Abstract
Mutualisms (reciprocally beneficial heterospecific interactions) are thought as susceptible to exploitation by “cheaters” receiving benefits from partners without fair return. Theoretical studies suggest that partner discrimination is one of the key mechanisms to prevent cheaters from prevailing, but recently, a paradox is suggested that costly partner discrimination might collapse as the result of decreasing in quality variation among potential partners imposed by partner discrimination itself. Here, I develop a simple general mathematical model that consists of two host strains (discriminators/indiscriminators) and two symbiont strains (cooperators/non-cooperators) to establish a framework for the coevolutionary dynamics of mutualisms. First, I present a basic model, a positive equilibrium of which is neutrally stable. Secondly, I derive a formula to describe how the equilibrium shifts with a change in arbitrary parameters: I show a counter-intuitive result that the equilibrium frequency of discriminator hosts decreases as discrimination efficiency increases. Finally, I examine how the equilibrium and its stability changes by adding dependence of fitness of symbionts or hosts on their own frequencies: I find that negative or positive frequency dependence makes the equilibrium asymptotically stable or unstable, respectively. I also find that mutation and immigration of symbionts always make the equilibrium asymptotically stable, even if it does not increase low-quality symbionts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutualisms are a type of heterospecific interactions in which each participant can gain benefits from each other. Various mutualisms play essential roles in a wide range of ecosystems on the earth including tropical rain forests (Janzen 1979; Mcguire 2007), temperate forests (Bennett et al. 2017; Kadowaki et al. 2018), coral reefs (Poulin and Vickery 1995; Rowan 2004), etc. As mutualisms seem susceptible to exploitation by less cooperative or completely uncooperative partners (“cheaters”) receiving benefits from partners without appropriate return, evolutionary ecologists have addressed those apparently paradoxical interactions to identify key mechanisms to prevent cheaters from prevailing and allow them to evolve and persist (Bull and Rice 1991; Sachs et al. 2004).
Partner discrimination, here I use this term in a wide sense including partner choice (Bull and Rice 1991; Sachs et al. 2004), sanction (West et al. 2002a, b; Kiers et al. 2003; Frederickson 2013), preferential allocation (Bever et al. 2009; Kiers et al. 2011), preferential rewarding (Heath and Tiffin 2009), etc., is thought as one of such mechanisms. In partner discrimination, individuals prefer to associate with more cooperative partners, which result in purging cheaters and less cooperative partners from a potential partner population. West et al. (2002a, b) theoretically demonstrated that the presence of low-quality symbionts can select for partner discrimination by hosts (West et al. 2002a) and that the presence of hosts discriminating symbionts can select for more cooperative symbionts (West et al. 2002b). However, Foster and Kokko (2006) paradoxically suggests that those two results do not guarantee stability of mutualistic systems between hosts and symbionts in coevolutionary dynamics; the variation in quality of symbionts within the symbiont population monotonically decreases as the result of partner discrimination, which in turn diminishes the advantage of partner discrimination for hosts. Thus, it is difficult for mutualisms to persist stably unless that variation is maintained via constant immigration or biased mutation introducing low-quality symbionts into the symbiont populations. Further theoretical studies are necessary to identify how and when costly partner discrimination by hosts can emerge and persist in coevolutionary dynamics of mutualistic systems.
To date, mathematical models assuming continuous distribution of the strength of partner discrimination in the host population and cooperation in the symbiont population have been proposed by previous studies. However, they are analytically intractable, and computer simulations are the main methods available for analysis, which can substantially restrict their predictive capability (Foster and Kokko 2006; Ezoe 2016). An alternative framework is a “two-by-two” model, in which two discrete genotypes in each of host and symbiont populations (“discriminator”/ “indiscriminator” host strains and “cooperator”/ “non-cooperator” symbiont strains) are competing within each population (Steidinger and Bever 2014; Uchiumi et al. 2017). This type of models (her,e I temporarily name them “DICN models”) is analytically tractable and has a good potential for giving comprehensive insights into the coevolutionary dynamics of mutualistic systems.
