, Furtado IJ (2021) Characterization of multicomponent antioxidants from Haloferax alexandrinus GUSF-1 (KF796625). 3 Biotech 11:58. https://doi.org/10.1007/s13205-020-02584-9
Vázquez-Madrigal AS, Barbachano-Torres A, Arellano-Plaza M et al (2021) Effect of carbon sources in carotenoid production from Haloarcula sp. M1, Halolamina sp. M3 and Halorubrum sp. M5, halophilic archaea isolated from Sonora Saltern Mexico. Microorganisms 9:1096. https://doi.org/10.3390/microorganisms9051096
Verma DK, Chaudhary C, Singh L et al (2020) Isolation and taxonomic characterization of novel haloarchaeal isolates from Indian Solar Saltern: a brief review on distribution of bacteriorhodopsins and V-Type ATPases in Haloarchaea. Front Microbiol. https://doi.org/10.3389/fmicb.2020.554927
Flores N, Hoyos S, Venegas M et al (2020) Haloterrigena sp. Strain SGH1, a bacterioruberin-rich, perchlorate-tolerant halophilic archaeon isolated from halite microbial communities, Atacama Desert, Chile. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00324
Flegler A, Runzheimer K, Kombeitz V et al (2020) Arthrobacter bussei sp. nov., a pink-coloured organism isolated from cheese made of cow’s milk. Int J Syst Evol Microbiol 70:3027–3036. https://doi.org/10.1099/ijsem.0.004125
Haque RU, Paradisi F, Allers T (2020) Haloferax volcanii for biotechnology applications: challenges, current state and perspectives. Appl Microbiol Biotechnol 104:1371–1382. https://doi.org/10.1007/s00253-019-10314-2
Silva TR, Tavares RSN, Canela-Garayoa R et al (2019) Chemical characterization and biotechnological applicability of pigments isolated from antarctic bacteria. Mar Biotechnol 21:416–429. https://doi.org/10.1007/s10126-019-09892-z
Fariq A, Yasmin A, Jamil M (2019) Production, characterization and antimicrobial activities of bio-pigments by Aquisalibacillus elongatus MB592, Salinicoccus sesuvii MB597, and Halomonas aquamarina MB598 isolated from Khewra Salt Range, Pakistan. Extremophiles 23:435–449. https://doi.org/10.1007/s00792-019-01095-7
Hou J, Cui H-L (2018) In vitro antioxidant, antihemolytic, and anticancer activity of the carotenoids from halophilic archaea. Curr Microbiol 75:266–271. https://doi.org/10.1007/s00284-017-1374-z
Montero-Lobato Z, Ramos-Merchante A, Fuentes JL et al (2018) Optimization of growth and carotenoid production by Haloferax mediterranei using response surface methodology. Mar Drugs 16:372. https://doi.org/10.3390/md16100372
Squillaci G, Parrella R, Carbone V et al (2017) Carotenoids from the extreme halophilic archaeon Haloterrigena turkmenica: identification and antioxidant activity. Extremophiles 21:933–945. https://doi.org/10.1007/s00792-017-0954-y
De La Vega M, Sayago A, Ariza J et al (2016) Characterization of a bacterioruberin-producing Haloarchaea isolated from the marshlands of the Odiel river in the southwest of Spain. Biotechnol Prog 32:592–600. https://doi.org/10.1002/btpr.2248
Naziri D, Hamidi M, Hassanzadeh S et al (2014) Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from Urmia Lake. Adv Pharm Bull 4:61–67. https://doi.org/10.5681/apb.2014.010
Yatsunami R, Ando A, Yang Y et al (2014) Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front Microbiol. https://doi.org/10.3389/fmicb.2014.00100
Sui L, Liu L, Deng Y (2014) Characterization of halophilic C50 carotenoid-producing archaea isolated from solar saltworks in Bohai Bay, China. Chin J Oceanol Limnol 32:1280–1287. https://doi.org/10.1007/s00343-015-4033-x
Lorantfy B, Renkecz T, Koch C et al (2014) Identification of lipophilic bioproduct portfolio from bioreactor samples of extreme halophilic archaea with HPLC-MS/MS. Anal Bioanal Chem 406:2421–2432. https://doi.org/10.1007/s00216-014-7626-x
Abbes M, Baati H, Guermazi S et al (2013) Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement Altern Med 13:255. https://doi.org/10.1186/1472-6882-13-255
Mandelli F, Miranda VS, Rodrigues E, Mercadante AZ (2012) Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J Microbiol Biotechnol 28:1781–1790. https://doi.org/10.1007/s11274-011-0993-y
Rajurkar A, Pathak A (2012) Isolation and characterization of carotenoid producing haloarchaea from solar saltern of Mulund, Mumbai, Indian J Nat Prod Resour 3:483–488. https://nopr.niscpr.res.in/handle/123456789/15575
Fang C-J, Ku K-L, Lee M-H, Su N-W (2010) Influence of nutritive factors on C50 carotenoids production by Haloferax mediterranei ATCC 33500 with two-stage cultivation. Bioresour Technol 101:6487–6493. https://doi.org/10.1016/j.biortech.2010.03.044
Yachai M (2009) Carotenoid production by halophilic archaea and its applications. PhD Thesis, Prince of Songkla University.
