Abstract
“One-to-many” mutualisms are often observed in nature. In this type of mutualism, each host individual can interact with many symbionts, whereas each individual symbiont can interact with only one host individual. Partner choice by the host is a potentially critical mechanism for maintaining such systems; however, its long-term effects on the coevolution between the hosts and symbionts have not been completely explored. In this study, I developed a simple mathematical model to describe the coevolutionary dynamics between hosts and symbionts in a one-to-many mutualism. I assumed that each host chooses a constant number of symbionts from a potential symbiont population, a fraction of which are chosen through preferential choice on the basis of the cooperativeness of the symbionts and the rest are chosen randomly. Using numerical calculations, I found that mutualism is maintained when the preferential choice is not very costly and the mutation rate of symbionts is large. I also found that symbionts that receive benefits from hosts without a return (cheater symbionts) and hosts that do not engage in preferential partner choice (indiscriminator hosts) can coexist with mutualist symbionts and discriminator hosts, respectively. The parameter domain of pure mutualism, i.e., free from cheater symbionts and indiscriminator hosts, can be narrower than the whole domain where the mutualism persists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutualisms are intimate associations between individuals of two different species, where each individual benefits from the activity of the other. Among the full variety of mutualisms in nature, we often find a class in which each host individual can interact with many symbiont individuals (or symbiont strains), whereas each individual symbiont can interact with a particular host individual. Here, I refer to this type of mutualism as “one-to-many” mutualisms, which include well-known mutualistic associations such as legume–rhizobia (Denison 2000; Simms and Taylor 2002; Kiers et al. 2003, 2006; Denison and Kiers 2004; Heath and Tiffin 2007, 2009; Sachs and Simms 2008; Oono et al. 2009; Friesen and Mathias 2010; Friesen 2012) and obligate pollination mutualisms (Pellmyr and Huth 1994; West and Herre 1994; Goto et al. 2010; Jandér and Herre 2010; Jandér et al. 2010).
Mutualisms are of particular interest in the field of evolutionary ecology because they are thought to be vulnerable to invasions of individuals that reap benefits from their partners without return (cheaters). Theoretically, the origin and maintenance of mutualisms can be often explained by the general mechanism of partner fidelity feedback (Bull and Rice 1991; Frank 1994; Sachs et al. 2004; Fujita et al. 2014). Partner fidelity feedback operates when the association between partners lasts for so long that the donation from one partner to the other affects its own fitness through positive feedback from the other. For one-to-many mutualisms, however, partner fidelity feedback is inefficient for facilitating mutualisms because of “the tragedy of the commons” (Rankin et al. 2007; Oono et al. 2009). It occurs when the contribution of an individual symbiont (or a strain of symbionts) to its host benefits all symbionts that share the same host through the feedback; cheating symbionts profit the most from the tragedy of the commons.
Therefore, partner choice is theoretically suggested to be a more plausible mechanism for one-to-many mutualisms (Bull and Rice 1991; Noë and Hammerstein 1994, 1995; West et al. 2002a, b; Foster and Kokko 2006; Ezoe 2012; Bever 2015). Following the classification used by Sachs et al. (2004), I use the term “partner choice” in a broader sense to include host sanction or preferential allocation of resources to partners. If cheaters or less cooperative individuals are less likely to be chosen to associate with (alternatively, be selectively sanctioned or less rewarded by) the partners, they cannot sufficiently exploit the association. A number of empirical supports for a host’s partner choice have been reported in some one-to-many mutualistic systems, including legume–rhizobia (Simms and Taylor 2002; Kiers et al. 2003, 2006; Sachs and Simms 2008), yucca–yucca moth (Pellmyr and Huth 1994), fig–fig wasp (Jandér and Herre 2010), and Glochidion tree–Epicephala moth (Goto et al. 2010) systems. Partner choice also helps to maintain mutualism in many-to-many mutualisms, such as plant–mycorrhiza systems (Kiers et al. 2011).