In this study, I develop a generic DICN model to establish a framework for the dynamics of mutualistic systems based on partner discrimination by hosts without specifying detailed function forms (Fig. 1). This model describes the dynamics of the frequencies of two host and two symbiont strains by a replicator dynamics system (Hofbauer and Sigmund 1998), assuming that the strength of partner discrimination by discriminator hosts and cooperation by cooperator symbionts are set to fixed values and do not change in time. Similar formulation is adopted by previous studies (Steidinger and Bever 2014; Uchiumi et al. 2017), and the aim of this study is to generalize them. First, I present a basic model in which fitness of both host and symbiont individuals is independent from the frequencies of their own strains and show that its equilibrium is neutrally stable. Secondly, I derive a formula to describe in which direction the equilibrium shifts with a slight change in values of involving parameters. Finally, I examine how the stability of the equilibrium changes by considering frequency dependence in the fitness of host and symbiont individuals.
Model and analysis
I assume a mutualistic system between a host population and a symbiont population (Fig. 1). Each host individual can associate with many symbiont individuals horizontally transmitted among hosts, while each symbiont can associate with only a single host (one-to-many mutualism). This type of mutualisms includes many well-known examples such as plant–pollinating seed predator (Janzen 1979; Pellmyr and Huth 1994) and legume–rhizobium systems (Denison 2000; Heath and Tiffin 2007, 2009).
The host population consists of two distinct strains: “discriminator” hosts preferentially associate with symbionts beneficial to themselves, while “indiscriminator” hosts do not show such preference. The symbiont population also consists of two strains: “cooperator” symbionts contribute to their associating hosts at the expense of a part of benefit that they receive from the hosts, while “non-cooperator” symbionts receive benefit from the hosts without return.
The frequencies of discriminator and indiscriminator hosts in the host population are denoted by H and 1-H, respectively (0 ≤ H ≤ 1). Similarly, the frequencies of cooperator and non-cooperator symbionts are denoted by S and 1-S, respectively (0 ≤ S ≤ 1). The expected fitnesses of each discriminator host, indiscriminator host, cooperator symbiont, and noncooperator symbiont are denoted by φD, φI, ψC, and ψN, respectively, which are assumed continuously partially differentiable functions with respect to H and S.
I assume that partner preference by discriminator hosts incurs costs to themselves. Therefore, when all symbionts are cooperators (S = 1), indiscriminator hosts are favored over discriminator ones, because there is no variation in quality among symbionts, so that the partner preference has no advantage: φ = φD-φI < 0. In addition, I also assume that ψ = ψC-ψN < 0 at H = 0 and ψH = ∂ψ/∂H > 0 for 0 < H < 1, because noncooperator symbionts freely exploit hosts when all hosts are indiscriminators, while such exploitation becomes more difficult as the frequency of discriminator hosts increases in the host population.
The coupled dynamics of H and S is described by a replicator equation system, which is a framework widely adopted to study common evolutionary dynamics (Hofbauer and Sigmund 1998):
where \(\overline{\mathrm{\varphi } }\)=HφD + (1-H)φI and \(\overline{\uppsi }\)=SψC + (1-S)ψN.
For the system Eqs. (1) and(2), I assume that the discriminator hosts are favored (φ > 0) when the frequency of non-cooperator symbionts is at an intermediate level, because discriminator hosts are less susceptible to exploitation by non-cooperator symbionts. Since I have assumed that φ is a continuous function and φ < 0 at S = 1, there should be a value 0 < S* < 1 satisfying φ = 0 at S = S* and φS = ∂φ/∂S < 0 in a neighborhood of S*. Similarly, I reasonably assume that that there should be a value 0 < H* < 1 satisfying ψ = 0 at H = H* and ψH = ∂ψ/∂H > 0 in a neighborhood of H*. On the other hand, if φ and ψ are negative over 0 ≤ H ≤ 1 and 0 ≤ S ≤ 1, (H, S) = (0, 0) should be a globally stable equilibrium, so that the mutualistic system eventually collapses.
The basic model
In this section, I focus on a simple case by making an additional assumption that fitnesses of host and symbiont individuals are independent from frequencies of their own strains:
Therefore we have,
In this case, H* and S* are independent from S and H, respectively, and (H*, S*) is an internal equilibrium of Eqs. (1) and (2).