LoBasso S, LoPalco P, Mascolo G, Corcelli A (2008) Lipids of the ultra-thin square halophilic archaeon Haloquadratum walsbyi. Archaea 2:177–183. https://doi.org/10.1155/2008/870191
Asker D, Awad T, Ohta Y (2002) Lipids of Haloferax alexandrinus strain TMT: an extremely halophilic canthaxanthin-producing archaeon. J Biosci Bioeng 93:37–43. https://doi.org/10.1016/S1389-1723(02)80051-2
Asker D, Ohta Y (1999) Production of canthaxanthin by extremely halophilic bacteria. J Biosci Bioeng 88:617–621. https://doi.org/10.1016/s1389-1723(00)87089-9
Häberli A, Bircher C, Pfander H (2000) Isolation of a new carotenoid and two new carotenoid glycosides from Curtobacterium flaccumfaciens pvar poinsettiae. Helv Chim Acta 83:328–335. https://doi.org/10.1002/(SICI)1522-2675(20000216)83:2%3c328::AID-HLCA328%3e3.0.CO;2-N
Shahmohammadi HR, Asgarani E, Terato H et al (1998) Protective roles of bacterioruberin and intracellular KCl in the resistance of Halobacterium salinarium against DNA-damaging agents. J Radiat Res (Tokyo) 39:251–262. https://doi.org/10.1269/jrr.39.251
Strand A, Shivaji S, Liaaen-Jensen S (1997) Bacterial carotenoids 55. C50-carotenoids 25.† revised structures of carotenoids associated with membranes in psychrotrophic Micrococcus roseus. Biochem Syst Ecol 25:547–552. https://doi.org/10.1016/S0305-1978(97)00039-2
Chattopadhyay MK, Jagannadham MV, Vairamani M, Shivaji S (1997) Carotenoid pigments of an antarctic psychrotrophic bacterium micrococcus roseus: temperature dependent biosynthesis, structure, and interaction with synthetic membranes. Biochem Biophys Res Commun 239:85–90. https://doi.org/10.1006/bbrc.1997.7433
D’Souza SE, Altekar W, D’Souza SF (1997) Adaptive response of Haloferax mediterranei to low concentrations of NaCl (< 20%) in the growth medium. Arch Microbiol 168:68–71. https://doi.org/10.1007/s002030050471
Rønnekleiv M, Liaaen-Jensen S, Meshkov SV et al (1992) Bacterial carotenoids. 52. C50-Carotenoids 22. Naturally occurring geometrical isomers of bacterioruberin. Acta Chem Scand 46:1092–1095. https://doi.org/10.3891/acta.chem.scand.46-1092
Saito T, Terato H, Yamamoto O (1994) Pigments of Rubrobacter radiotolerans. Arch Microbiol 162:414–421. https://doi.org/10.1007/BF00282106
Tindall BJ, Tomlinson GA, Hochstein LI (1989) Transfer of Halobacterium denitrificans (Tomlinson, Jahnke, and Hochstein) to the genus Haloferax as Haloferax denitrificans comb. nov. Int J Syst Bacteriol 39:359–360. https://doi.org/10.1099/00207713-39-3-359
Juez G, Rodriguez-Valera F, Ventosa A, Kushner DJ (1986) Haloarcula hispanica spec. nov. and Haloferax gibbonsii spec, nov., Two new species of extremely Halophilic Archaebacteria. Syst Appl Microbiol 8:75–79. https://doi.org/10.1016/S0723-2020(86)80152-7
Tomlinson GA, Jahnke LL, Hochstein LI (1986) Halobacterium denitrificans sp. nov., an extremely halophilic denitrifying bacterium. Int J Syst Bacteriol 36:66–70. https://doi.org/10.1099/00207713-36-1-66