Partner choice, however, would have a different effect on most one-to-many mutualisms than many-to-many mutualisms because of their asymmetric structure; in one-to-many mutualisms, it is very often the case that only hosts have the option of choosing more effective symbionts. This favors symbionts that contribute the most to their hosts and hence secure the least profit for themselves from the association. In other words, symbionts are inevitably “enslaved” by their hosts (Kiers et al. 2011), which can harm the stability of the mutualistic system, particularly when symbionts can freely be recruited from the environment so that partner fidelity feedback operates insufficiently. In such cases, there are no incentives for host individuals to ensure benefits to their symbionts. Because of the unilateral exploitation by hosts, the symbiont population might shrink and eventually go extinct by a small change in environmental conditions. Otherwise, symbionts might withdraw from the unprofitable associations to become free-living or parasitic if they are capable to do so (Sachs and Simms 2006). This potential for failure of mutualisms can be considered as the second tragedy of the commons in one-to-many mutualisms, although it has rarely been recognized.
To focus on this problem, previously I developed a simple theoretical model for evolutionary dynamics of the cooperativeness of symbionts in one-to-many mutualisms with assuming that hosts have a limited ability to discriminate among symbionts (Ezoe 2012). Specifically, I divided the symbiont partner choice by hosts into two components: one is preferential choice, in which more cooperative symbiont individuals are more likely to be chosen, and the other is random choice, in which they are chosen independently of their cooperativeness. I showed that cheater symbionts that receive benefits from their hosts with no return can invade when the discrimination in the preferential choice is strict. This is because more strict preferential choice favors more cooperative symbionts that secure less profit for themselves, which gives competitive advantage to cheaters chosen in the random choice. This result seems paradoxical but is consistent with empirical evidences that cheater symbionts often coexist with the mutualist ones and are not necessarily punished by hosts in mutualistic systems (West and Herre 1994; Denison and Kiers 2004; Edwards et al. 2010; Jandér and Herre 2010). Nevertheless, my previous study was insufficient to fully explain coevolutionary stability of one-to-many mutualisms, as I did not consider the evolution on the host side explicitly.
In this article, I extend my previous model by considering the evolution of the two traits on the accuracy of the partner choice by hosts, the fraction of the preferential choice, and the strength of the preferential choice. Using numerical calculations, I explore the long-term coevolutionary dynamics between hosts and symbionts. I mainly examine the effects of cost coefficients for the accuracy of the partner choice and the mutation rate of symbionts on the outcome of the coevolutionary dynamics.
Mathematical model
I extend my previous model (Ezoe 2012) to allow the partner preference trait of hosts to evolve. First, I assume an obligate mutualistic system where each host interacts with many symbionts, whereas each symbiont can interact with a particular host. The symbiont population is sufficiently large and consists of many strains of varying cooperativeness values, x (0 ≤ x ≤ 1), with the host. The cooperativeness of the strain i is denoted by x i . The partner choice trait of a host is represented by a pair of numbers (c, k), where 0 ≤ c ≤ 1 is a fraction of the preferential choice of a host and k ≥ 0 is the strength of the preference. I assume that the preferential choice is genetically determined. The trait of a host individual of strain j is denoted by (c j , k j ).
Generations of symbionts are discrete, and their population turns over per unit time. The association between hosts and symbionts is renewed with every generation of symbionts. At the beginning of the generation, each host chooses a sufficiently large constant number, N, of symbiont partners from the potential symbiont population in the environment (Fig. 1a). The choice by a host individual, j, consists of two components: the fraction, c j , of partners chosen on the basis of their cooperativeness (preferential choice), and the remaining, 1 − c j , chosen independently (random choice). For each preferential choice, the probability that a symbiont of strain i is chosen is
where s i and S are the population densities of strain i and all symbionts (S = Σ i s i ), respectively. The function f(x, k) represents the preferential weight for choosing more cooperative symbionts, and \( {\overline{f}}_{k_j} \) is the average of f(x, k j ) over the symbiont population in the environment, i.e., \( {\overline{f}}_{k_j} \) = Σ i s i f(x i , k j )/S. I assume a specific function form f(x, k) = x k, whereas Ezoe (2012) analyzed with more general forms of f. On the other hand, for each random choice, the probability that a symbiont individual of strain i is chosen is equal to the frequency of the strain s i /S. Therefore, the expected number of symbionts of strain i in the N symbiont partners is equal to
A schematic representation of the mutualistic system assumed in the model. a At the beginning of the generation of the symbiont population, each host chooses N partners from an environmental symbiont population (other host individuals are not shown). The host chooses partners as follows: a fraction of partners c j are chosen depending on the cooperativeness of the symbionts (preferential choice) and the remaining 1 − c j are randomly chosen (random choice), where j indicates the strain of the host. b After establishing an association between the hosts and symbionts, each symbiont receives a constant amount of resources, R, from the host. A symbiont of strain i spends 1 − x i of the resource on its own reproduction and contributes the remaining x i for the fitness gains of the host
After establishing associations with symbionts, the host offers a constant amount of resources, R, to each symbiont. A symbiont of strain i spends (1 − x i ) resources on their own reproduction and contributes x i to the fitness gain of the host (Fig. 1b). More cooperative (higher x) symbionts gain less reproductive success but are more likely to be chosen by hosts. Note that if no hosts choose symbionts preferentially, symbionts making any contribution to their hosts should not be favored. Therefore, to maintain a mutualistic association between hosts and symbionts, at least some hosts must engage in the preferential symbiont choice.