The equilibrium (H*, S*) can be shown neutrally stable by constructing a Lyapunov function (Hofbauer and Sigmund 1998). The total derivative of the Lyapunov function V(H, S) is given by:
The function V is time-independent, and has a local minimum at (H*, S*) (Appendix 1). Thus, the solutions of Eqs. (1) and (2) starting from any point in a neighborhood of (H*, S*) move along closed curves around the equilibrium in a counter-clockwise direction on the H–S phase plane.
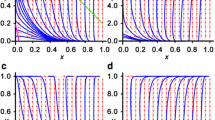
An example is shown in Fig. 2, which is based on “one-shot discrimination” model in Uchiumi et al. (2017) with setting the mutation rate of symbionts to 0:
An example of solution trajectories for the basic model after Uchiumi et al. (2017). The three gray closed curves around an internal neutrally stable equilibrium (H*, S*) (the filled circle) represent the solutions of Eqs. (1) and (2) with different initial values ((H(0), S(0)) = (0.5, 0.1), (0.5, 0.3), (0.5, 0.5)). The values of other parameters are: B = 1.0 × 10−4, C = 5.0 × 10−5, b = 1.0 × 10−4, c = 1.0 × 10−5, ΔE = 1.0 × 10−5, and ΔM = 5.0 × 10−6
Parameter dependence of the equilibrium
Next, I examine how (H*, S*), a neutrally stable equilibrium of Eq. (2), depends on parameter values of the basic model explained above. Note that any change in parameter values does not affect the stability of the equilibrium if Eq. (3) holds.
Considering H* and S* as the functions of an arbitrary parameter p, differentiation of both sides of equations φ(H*, S*) = 0 and ψ(H*, S*) = 0 with respect to p leads to:
respectively. Solving Eqs. (5) and (6) for the derivatives of H*and S*, I have
where φp = ∂φ/∂p and ψp = ∂ψ/∂p. For the basic model, I have assumed φH = ψS = 0, then
Equation (8) indicates how the equilibrium (H*, S*) shifts as the parameter p slightly changes.Although the equilibrium is neutrally stable for the basic model, some additional (sufficiently small) factor can make the equilibrium asymptotically stable. In such cases, Eq. (8) allows us to predict how the stable equilibrium eventually moves after a small change in the value of any parameter.
One counter-intuitive consequence of the above analysis is a change in parameters related to either hosts or symbionts does not affect its own equilibrium but others. For example, when p is the cost for symbionts to cooperate with hosts, it is reasonable to assume ψp < 0. Given that φS < 0 and ψH > 0, Eq. (8) predicts that H* increases as p increases, although S* remains constant if φp = 0.
Furthermore, an increase in “efficiency” of partner discrimination by discriminator hosts is found to result in a decrease in the equilibrium frequency of discriminator hosts as well as an increase in that of cooperator symbionts. This situation is similar to “Volterra’s principle” for Lotka–Volterra predator–prey equation (Hofbauer and Sigmund 1998). In fact, when p is the efficiency parameter, we suppose that φp > 0 and ψp > 0, because an increase in p should favor both discriminator hosts and cooperator symbionts over indiscriminator hosts and noncooperator symbionts, respectively, which result in dH*/dp < 0 and dS*/dp > 0 from Eq. (8). Such an eventually decreasing transition of the frequency of discriminator hosts from a previous equilibrium to the new one often starts with a temporal increase for a short period of time, because when p abruptly increases, the previous equilibrium is likely to be left in the lower-right region of the new equilibrium, where φ > 0 (Fig. 3).
The equilibrium shift with a change in the efficiency of partner discrimination by hosts. This example is based on the same model as Fig. 2 with a positive mutation rate of symbionts (μ = 2.0 × 10−7) (Uchiumi et al. 2017), then the equilibrium is asymptotically stable. a the H–S phase plane, and b the time courses of the frequencies of discriminator hosts (the solid curve) and cooperator symbionts (the dashed curve). After an abrupt shift in the parameter for efficiency of partner discrimination by discriminator hosts from δ = 0.4 to δ = 0.6 at time t = 0, the trajectory of (H, S) starting from a previous equilibrium (the gray circle in Fig. 3a) converges to the new one (the black circle). Other parameter values are the same as Fig. 2
Modification to the basic model
In the following sections, I consider additive factors to the basic model Eqs. (1) and (2) assuming Eq. (3) to examine how they affect the equilibrium.