Kushwaha S, Juez-Perez G, Rodrigu doiez-Valera F., et al (1982) Survey of lipids of a new group of extremely halophilic bacteria from salt ponds in Spain. Can J Microbiol 28:1365–1372. https://doi.org/10.1139/m82-203
Nur I, Steinitz YL, Okon Y, Henis Y (1981) Carotenoid composition and function in nitrogen-fixing bacteria of the genus Azospirillum. Microbiology 122:27–32. https://doi.org/10.1099/00221287-122-1-27
Evans RW, Kushwaha SC, Kates M (1980) The lipids of Halobacterium marismortui, an extremely halophilic bacterium in the dead sea. Biochim Biophys Acta BBA - Lipids Lipid Metab 619:533–544. https://doi.org/10.1016/0005-2760(80)90105-8
Kushwaha SC, Kramer JKG, Kates M (1975) Isolation and characterization of C50-carotenoid pigments and other polar isoprenoids from Halobacterium cutirubrum. Biochim Biophys Acta BBA - Lipids Lipid Metab 398:303–314. https://doi.org/10.1016/0005-2760(75)90146-0
Arpin N, Fiasson J-L, Norgård S et al (1975) Bacterial carotenoids. XLVI. C50-carotenoids. 14. C50-Carotenoids from Arthrobacter glacialis. Acta Chem Scand 29:921–926. https://doi.org/10.3891/acta.chem.scand.29b-0921
Kushwaha SC, Gochnauer MB, Kushner DJ, Kates M (1974) Pigments and isoprenoid compounds in extremely and moderately halophilic bacteria. Can J Microbiol 20:241–245. https://doi.org/10.1139/m74-038
Gochnauer MB, Kushwaha SC, Kates M, Kushner DJ (1972) Nutritional control of pigment and isoprenoid compound formation in extremely halophilic bacteria. Arch Für Mikrobiol 84:339–349. https://doi.org/10.1007/BF00409082
Mullakhanbhai MF, Francis GW, Upadhyay RR et al (1972) Bacterial lipids. 1. Lipid constituents of a moderately halophilic bacterium. Acta Chem Scand 26:1399–1410. https://doi.org/10.3891/acta.chem.scand.26-1399
Borch G, Norgård S, Liaaen-Jensen S et al (1972) C50-carotenoids. 8. circular dichroism and relative configuration of C50-carotenoids. Acta Chem Scand 26:402–403. https://doi.org/10.3891/acta.chem.scand.26-0402
Arpin N, Fiasson J-L, Liaaen-Jensen S et al (1972) Bacterial carotenoids. XXXIX. C5O-carotenoids 10. bacterioruberin mono- and diglucoside. Acta Chem Scand 26:2526–2528. https://doi.org/10.3891/acta.chem.scand.26-2526
Norgård S, Aasen AJ, Liaaen-Jensen S et al (1970) Bacterial carotenoids. XXXII. C50-carotenoids 6. Carotenoids from Corynebacterium poinsettiae including four new C50-Diols. Acta Chem Scand 24:2183–2197. https://doi.org/10.3891/acta.chem.scand.24-2183
Kelly M, Norgård S, Liaaen-Jensen S (1970) Bacterial carotenoids. 31. C50-carotenoids 5. Carotenoids of Halobacterium salinarium, especially bacterioruberin. Acta Chem Scand 24:2169–2182. https://doi.org/10.3891/acta.chem.scand.24-2169
Martínez-Espinosa RM, Torregrosa-Crespo J (2021) Haloarchaea may contribute to the colour of Avian Plumage in Marine ecosystems. In: Birds - challenges and opportunities for business, conservation and research. IntechOpen.