At the end of each generation, the offspring of symbionts disperse over the environment and completely mix with other strains. I assume that the cooperativeness of individual symbionts can change by mutation; therefore, the density of strain i at the beginning of the next generation s i ′ is
where μ ii ′ is the mutation rate from strain i to i′ and σ i is the density of the offspring of strain i at the end of the generation:
where g is a conversion rate constant from the resource to the symbiont offspring and h j is the host frequency of strain j.
The expected fitness gain of a host of strain j is equal to the total return from its symbionts minus the cost of the partner choice:
where a is a conversion rate constant from the resource to the host through the symbionts and χ(c, k) is a cost function of the symbiont choice by the host. I assume that χ(c, k) is an increasing function with respect to c and k:
where ρ c , ρ k , C, and K are positive constants and the upper limits of c and k are 0 < C ≤ 1 and 0 < K, respectively. The terms on the right-hand side of Eq. (6) represent a reasonable assumption that the ability of the host to discriminate among symbionts is imperfect with a finite cost.
I assume that the population density of hosts remains unity over time (Σ j h j = 1) and that mutations occur at a small rate per unit of time. Then, the frequency of the host strain j in the next time unit h j ′ is
where ν jj ′ denotes the mutation rate from strain j to j′ and 0 < α ≤ 1 is the turnover rate of the host population. The parameter α reflects the difference in the turnover time between the host and symbiont populations, although its value does not affect the qualitative results of the dynamics in preliminary calculations.
Direct numerical calculations were conducted on the long-term dynamics of the model described above. The details on the calculations are provided in the Appendix. After non-dimensionalizing the model, it is found that the values of aN, bN, and R do not affect the dynamics. Therefore, the independent parameters are mutation rates of symbionts and hosts (μ ii ′ and ν jj ′, respectively), cost coefficients (ρ c and ρ k ), and upper limits (C and K) of the traits c and k in the preferential choice and in the turnover rate of the host population α. I fixed ν jj ′ and α because changing their values did not affect the results qualitatively in preliminary calculations and examined how the rest of the parameters affect the coevolutionary dynamics between hosts and symbionts.
Results
In the final states of the numerical calculations, the distribution of both the hosts and symbionts tended to converge around one or two sharp peaks. In the symbiont population, one peak occurred at x = 0, which corresponds to symbionts that make no contribution to their hosts (cheaters), whereas the other peak occurred in the domain x > 0 (mutualists). Similarly, in the host population, one peak occurred at c = k = 0, which corresponds to hosts that do not choose their symbionts with any cost (indiscriminators), whereas the other peak occurred in the domain c > 0 and k > 0 (discriminators).
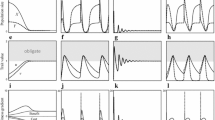
Figure 2 shows how the final states of the numerical calculations qualitatively depended on the cost coefficients of the preferential choice of the hosts ρ c and ρ k . Mutualism persisted when both ρ c and ρ k were small, whereas the final states were either stable or oscillating. When the cost coefficient of the strength of preferential choice ρ k was small compared with the cost coefficient of the fraction of preferential choice ρ c , cheater symbionts (x = 0) coexisted with mutualist symbionts (x > 0). On the other hand, when ρ c was small compared with ρ k , indiscriminator hosts (c = k = 0) coexisted with discriminator hosts. Indiscriminator hosts could persist because the presence of discriminator hosts prevented cheater symbionts from increasing. Therefore, they are considered as “free-rider” hosts. They did not invade when ρ c was large because of the presence of cheater symbionts, to which they were more susceptible than discriminator hosts.