Frequency dependence of host and symbiont strain fitness
First, I examine how the dependence of the host and symbiont fitness on their own frequencies affects the equilibrium. In general, host or symbiont individuals belonging to the same strain (discriminators, indiscriminators, cooperators, or non-cooperators) are likely to be genetically more similar to each other than to the individuals of the different strains. Then, interactions between individuals are greater within a strain than between different strains in the host or symbiont population. If it is the case, the fitness of individuals positively or negatively depends on the frequency of their own strain in the population.
Similar to the result of the previous section, adding the frequency dependence of symbiont or host strains to the basic model does not shift the equilibrium of their own frequencies but that of the others (Appendix 2).
To examine a change in stability of the equilibrium, first I consider sufficiently small positive frequency dependence of symbionts only, with replacing Eq. (2) with:
where ψF is the difference between the frequency-dependent fitness of a cooperator symbiont ψFC and that of a noncooperator symbiont ψFN: ψF = ψFC-ψFN. Assuming positive frequency dependence, ∂ψF/∂S > 0. Note that the equilibrium of the symbiont frequency remains S = S*, because the dynamics of symbionts Eq. (2) is unchanged. As the magnitude of frequency dependence is sufficiently small, I expect that there is still an equilibrium of the host H** nearby H* (0 < H** < 1).
At the new equilibrium (H**, S*), the components of the Jacobian matrix of Eqs. (2) and (9) are
where the sign of ∂ψF/∂H is the same as that of ∂ψ/∂S if density dependence is sufficiently small. From Eq. (10) and (13), the trace of the Jacobian matrix is.
which indicates that the equilibrium is unstable. On the other hand, when I consider negative frequency dependence of symbionts, the sign of the left-hand side of Eq. (13) turns negative because ∂ψF/∂S < 0. From Eqs. (10) to (12), the trace and the determinant of the Jacobian matrix are
respectively, which indicates that the equilibrium is asymptotically stable.
Similarly, it can be shown that positive (sufficiently small) frequency dependence of host strains tends to destabilize the equilibrium, while the negative one tends to stabilize it (Appendix 3).
Mutation and immigration of symbionts
Mutation of symbionts, especially biased mutation (deteriorating the level of cooperation of mutants to their hosts), has been thought to be a factor to maintain partner discrimination of hosts and stabilize mutualistic systems, because it reintroduces the variation in quality into the symbiont population against the selection imposed by partner discrimination by hosts (Foster and Kokko 2006; Heath and Stinchcombe 2013; Uchiumi et al. 2017).
Considering mutation terms of the symbionts in the present model, Eq. (2) is modified to:
where μ+ and μ- are ameliorating (from noncooperator to cooperator symbionts) and deteriorating (from cooperator to noncooperator symbionts) mutation rates, respectively. I assume that they are sufficiently small that there is still an internal equilibrium nearby (H*, S*), the internal equilibrium of Eqs. (1) and (2).
Note that as the dynamics of host frequency Eq. (2) is unchanged, so is the equilibrium of symbiont frequency as in the previous section. It can be proved that
where (H**, S*) is an equilibrium of Eqs. (2) and (15) (Appendix 2). Equation (16) indicates that dominance of ameliorating mutation of symbionts (μ-/μ+ < (1-S*)/S*) makes the equilibrium of host frequency decrease, while that of deteriorating mutation makes it increase.
It can be shown that Eq. (15) is a special case of negative frequency dependence that I have considered in the previous section. In fact, putting
Equation (15) results in Eq. (9). In addition, the function ψF satisfies
at the equilibrium (H**, S*). The last equality in Eq. (18) is held only when μ+ = μ− = 0. Thus, the mutation of symbionts should always render the equilibrium asymptotically stable, regardless of whether it is biased or not. In particular, it is interesting that ameliorating mutation as well as the deteriorating one can stabilize the equilibrium.