Yim KJ, Kwon J, Cha I-T et al (2015) Occurrence of viable, red-pigmented haloarchaea in the plumage of captive flamingoes. Sci Rep 5:16425. https://doi.org/10.1038/srep16425
Heider SAE, Peters-Wendisch P, Wendisch VF (2012) Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiol 12:198. https://doi.org/10.1186/1471-2180-12-198
Heider SAE, Peters-Wendisch P, Netzer R et al (2014) Production and glucosylation of C50 and C40 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol 98:1223–1235. https://doi.org/10.1007/s00253-013-5359-y
Netzer R, Stafsnes MH, Andreassen T et al (2010) Biosynthetic pathway for γ-Cyclic Sarcinaxanthin in Micrococcus luteus: heterologous expression and evidence for diverse and multiple catalytic functions of C50 carotenoid cyclases. J Bacteriol 192:5688–5699. https://doi.org/10.1128/jb.00724-10
Tao L, Yao H, Cheng Q (2007) Genes from a Dietzia sp. for synthesis of C40 and C50 β-cyclic carotenoids. Gene 386:90–97. https://doi.org/10.1016/j.gene.2006.08.006
Yang Y, Yatsunami R, Ando A et al (2015) Complete biosynthetic pathway of the C50 carotenoid bacterioruberin from Lycopene in the extremely halophilic archaeon Haloarcula japonica. J Bacteriol 197:1614–1623. https://doi.org/10.1128/jb.02523-14
Yang J, Guo L (2014) Biosynthesis of β-carotene in engineered E coli using the MEP and MVA pathways. Microb Cell Factories 13:160. https://doi.org/10.1186/s12934-014-0160-x
Giani M, Miralles-Robledillo JM, Peiró G et al (2020) Deciphering pathways for carotenogenesis in haloarchaea. Molecules 25:1197. https://doi.org/10.3390/molecules25051197
Cerletti M, Martínez MJ, Giménez MI et al (2014) The LonB protease controls membrane lipids composition and is essential for viability in the extremophilic haloarchaeon Haloferax volcanii. Environ Microbiol 16:1779–1792. https://doi.org/10.1111/1462-2920.12385
Cerletti M, Paggi RA, Guevara CR et al (2015) Global role of the membrane protease LonB in Archaea: potential protease targets revealed by quantitative proteome analysis of a lonB mutant in Haloferax volcanii. J Proteomics 121:1–14. https://doi.org/10.1016/j.jprot.2015.03.016
Cerletti M, Paggi R, Troetschel C et al (2018) LonB protease is a novel regulator of carotenogenesis controlling degradation of phytoene synthase in Haloferax volcanii. J Proteome Res 17:1158–1171. https://doi.org/10.1021/acs.jproteome.7b00809
Cerletti M, Rabino A, Paggi RA et al (2022) The C-terminal region of phytoene synthase is a key element to control carotenoid biosynthesis in the haloarchaeon Haloferax volcanii. Biochem J 479:2365–2377. https://doi.org/10.1042/BCJ20220403
Rodrigo-Baños M, Garbayo I, Vílchez C et al (2015) Carotenoids from haloarchaea and their potential in biotechnology. Mar Drugs 13:5508–5532. https://doi.org/10.3390/md13095508
Sasaki T, Razak NWA, Kato N, Mukai Y (2012) Characteristics of halorhodopsin-bacterioruberin complex from Natronomonas pharaonis membrane in the solubilized system. Biochemistry 51:2785–2794. https://doi.org/10.1021/bi201876p
Fenselau R, Bertholf K (2022) Left on red: the chemistry of color. Synap Intercoll Sci Mag 30:
Liaaen-Jensen S, Lutnaes BF (2005) Charged Carotenoid Species. In: Atta-ur-Rahman (ed) Studies in Natural Products Chemistry. Elsevier, Amsterdam, pp 515–557
Lazrak T, Wolff G, Albrecht A-M et al (1988) Bacterioruberins reinforce reconstituted Halobacterium lipid membranes. Biochim Biophys Acta BBA - Biomembr 939:160–162. https://doi.org/10.1016/0005-2736(88)90057-0
Britton G (1995) Structure and properties of carotenoids in relation to function. FASEB J 9:1551–1558. https://doi.org/10.1096/fasebj.9.15.8529834
Salvador-Castell M, Tourte M, Oger PM (2019) In search for the membrane regulators of archaea. Int J Mol Sci 20:4434. https://doi.org/10.3390/ijms20184434
Baxter RM (1960) Carotenoid pigments of halophilic bacteria. Can J Microbiol 6:417–424. https://doi.org/10.1139/m60-047
Sun C, Ding X, Cui H et al (2018) In situ study of the function of bacterioruberin in the dual-chromophore photoreceptor archaerhodopsin-4. Angew Chem Int Ed 57:8937–8941. https://doi.org/10.1002/anie.201803195