Dependency of the final states of numerical calculations on the parameter values. Blank regions indicate the states of pure mutualism [(−, −) states] where the symbiont population consists of mutualists, and all individuals in the host population engage in preferential partner choices (discriminator hosts). Horizontally and vertically hatched regions indicate the states where cheater symbionts (x = 0) (+, −) and indiscriminator hosts (c, k = 0) (−, +) coexist with mutualistic symbionts and discriminator hosts, respectively. Cross-hatched regions indicate the states where both cheater mutualists (x = 0) and indiscriminator hosts are included (+, +). Dark shaded regions indicate states without mutualistic symbionts. Light shaded domains indicate final oscillatory states. The values of parameters are as follows: ν 0 = 0.0001, α = 1, C = 1.0, K = 10.0; a μ 0 = 0.00001, b μ 0 = 0.0001, c μ 0 = 0.001
Overall, mutualism persisted over a wide range of cost parameters, whereas “pure mutualism,” consisting of only discriminator hosts and only mutualistic symbionts, occurred in a relatively small region. A comparison of Fig. 2a–c shows that mutualism persistence increases as the mutation rate of the symbionts increases. A high mutation rate of symbionts maintained a large variation in their cooperativeness, which favored the costly preferential choice by hosts.
As mentioned above, the final states of the numerical calculations could be oscillating (Fig. 3). When the costs of preferential choice by the hosts are high, the magnitude of the genetic variation among mutualistic symbionts generated by mutation is not enough to maintain the preferential choice; thus, the traits of the choice gradually decrease (Fig. 3a). This triggers a quick rise of cheater symbionts (Fig. 3b), resulting in the increase of genetic variation among symbionts so that the preferential choice by hosts recovers. Foster and Kokko (2006) also found similar oscillations in a parameter region between the regions where mutualism is stable and unstable.
An example of oscillating coevolutionary dynamics of the traits of a hosts and b symbionts. The values of the parameters are ν 0 = 0.0001, α = 1, C = 1.0, K = 10.0, μ 0 = 0.001, ρ k = 10−3.4 = 0.000398, ρ c = 10−2.4 = 0.00398. Note that \( \widehat{x}={\displaystyle {\sum}_{i\ne 0}{x}_i{s}_i/{\displaystyle \sum {s}_i}} \) denotes the mean cooperativeness over only mutualist symbionts
Finally, I changed the upper limits of the parameters of the preferential choice (Fig. 4). When I decreased the limit of the strength of preference K, the parameter region in which mutualism persisted shrank, although the region in which pure mutualism persisted expanded toward the region in which mutualist and cheater symbionts coexisted (Fig. 4a). When I decreased K further, only pure mutualism persisted (Fig. 4b). These results might seem counterintuitive, but are consistent with the condition for cheaters not to invade k(1 − c) < 1 (Ezoe 2012). As K decreased, the strength of preference k of discriminator hosts also decreased because the cost of having high k increased. This prevented mutualism from persisting when the cost coefficient of the strength of preference ρ k was high. At the same time, however, decrease in k favored lower cooperativeness among mutualistic symbionts, which increased their fitness and consequently prevented cheater symbionts from invading. Similarly, when I decreased the limit of the fraction of preferential choice C, the region in which mutualist and cheater symbionts coexisted expanded (Fig. 4c). This was because lower c allowed cheater symbionts to invade through random choice by the hosts.
Discussion
To investigate the long-term stability of one-to-many mutualisms, I expanded the theoretical model developed by Ezoe (2012) to describe coevolutionary dynamics between cooperativeness of symbionts and partner choice of hosts. By conducting numerical calculations of the model, I found that mutualism persisted when the preferential choice by hosts were not very costly and when the mutation rate of symbionts was large. Over a large parameter region, however, symbionts that received benefits from hosts without return (cheaters) or hosts that did not engage in costly preferential partner choices (indiscriminators) coexisted with mutualist symbionts and discriminator hosts. The parameter region where pure mutualism (i.e., free from cheaters and indiscriminators) occurred was small compared with the whole region where mutualism persisted.