Immigration of low-quality symbionts from an outside source population has also been suggested as a factor maintaining variation in quality of symbionts (Foster and Kokko 2006). Considering immigration from outside source symbiont populations, Eq. (2) is modified to:
where m > 0 is the immigration rate from outside source populations and σ is the proportion of cooperator symbionts in the immigrating symbionts. Here, I assume m and σ to be constants. Again, putting
Equation (20) results in Eq. (9). At the equilibrium (H**, S*), I have
because 0 < S* < 1, m > 0, and 0 ≤ σ ≤ 1. Equation (21) shows that the constant immigration of symbionts also tends to stabilize the equilibrium, regardless of the proportion of cooperator symbionts in the immigrant population.
Discussion
In this study, I have developed a general mathematical model (DICN model) for the coevolutionary dynamics of mutualistic systems consisting of two host strains (discriminators/indiscriminators) and two symbiont strains (cooperators/noncooperators). First, I have constructed a basic model an equilibrium of which is neutrally stable. Next, I have derived a formula to describe how the equilibrium shifts with a change in an arbitrary parameter and shown that at the equilibrium frequency of discriminator hosts decreases as discrimination efficiency increases. Finally, I have examined how position and stability of the equilibrium change by adding dependence of fitness of hosts or symbionts on their own frequencies to show that positive frequency dependence of hosts or symbionts makes the equilibrium unstable, while the negative one makes it asymptotically stable. I have also shown that mutation and immigration of symbionts make the equilibrium asymptotically stable, irrespective of whether they increase low-quality symbionts in the symbiont population or not.
Relevance to Volterra’s principle
I have found that at the equilibrium, the frequency of discriminator hosts decreases as the discrimination efficiency of hosts increases in the DICN model. Based on analysis of the Lotka–Volterra predator–prey (LVPP) equation, Volterra’s principle states that catching both predators and prey leads to a decrease of predators and an increase of prey (Hofbauer and Sigmund 1998). The DICN model seems very different from the LVPP model, but they are both special cases of Kolmogorov model (Brauer and Castillo-Chavez 2012) and their fundamental structures are similar to each other: both generate negative feedback between two variables (the frequencies of the discriminator host and cooperator symbiont strains in the DICN model, and the predator and prey population densities in the LVPP model).
One possible translation between them is that indiscriminator hosts and non-cooperator symbionts in the DICN model corresponds to prey and predators in the LVPP model, respectively, since the former are exploited by the latter in both models. In the DICN model, an increase in discrimination efficiency enhances the fitness of discriminator hosts and cooperator symbionts, which means a decrease in the relative fitness of indiscriminator hosts and non-cooperator symbionts. Then, that would result in a decrease of non-cooperator symbionts as well as an increase of indiscriminator hosts at the equilibrium.
Factors stabilizing or destabilizing the equilibrium
Biased mutation or immigration from an outside source population to restore lower quality symbionts has been considered as a key to maintain costly discrimination in previous studies (Foster and Kokko 2006; Heath and Stinchcombe 2013; Uchiumi et al. 2017). In this study, however, I have shown that mutation and immigration always stabilize the equilibrium irrespective of whether they increase the frequency of non-cooperator symbionts in the symbiont population. I have clarified that a more general criterion to determine stability of the equilibrium is dependency of host and symbiont “fitnesses” on the frequency of their own strains in each population; positive frequency dependence tends to destabilize the equilibrium, while the negative one tends to stabilize it.
Examining the frequency dependence of other factors, we can discuss whether they would stabilize or destabilize the equilibrium. Uchiumi et al. (2017) considers a “resampling” strategy of discriminator hosts in which the hosts reacquire symbionts after purging associating non-cooperator symbionts and suggests that strategy destabilizes the equilibrium by inducing positive feedback in frequency of cooperator symbionts.
In contrast, Akçay (2017) assumes that hosts have a fixed target number of associating symbionts and continue sequential sampling of symbionts until they reach that number. This assumption leads to negative frequency dependence of the symbiont fitness, because the relative advantage of cooperative symbionts in the probability chosen by hosts decreases as their own frequency increases.