Cao Z, Ding X, Peng B et al (2015) Novel expression and characterization of a light driven proton pump archaerhodopsin 4 in a Halobacterium salinarum strain. Biochim Biophys Acta BBA - Bioenerg 1847:390–398. https://doi.org/10.1016/j.bbabio.2014.12.008
Li Q, Sun Q, Zhao W et al (2000) Newly isolated archaerhodopsin from a strain of Chinese halobacteria and its proton pumping behavior. Biochim Biophys Acta BBA - Biomembr 1466:260–266. https://doi.org/10.1016/S0005-2736(00)00188-7
Yoshimura K, Kouyama T (2008) Structural role of bacterioruberin in the trimeric structure of archaerhodopsin-2. J Mol Biol 375:1267–1281. https://doi.org/10.1016/j.jmb.2007.11.039
Chan SK, Kitajima-Ihara T, Fujii R et al (2014) Crystal structure of cruxrhodopsin-3 from Haloarcula vallismortis. PLoS ONE 9:e108362. https://doi.org/10.1371/journal.pone.0108362
Zhang J, Mizuno K, Murata Y et al (2013) Crystal structure of deltarhodopsin-3 from Haloterrigena thermotolerans. Proteins Struct Funct Bioinforma 81:1585–1592. https://doi.org/10.1002/prot.24316
Kouyama T, Kanada S, Takeguchi Y et al (2010) Crystal structure of the light-driven chloride pump halorhodopsin from Natronomonas pharaonis. J Mol Biol 396:564–579. https://doi.org/10.1016/j.jmb.2009.11.061
Calegari-Santos R, Diogo RA, Fontana JD, Bonfim TMB (2016) Carotenoid production by halophilic archaea under different culture conditions. Curr Microbiol 72:641–651. https://doi.org/10.1007/s00284-015-0974-8
Raghavan TM, Furtado I (2005) Expression of carotenoid pigments of haloarchaeal cultures exposed to aniline. Environ Toxicol 20:165–169. https://doi.org/10.1002/tox.20091
Bidle KA, Hanson TE, Howell K, Nannen J (2007) HMG-CoA reductase is regulated by salinity at the level of transcription in Haloferaxvolcanii. Extremophiles 11:49–55. https://doi.org/10.1007/s00792-006-0008-3
Chen C, Hsu S-H, Lin M-T, Hsu Y-H (2015) Mass production of C50 carotenoids by Haloferax mediterranei in using extruded rice bran and starch under optimal conductivity of brined medium. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-015-1471-y
Zalazar L, Pagola P, Miró MV et al (2019) Bacterioruberin extracts from a genetically modified hyperpigmented Haloferax volcanii strain: antioxidant activity and bioactive properties on sperm cells. J Appl Microbiol 126:796–810. https://doi.org/10.1111/jam.14160
Gong X, Luo H, Wu X et al (2022) Production of Red pigments by a newly isolated Talaromyces aurantiacus strain with LED stimulation for screen printing. Indian J Microbiol 62:280–292. https://doi.org/10.1007/s12088-022-01008-x
El-Sayed WSM, Takaichi S, Saida H et al (2002) Effects of light and low oxygen tension on pigment biosynthesis in Halobacterium salinarum, revealed by a novel method to quantify both retinal and carotenoids. Plant Cell Physiol 43:379–383. https://doi.org/10.1093/pcp/pcf044
Ma Y-C, Su W-P, Sun Z-S et al (2023) Optimization of extraction procedure and antioxidant activity of C50 carotenoids from Halorubrum sp. HRM-150. Process Biochem 130:577–583. https://doi.org/10.1016/j.procbio.2023.05.014
Vaz BMC, Kholany M, Pinto DCGA et al (2022) Recovery of bacterioruberin and proteins using aqueous solutions of surface-active compounds. RSC Adv 12:30278–30286. https://doi.org/10.1039/D2RA02581G
Kholany M, Schaeffer N, Macário IPE et al (2023) Unveiling the use of hydrophobic eutectic solutions as task-specific solvents to recover bacterioruberin from Haloferax mediterranei. ACS Sustain Chem Eng 11:13594–13605. https://doi.org/10.1021/acssuschemeng.3c02997
Caimi AT, Yasynska O, Rivas Rojas PC et al (2022) Improved stability and biological activity of bacterioruberin in nanovesicles. J Drug Deliv Sci Technol 77:103896. https://doi.org/10.1016/j.jddst.2022.103896
Oliyaei N, Moosavi-Nasab M, Tanideh N, Iraji A (2023) Multiple roles of fucoxanthin and astaxanthin against Alzheimer’s disease: Their pharmacological potential and therapeutic insights. Brain Res Bull 193:11–21. https://doi.org/10.1016/j.brainresbull.2022.11.018