To date, a number of theoretical studies have investigated the evolutionary dynamics of one-to-many mutualisms. Some of them examined the evolutionary stability of the cooperativeness of symbionts for a given level of the partner choice by hosts (West et al. 2002a; Friesen and Mathias 2010; Ezoe 2012), while West et al. (2002b) examined the evolutionary stability of costly partner choice by hosts for a given distribution of cooperativeness of symbionts. From those results, however, I cannot conclude immediately that the cooperativeness of symbionts and costly partner choice by hosts are stable in the coevolutionary dynamics: partner choice by hosts reduces genetic variation in the symbiont population, which in turn decreases the incentive for costly partner choice (Heath and Stinchcombe 2013). Foster and Kokko (2006) developed a coevolutionary model in which the cooperativeness of symbionts and the costly partner choice of hosts coevolve. They found that the mutualism is unstable without the constant immigration of less cooperative symbionts from the outside or biased mutation toward the degradation of the cooperativeness of symbionts. Similarly, Song and Feldman (2013) showed that non-heritable phenotypic variation in the symbiont population facilitates the maintenance of costly partner choice of hosts and thereby mutualistic systems.
In contrast, the present study suggests that the variation among symbionts in cooperativeness that is necessary to maintain the costly partner choice can result not only from those direct sources of the variation, but also from indirect mechanisms through the imperfect choice of hosts. I previously showed that the frequency of cheaters in the symbiont population increases with the strength of the preferential choice of hosts k as long as k(1 − c) > 1 (Ezoe 2012); strengthening the preferential choice causes the mutualistic symbionts to be more cooperative (larger x) at the expense of their own fitness, which is advantageous for cheating symbionts that are chosen through the random choice. In the present model, the variation in cooperativeness among symbionts favors higher k values initially. As k increases and exceeds 1/(1 − c), however, cheating symbionts begin to invade and increase in frequency. To reduce the chance of associating with cheaters, hosts must increase the fraction of preferential choice c. This incurs additional choice costs to the hosts to hinder further increases in k. Thus, the variation in cooperativeness among the symbionts is maintained.
It is difficult to identify the reasons that the mutualism cannot persist without the outer sources of variation among symbiont cooperativeness in Foster and Kokko (2006), while it can in the present model. A possible cause of the difference between the two models is an assumption how the contribution of symbionts to their hosts affects their own fitness. In my model, I assume that the fitness of symbionts is proportional to (1 − their cooperativeness); therefore, it can approach zero as the cooperativeness increases to the maximum. This assumption reduces the relative fitness of highly cooperative symbionts and helps cheater symbionts to invade, which facilitates maintenance of variation in the cooperativeness among symbionts. This assumption would be consistent with pollinating seed-consuming mutualisms in which symbionts cannot reproduce without the expense of host seeds or legume–rhizobia mutualisms in which carbohydrates that rhizobia consume to fix nitrogen are provided by hosts. In contrast, Foster and Kokko (2006) assume that the terms of costs for contribution to hosts and benefits for reward from hosts are separated, and the sum of the two terms is the fitness of symbionts. Therefore, the fitness of symbionts does not vanish even though the contribution is at the maximum as long as the association is so cooperative that their hosts properly reward them for their contribution, which would prevent cheater symbionts to invade until the variation among symbionts decreases under the critical level that can support symbiont choice of hosts. However, it is possible that other differences in assumptions between Foster and Kokko (2006) and my models might also affect the difference in their results: The former assumes the constant cost of mutualisms independent from the strength of partner choice, partner fidelity feedback between hosts and symbionts, and evolution of the amount of host reward, while the latter does not. Future studies might clarify the causes of the difference in the results and give a deeper insight into conditions for maintaining one-to-many mutualisms.
It seems paradoxical that the presence of cheaters can prevent mutualisms from ending up with an evolutionary breakdown. Nonetheless, some theoretical studies have found similar results in various contexts of the emergence and maintenance of mutualisms (Foster and Kokko 2006; Ferrière et al. 2002, 2007; Ezoe and Ikegawa 2013; Fujita et al. 2014; Steidinger and Bever 2014). In addition, empirical studies show that cheater symbionts persistently coexist with the mutualist ones in the legume–rhizobium (Denison 2000) and fig–fig wasp systems (Jandér et al. 2010).
As well as cheater symbionts, the present model also predicts the presence of indiscriminator hosts, which abandon the costly partner choice. The majority of hosts engage in the choice so that the average cooperativeness of symbionts is maintained above a certain level. Therefore, indiscriminator hosts are not severely exploited by less cooperative or cheater symbionts. The presence of indiscriminators might potentially relieve selective pressure on less cooperative symbionts and maintain variety within symbiont populations. This speculation is theoretically intriguing, although there are few empirical supports for this hypothesis to my knowledge. Nevertheless, the present model implies that diversity within host populations can play an important role in maintaining the variation in symbiont populations.