Similarly, Ezoe (2019) shows that the adaptive regulation in the number of associating symbionts by hosts counteracts the positive feedback between host strains and their beneficial symbiont strains in a mutualistic system consisting of two competing host–symbiont associations and promotes stable coexistence between them. In that study, the total number of symbionts that a single host associates to maximize its own fitness decreases as the frequency of the symbionts beneficial to the host increases, which results in negative frequency dependence of the symbiont fitness. Therefore, the adaptive regulation of the number of associating symbionts by host would also stabilize the equilibrium of the DICN model.
As well as recruitment of symbionts, reward offered by hosts can also induce positive or negative frequency dependence in symbiont populations. If the amount of net reward to each associating cooperative symbiont is an accelerating or decreasing function of its own frequency, it can stabilize or destabilize the equilibrium via positive or negative frequency feedback, respectively.
Partner fidelity feedback is considered as another major mechanism that can promote the evolution of mutualisms (Bull and Rice 1991; Sachs et al. 2004; Weyl et al 2010). However, partner fidelity feedback can promote positive feedback between host and symbiont strains; therefore, it can destabilize the equilibrium of mutualistic systems driven by partner discrimination (Shapiro and Turner 2014; Uchiumi et al. 2017).
Several studies suggest that spatial structure of populations can promote the evolution of mutualism, as it induces partner fidelity feedback via positively correlated spatial distribution between cooperative heterospecific partners (Doebeli and Knowlton 1998; Yamamura et al. 2004; Travis et al. 2006; Ezoe 2009; Ezoe and Ikegawa 2013). In contrast, Akçay (2017) demonstrates that spatial structure can destabilize mutualism with partner choice, although he suggests that it is because spatial structure decreases local variation in the cooperativeness trait of symbionts so that the benefit of partner choice for hosts diminishes, rather than spatial structure causes the partner fidelity feedback explained above.
Conclusion
The model I have developed in this study is a generalization of discrete-trait models consisting of discriminator/indiscriminator hosts and cooperator/non-cooperator symbionts (Steidinger and Bever 2014; Uchiumi et al. 2017). This model is sufficiently comprehensive as well as tractable that we can analytically derive general principles for the coevolutionary dynamics between host and symbiont populations irrespective of specific model details. My findings should be a helpful guide in analyzing of more realistic continuous-trait models for coevolutionary dynamics of mutualistic systems.
References
Akçay E (2017) Population structure reduces benefits from partner choice in mutualistic symbiosis. Proc R Soc B 284:20162317. https://doi.org/10.1098/rspb.2016.2317
Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017) Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355:181–184. https://doi.org/10.1126/science.aai8212
Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M (2009) Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol Lett 12:13–21. https://doi.org/10.1111/j.1461-0248.2008.01254.x
Brauer F, Castillo-Chavez C (2012) Mathematical models in population biology and epidemiology, 2nd edn. Springer, New York
Bull JJ, Rice WR (1991) Distinguishing mechanisms for the evolution of co-operation. J Theor Biol 149:63–74. https://doi.org/10.1016/S0022-5193(05)80072-4
Denison RF (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am Nat 156:567–576. https://doi.org/10.1086/316994
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. PNAS 95:8676–8680
Ezoe H (2009) Dual lattice model of the evolution of facultative symbiosis with continuous Prisoner’s Dilemma game. J Theor Biol 259:744–750. https://doi.org/10.1016/j.jtbi.2009.04.023
Ezoe H, Ikegawa Y (2013) Coexistence of mutualists and non-mutualists in a dual-lattice model. J Theor Biol 332:1–8. https://doi.org/10.1016/j.jtbi.2013.04.016