Miller NJ, Sampson J, Candeias LP et al (1996) Antioxidant activities of carotenes and xanthophylls. FEBS Lett 384:240–242. https://doi.org/10.1016/0014-5793(96)00323-7
Tian B, Xu Z, Sun Z et al (2007) Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochim Biophys Acta BBA - Gen Subj 1770:902–911. https://doi.org/10.1016/j.bbagen.2007.01.016
Saito T, Miyabe Y, Ide H, Yamamoto O (1997) Hydroxyl radical scavenging ability of bacterioruberin. Radiat Phys Chem 50:267–269. https://doi.org/10.1016/S0969-806X(97)00036-4
Hegazy GE, Abu-Serie MM, Abo-Elela GM et al (2020) In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6. Sci Rep 10:5986. https://doi.org/10.1038/s41598-020-62663-y
Giani M, Montoyo-Pujol YG, Peiró G, Martínez-Espinosa RM (2023) Haloarchaeal carotenoids exert an in vitro antiproliferative effect on human breast cancer cell lines. Sci Rep 13:7148. https://doi.org/10.1038/s41598-023-34419-x
Shahbazi S, Zargar M, Zolfaghari MR, Amoozegar MA (2023) Carotenoid pigment of Halophilic archaeon Haloarcula sp. A15 induces apoptosis of breast cancer cells. Cell Biochem Funct 41:344–354. https://doi.org/10.1002/cbf.3786
Shahbazi S, Zargar M, Zolfaghari MR, Amoozegar MA (2023) Evaluation of anti-cancer effect of carotenoids produced by a halophilic archaeon, Haloarcula Sp. Strain A15 isolated from saline environment of iran on breast cancer cells. In review.
Sahli K, Gomri MA, Esclapez J et al (2020) Bioprospecting and characterization of pigmented halophilic archaeal strains from Algerian hypersaline environments with analysis of carotenoids produced by Halorubrum sp. BS2. J Basic Microbiol 60:624–638. https://doi.org/10.1002/jobm.202000083
Egorenko MY, Baiguzhin GF, Kiseleva MA et al (2022) Cultivation of the Halobacterium salinarum biomass with high antioxidant activity for agricultural and food industry. IOP Conf Ser Earth Environ Sci 1112:012061. https://doi.org/10.1088/1755-1315/1112/1/012061
Simioni YR, Perez NS, Barbosa LRS et al (2022) Enhancing the anti-psoriatic activity of vitamin D3 employing nanostructured archaeolipid carriers. J Drug Deliv Sci Technol 73:103455. https://doi.org/10.1016/j.jddst.2022.103455
Higa LH, Schilrreff P, Briski AM et al (2020) Bacterioruberin from Haloarchaea plus dexamethasone in ultra-small macrophage-targeted nanoparticles as potential intestinal repairing agent. Colloids Surf B Biointerfaces 191:110961. https://doi.org/10.1016/j.colsurfb.2020.110961
Lopes-dos-Santos RMA, De Troch M, Bossier P, Van Stappen G (2019) Archivory in hypersaline aquatic environments: haloarchaea as a dietary source for the brine shrimp Artemia. FEMS Microbiol Ecol 95:fiz178. https://doi.org/10.1093/femsec/fiz178
Sui L, Ren B, Wang S et al (2020) Archaea Haloferax supplementation improves Artemia biomass production in hypersaline conditions. Aquaculture 528:735540. https://doi.org/10.1016/j.aquaculture.2020.735540
HALOTEK GmbH HG (2023) Halorubin | HALOTEK Applied biotechnologies. In: HALORUBIN – Bacterioruberin- powerful carotenoid strongest antioxidative Eff. https://halotek.de/halorubin/. Accessed 1 Jan 2024.
ADEKA A (2023) ADEKA - Cosmetic ingredients and formulation guide 2023. In: ADEKA Eur. GmbH. https://www.adeka.eu/pc.html. Accessed 11 Dec 2023.
Acknowledgements
We acknowledge the authors cited in references for their contributions to the research on bacterioruberin from extremophiles. We also acknowledge Mr. Parthiban Subramanian for plagiarism checking from Plant Genetic Engineering Laboratory, Bharathiar University, Coimbatore.
Author information
Authors and Affiliations
Contributions
MP performed data collection and analysis and wrote the draft. SR designed and revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Declarations
Not applicable.
Conflict of interest
No Conflict of Interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palanisamy, M., Ramalingam, S. Microbial Bacterioruberin: A Comprehensive Review. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01312-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01312-8