Steidinger and Bever (2014) developed a model for the coevolutionary dynamics of mutualisms with an assumption that both the traits of symbionts and hosts are binary (cheaters/mutualists for symbionts, discriminators/indiscriminators for hosts). They suggested that the coexistence of discriminators and indiscriminators in host populations facilitates the coexistence of cheaters and mutualists in symbiont populations and vice versa. Although my results are similar to theirs, I have also shown that whether and when cheater symbionts and indiscriminator hosts can coexist with mutualist symbionts and discriminator hosts, respectively. This is allowed by my assumption that the trait values of the symbiont and the host are continuous so that the variation among mutualist symbionts can solely support the partner choice by hosts. In their model, both the symbiont and host populations should be mixed because of their binary assumption. Other studies have shown that the dynamics of models of mutualism with continuous traits can be qualitatively different from the ones with binary traits (Doebeli and Knowlton 1998; Ezoe 2009).
However, interactions between hosts and symbionts can be more complex than the assumption in the present model. Empirical studies suggest that mutualisms can be context dependent, i.e., the degree of cooperation depends on the combination between genotypes of hosts and symbionts as well as environmental conditions (Heath and Tiffin 2007, 2009). When various host strains coexist within a population, a mutualistic symbiont strain for a particular host strain may be less effective or even a cheater for another host strain; as such, the diversity within the symbionts can be maintained as a whole (Bever 1999). The spatial structure of habitats can also harbor heterogeneity in congeniality between symbionts and hosts through localized coevolution (Parker 1999; Thompson 2005). Although studies on the evolution of mutualism have tended to concentrate on the intimate interactions between cooperative host and symbiont strains, I should focus more attention on the roles of diverse interactions between both host and symbiont assemblages in diffusive coevolutionary dynamics.
References
Bever JD (1999) Dynamics within mutualism and the maintenance of diversity: inference from a model of interguild frequency dependence. Ecol Lett 2:52–62
Bever JD (2015) Preferential allocation, physio-evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytol 205:1503–1514
Bull JJ, Rice WR (1991) Distinguishing mechanisms for the evolution of co-operation. J Theor Biol 149:63–74
Denison RF (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am Nat 156:567–576
Denison RF, Kiers ET (2004) Lifestyle alternatives for rhizobia: mutualism, parasitism, and forgoing symbiosis. FEMS Microbiol Lett 237:187–193
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. Proc Natl Acad Sci U S A 95:8676–8680
Edwards DP, Ansell FA, Woodcock P, Fayle TM, Chey VK, Hamer KC (2010) Can the failure to punish promote cheating in mutualism? Oikos 119:45–52
Ezoe H (2009) Dual lattice model of the evolution of facultative symbiosis with continuous Prisoner’s Dilemma game. J Theor Biol 259:744–750
Ezoe H (2012) Evolutionary stability of one-to-many mutualisms. J Theor Biol 314:138–144
Ezoe H, Ikegawa Y (2013) Coexistence of mutualists and non-mutualists in a dual-lattice model. J Theor Biol 332:1–8
Ferrière R, Bronsterin JL, Rinaldi S, Law R, Gauduchon M (2002) Cheating and the evolutionary stability of mutualisms. Proc R Soc Lond B 269:773–780
Ferrière R, Gauduchon M, Bronstein JL (2007) Evolution and persistence of obligate mutualists and exploiters: competition for partners and evolutionary immunization. Ecol Lett 10:115–126
Foster KR, Kokko H (2006) Cheating can stabilize cooperation in mutualisms. Proc R Soc Lond B 273:2233–2239
Frank SA (1994) Genetics of mutualism: the evolution of altruism between species. J Theor Biol 170:393–400
Friesen ML (2012) Widespread fitness alignment in the legume-rhizobium symbiosis. New Phytol 194:1096–1111
Friesen ML, Mathias A (2010) Mixed infections may promote diversification of mutualistic symbionts: why are there ineffective rhizobia? J Evol Biol 23:323–334
Fujita H, Aoki S, Kawaguchi M (2014) Evolutionary dynamics of nitrogen fixation in the legume–rhizobia symbiosis. PLoS ONE 9:e93670
Goto R, Okamoto T, Kiers ET, Kawakita A, Kato M (2010) Selective flower abortion maintains moth cooperation in a newly discovered pollination mutualism. Ecol Lett 13:321–329