Ezoe H (2016) Coevolutionary dynamics in one-to-many mutualistic systems. Theor Ecol 9:381–388. https://doi.org/10.1007/s12080-016-0296-x
Ezoe H (2019) Adaptive partner recruitment can help maintain an intra-guild diversity in mutualistic systems. J Theor Biol 478:40–47. https://doi.org/10.1016/j.jtbi.2019.06.017
Foster KR, Kokko H (2006) Cheating can stabilize cooperation in mutualisms. Proc R Sci B 273:2233–2239. https://doi.org/10.1098/rspb.2006.3571
Frederickson ME (2013) Rethinking mutualism stability: cheaters and the evolution of sanctions. Q Rev Biol 88:269–295. https://doi.org/10.1086/673757
Heath KD, Tiffin P (2007) Context dependence in the coevolution of plant and rhizobial mutualists. Proc R Soc B 274:1905–1912. https://doi.org/10.1098/rspb.2007.0495
Heath KD, Tiffin P (2009) Stabilizing mechanisms in a legume-rhizobium mutualism. Evolution 63:652–662. https://doi.org/10.1111/j.1558-5646.2008.00582.x
Heath KD, Stinchcombe JR (2013) Explaining mutualism variation: a new evolutionary paradox? Evolution 68:309–317. https://doi.org/10.1111/evo.12292
Hofbauer J, Sigmund K (1998) Evolutionary Games and Population Dynamics. Cambridge University Press, Cambridge, UK
Janzen DH (1979) How to be a fig. Ann Rev Ecol Syst 10:13–51. https://doi.org/10.1146/annurev.es.10.110179.000305
Kadowaki K, Yamamoto S, Sato H, Tanabe AS, Hidaka A, Toju H (2018) Mycorrhizal fungi mediate the direction and strength of plant–soil feedbacks differently between arbuscular mycorrhizal and ectomycorrhizal communities. Commun Biol 1:196. https://doi.org/10.1038/s42003-018-0201-9
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume-rhizobia mutualism. Nature 425:78–81. https://doi.org/10.1038/nature01931
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O et al (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882. https://doi.org/10.1126/science.1208473
Mcguire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574. https://doi.org/10.1890/05-1173
Pellmyr O, Huth CJ (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372:257–260. https://doi.org/10.1038/372257a0
Poulin R, Vickery WL (1995) Cleaning symbiosis as an evolutionary game: to cheat or not to cheat? J Theor Biol 175:63–70. https://doi.org/10.1006/jtbi.1995.0121
Rowan R (2004) Coral bleaching: thermal adaptation in reef coral symbionts. Nature 430:742. https://doi.org/10.1038/430742a
Sachs JL, Mueller UG, Wilcox TP, Bull JJ (2004) The evolution of cooperation. Q Rev Biol 79:135–160. https://doi.org/10.1086/383541
Shapiro JW, Turner PE (2014) The impact of transmission mode on the evolution of benefits provided by microbial symbionts. Ecol Evol 4:3350–3361. https://doi.org/10.1002/ece3.1166
Steidinger BS, Bever JD (2014) The coexistence of hosts with different abilities to discriminate against cheater partners: an evolutionary game-theory approach. Am Nat 183:762–770. https://doi.org/10.1086/675859
Travis JMJ, Brooker RW, Clark EJ, Dytham C (2006) The distribution of positive and negative species interactions across environmental gradients on a dual-lattice model. J Theor Biol 241:896–902. https://doi.org/10.1016/j.jtbi.2006.01.025
Uchiumi Y, Ohtsuki H, Sasaki A (2017) Evolutionary emergence and maintenance of horizontally transmitted mutualism that do not rely on the supply of standing variation in symbiont quality. J Evol Biol 30:2211–2221. https://doi.org/10.1111/jeb.13187
West SA, Kiers ET, Pen I, Denison RF (2002a) Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J Evol Biol 15:830–837. https://doi.org/10.1046/j.1420-9101.2002.00441.x
West SA, Kiers ET, Simms EL, Denison RF (2002b) Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc R Soc B 269:685–694. https://doi.org/10.1098/rspb.2001.1878
Weyl EG, Frederickson ME, Yu DW, Pierce NE (2010) Economic contract theory tests models of mutualism. Proc Natl Acad Sci USA 107:15712–15716. https://doi.org/10.1073/pnas.1005294107
Yamamura N, Higashi M, Behera N, Wakano JW (2004) Evolution of mutualism through spatial effects. J Theor Biol 226:421–428. https://doi.org/10.1016/j.jtbi.2003.09.016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The author declares no competing interests.