Heath KD, Tiffin P (2007) Context dependence in the coevolution of plant and rhizobial mutualists. Proc R Soc B 274:1905–1912
Heath KD, Tiffin P (2009) Stabilizing mechanisms in a legume-rhizobium mutualism. Evolution 63:652–662
Heath KD, Stinchcombe JR (2013) Explaining mutualism variation: a new evolutionary paradox? Evolution 68:309–317
Jandér KC, Herre EA (2010) Host sanctions and pollinator cheating in the fig tree-fig wasp mutualism. Proc R Soc B 277:1481–1488
Jandér KC, Herre EA, Simms EL, Irwin R (2010) Precision of host sanctions in the fig tree-fig wasp mutualism: consequences for uncooperative symbionts. Ecol Lett 15:1362–1369
Kiers ET, Rousseau RA, Denison RF (2006) Measured sanctions: legume hosts detect quantitative variation in rhizobium cooperation and punish accordingly. Evol Ecol Res 8:1077–1086
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425:78–81
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H et al (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Noë R, Hammerstein P (1994) Biological markets: supply-and-demand determine the effect of partner choice in cooperation, mutualism and mating. Behave Ecol Sociobiol 35:1–11
Noë R, Hammerstein P (1995) Biological markets. Trends Ecol Evol 10:336–339
Oono R, Denison RF, Kiers ET (2009) Controlling the reproductive fate of rhizobia: how universal are legume sanctions? New Phytol 183:967–979
Parker MA (1999) Mutualism in metapopulations of legumes and rhizobia. Am Nat 153:S48–S60
Pellmyr O, Huth CJ (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372:257–260
Rankin DJ, Bargum K, Kokko H (2007) The tragedy of the commons in evolutionary biology. Trends Ecol Evol 22:643–651
Sachs JL, Mueller UG, Wilcox TP, Bull JJ (2004) The evolution of cooperation. Q Rev Biol 79:135–160
Sachs JL, Simms EL (2006) Pathways to mutualism breakdown. Trends Ecol Evol 21:585–592
Sachs JL, Simms EL (2008) The origins of uncooperative rhizobia. Oikos 117:961–966
Simms EL, Taylor DL (2002) Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integr Comp Biol 42:369–380
Song Z, Feldman MW (2013) Plant-animal mutualism in biological markets: evolutionary and ecological dynamics driven by non-heritable phenotypic variance. Theor Popul Biol 88:20–30
Steidinger B, Bever J (2014) The coexistence of hosts with different abilities to discriminate against cheater partners: an evolutionary game-theory approach. Am Nat 183:762–770
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
West SA, Herre EA (1994) The Ecology of the New World fig-parasitizing wasps Idarnes and implications for the evolution of the fig-pollinator mutualism. Proc R Soc Lond B 258:67–72
West SA, Kiers ET, Simms EL, Denison RF (2002a) Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc R Soc Lond B 269:685–694
West SA, Kiers ET, Pen I, Denison RF (2002b) Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? J Evol Biol 15:830–837
Acknowledgments
This work was supported by the Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS) KAKENHI 23570034.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The cooperativeness of symbionts was set to x i = i/40 (i = 0, 1, …, 39). The symbiont choice trait of hosts was two-dimensional, where j = (j 1, j 2) and (c j , k j ) = (C j 1/40, K j 2/40) (j 1, j 2 = 0, 1, …, 39). The mutation rates of symbionts and hosts were
respectively. Throughout the numerical calculations, the value of ν 0 was 0.0001, and the turnover rate of the host population was α = 1.
Each numerical calculation began from the initial distributions of symbionts and hosts, where s i = 0.025 (i = 0, 1, …, 39) and
respectively. Preliminary calculations showed that the initial distributions scarcely affected the final states of dynamics. Two million time units were sufficient for the dynamics to converge to a stable equilibrium or periodic oscillation.
In the final stages of the calculations, frequencies of the symbiont individuals of any trait value were not exactly equal to zero because of mutation. I determined that mutualist symbionts persisted when the frequency of cheater symbionts q = s 0/Σs i was smaller than 0.995, whereas the cheaters were extinct when it was less than 0.005; cheaters and mutualists coexisted otherwise. Similarly, indiscriminator hosts became extinct when their frequency was less than 0.005.
Rights and permissions
About this article
Cite this article
Ezoe, H. Coevolutionary dynamics in one-to-many mutualistic systems. Theor Ecol 9, 381–388 (2016). https://doi.org/10.1007/s12080-016-0296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-016-0296-x