Appendices
Appendix 1 Local stability of the equilibrium of the basic model
By the definition of equilibrium, φ = ψ = 0 at the equilibrium (H, S) = (H*, S*). In addition, I assume that the finesses of hosts and symbionts are independent from the frequencies of their own strains: ∂φ/∂H = ∂ψ/∂S = 0. From Eq. (4), I have
at the equilibrium (H*, S*). In addition,
because ψH = ∂ψ/∂H > 0 and φS = ∂φ/∂S < 0 at (H*, S*). Then
Equations (22) and (23) indicate that V has a local minimum at (H*, S*). Moreover, from Eqs. (1), (2) and (4),
at any points (H, S). Equation (24) indicates that the value of V is time invariant. Thus (H*, S*) is a neutrally stable equilibrium of Eqs. (1) and (2).
Appendix 2 Effects of frequency dependence on the position of the equilibrium
Let u be a continuous parameter for the degree of frequency dependence of symbiont strains; if u is positive (negative), the degree of the positive (negative) frequency dependence of symbiont strains monotonically increases with the magnitude of u. Functions ψFC and ψFN denote the frequency-dependent fitnesses of cooperator and non-cooperator symbionts, respectively. I assume that they are continuously partially differentiable with respect to u, and that ψFC = ψC and ψFN = ψN (and therefore ψF = ψC-ψN = ψ) when u = 0, where ψC and ψN are frequency-independent fitness functions of cooperator and non-cooperator symbionts, respectively.
The equilibrium of Eqs. (1) and (9) is denoted by (H**, S*). Note that H** = H* when u = 0. Applying the same procedure as Eqs. (5) and (6), I have
where \({\psi }_{H}^{F}=\partial {\psi }^{F}/\partial H\), \({\psi }_{S}^{F}=\partial {\psi }^{F}/\partial S\), \({\psi }_{u}^{F}=\partial {\psi }^{F}/\partial u\), and φu = ∂φ/∂u. As I have assumed φH = φu = 0, then at (H**, S*),
I have also assumed that ψH is positive at (H*, S*), the equilibrium of the frequency-independent system Eqs. (1) and (2). Therefore, if the magnitude of density dependence is sufficiently small, it should be \({\psi }_{H}^{F}>0\) at (H**, S*). Equation (26) indicates that the sign of \(\mathrm{d}\) H**/du is the opposite to the sign of \({\psi }_{u}^{F}\) at the equilibrium, which depends on the detail of the functions ψFC and ψFN.
To consider mutation of symbionts, I set k = μ-/μ+. Then Eq. (17) becomes
Equation (27) is continuously partially differentiable with respect to μ+ and ψF = ψ when μ+ = 0. Therefore, from Eq. (26),
Given ψH > 0 and ψ(H*) = 0, Eq. (28) is followed by Eq. (16).
Similarly, I consider (sufficiently small) positive frequency dependence of host strains with replacing Eq. (2) with
where φF = φFD-φFI. In addition, I introduce a continuous parameter v and assume that φFD and φFI are continuously partially differentiable with respect to v, and that φFD = φD and φFI = φI when v = 0, where φD and φI are frequency-independent fitness functions of discriminator and indiscriminator hosts, respectively. A derivation similar to the above leads to
where (H*, S**) is the equilibrium of Eqs. (2) and (29). Given \({\varphi }_{S}^{F}<0\) at the equilibrium, Eq. (30) indicates that the sign of dS**/dv is the same as the sign of \({\varphi }_{v}^{F}=\partial {\varphi }^{F}/\partial v\) at the equilibrium, which again depends on the detail of the functions φFD and φFI.
Appendix 3 Stability of the equilibrium and frequency dependence of host strains
Here I assume sufficiently small frequency dependence of host fitness on the frequency of its own strain in the population. The sign of the density dependence is the same as the sign of ∂φF/∂H. From Eqs. (2) and (29), I have the following components of the Jacobian matrix at the equilibrium (H*, S**):
If ∂φF/∂H > 0 (positive frequency dependence), it is found from Eqs. (31) and (34) that the trace of the Jacobian matrix.
which indicates that the equilibrium is unstable. On the other hand, if ∂φF/∂H < 0 (negative frequency dependence), Eq. (14) is satisfied so that the equilibrium is asymptotically stable.
Rights and permissions
About this article
Cite this article
Ezoe, H. A general mathematical model for coevolutionary dynamics of mutualisms with partner discrimination. Theor Ecol 15, 203–211 (2022). https://doi.org/10.1007/s12080-022-00537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-022-00537-x