Abstract
New technologies are increasingly widespread in medical practice. Particularly, the 3D view is considered among the most useful innovations for surgery. It allows the operator to reconstruct the patient’s anatomy in his own mind, going beyond his personal imagination. In the last few years, a new facility has been experienced, it’s the Exoscopy. Exoscopy is a magnified vision system, similar to Microscopy, but which also allows a tridimensional vision of the surgical anatomy. Despite Exoscopy having been used for years in Neurosurgery, it has been just rarely described in parotid surgery. We intend to report our experience with Exoscope Aesculap AEOS used to remove benign tumors of the parotid gland. We treated 14 patients with benign tumors of the parotid gland, since September 2023 to November 2023. Each surgery was conducted by the same expert surgeon which also reported his experience about intra-operative complications (as bleeding) in comparison to the traditional procedure without Exoscope. We evaluated the learning curve of Exoscope-Assisted Parotid Surgery comparing, among them, the operative times of the same procedures performed in chronological order. Each patient underwent the same follow-up which included three checks at one month, three months and six months. The follow-up was especially about the evaluation of palsy of the VII C.N. which was assessed through House-Brackmann score (H-B score). The results of our experience reports that the Exoscope is a useful tool for parotid gland surgery. It allows an excellent visualization of the facial nerve main trunk and its branches. Although the first procedures presented longer times in comparison to traditional surgery, the progressive reduction of the operative times demonstrates that the learning curve of Exoscopy is very fast. Certainly, more experience is required for the full introduction of Exoscopy in surgery practice of parotid gland but, now, its potentialities are highly exciting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New technologies are, nowadays, increasingly widespread in all surgical specialities. Augmented Reality, Artificial Intelligence, Navigation-Assisted, Robot-Assisted, Microscopy-Assisted are all new tools fast-changing. They have proven to be very useful in surgery for improving accuracy of surgical procedures, avoiding complications and speeding up the operative times.
Moreover, in the last few years, also Exoscopy has been emerging as a promising innovation in surgery.
Exoscopy is a magnified vision system, similar to Microscopy, but with the relevant difference of allowing a 3-D view not just for the surgical operator but, also, for all those who are present into the operating room. All that it’s possible thanks to a camera maneuvers by the surgeon, who moves the lens on the surgical field and can enlarge the field of view until 8-30x. Everything that is captured by the camera is aired on one, or more, 4 K monitor and can be visualized in a 3D view thanks to special polarized glasses worn by operators and all assistance staff.
In this work we report our experience about use of Exoscope Aesculap AEOS for treatment of benign lesions of parotid gland and, particularly, we report the optimal visibility of the Facial Nerve and its branches.
Tumors of parotid gland are a rare occurrence among head and neck tumor (3–8%) and most of them are benign tumors [1, 2].
The most frequent benign tumor of parotid gland is Pleomorphic Adenoma (PA), followed by Warthin’s Tumor (WT). They involve, generally, the superficial lobe of parotid gland [2].
The gold standard for treatment of benign parotid gland tumors is surgery. However, the topic of surgical procedure is full of controversies [2]. The main difficulties during surgical approach to parotid gland concern the presence of many critical anatomical structures which contract close relationships with the gland, as AuricularTemporal Nerve (ATN), External Carotide Artery (ECA), Transverse Facial Artery (TFA), External Jugular Vein (EJV), Superficial Temporal Artery (STA), Superficial Temporal Vein (STV), Great Auricular Nerve (GAN), RetroMandibular Vein (RMV), Internal Maxillary Artery (IMA), Posterior Auricular Artery (PAA).
However, the main problem during surgical approach to parotid gland is that to find the Facial Nerve (FN). This must be considered a real phase of the parotid surgery and it’s crucial to avoid injuries to main trunk of the nerve and its branches.
Surgical Anatomy of the Parotid Gland
According to the academic anatomy, the parotid gland is divided into two lobes, superficial and deep lobe, following the course of the Facial Nerve (FN) [3, 4]. However, the classic division of the parotid gland into two lobes has been considered unsuitable for the complex surgical necessity required for parotidectomy procedure. For this reason, in 2016, the ESGS (European Salivary Gland Society) proposed a new interpretation of the parotid anatomy, intended as a modification of Quer’s levels of parotid gland [5].
According to ESGS classification, five levels of parotid gland exist:
-
Level I (lateral superior)
-
Level II (lateral inferior)
-
Level III (medial inferior)
-
Level IV (medial superior)
-
Level V (accessory).
The levels division is based on the course of FN main branch, which divides the gland into superior and inferior levels and into superficial and deep levels. The fifth lobe, accessory, is variable and it lies along the course of the Stensen’s duct [5].
The procedures of treatment for benign tumors of parotid gland are Enucleation, ExtraCapsular Dissection, Superficial Parotidectomy and Total Parotidectomy. The risk of FN injury is higher as well as more extended the procedure is.
However, nerve sparing is mandatory during treatment of benigne lesions, even for Total Parotidectomy. So, functional loss of FN after surgery for benign tumors of the parotid gland has to be considered an unpleasant complication [6]. Other complications reported are hypoaesthesia of the auricle (injury of GAN), Frey’s syndrome, sialocele and salivary fistula [6].
The risk of sialocele or salivary fistula is esteemed in 10.4% [7].
The incidence of Frey’s syndrome is reported in a variable between 22 and 27% [8].
A less incidence of sialocele, salivary fistula and Frey’s syndrome are reported when the surgical approach to parotid gland is performed with SMAS flap coverage technique [9].
Surgical Anatomy of FN
Facial Nerve (FN) is the VII CN.
It is a mixed nerve, both motor and sensitive, both visceral and somatic. The somatomotor component is the most represented and it’s aimed to the innervation of the mimic muscles, stapedius, stylohyoid muscle, occipitofrontal muscle and posterior belly of the digastric muscle. The visceromotor component, as the viscerosensitive one, are directed to the lacrimal glands, the submandibular and sublingual glands, the nasal and palate glands. The somatosensitive component is general for the auricle and specific for the taste of 2/3 anterior of the tongue.
Both components somatosensitive and viscerosensitive and visceromotor are conducted by Wrisberg’s nerve (Intermediate nerve) which is part of VII CN.
The FN exits through the Stylomastoid Foramen of the Temporal Bone at the skull base. The main trunk makes a curve downward, passing beneath the external auditory canal, and continues forward. Once reached the mid portion of the earlobe, it deviates its course, becomes superficial and, just it enters the parotid gland, it divides into a superior (temporofacial) and an inferior branch (cervicofacial). After 1 cm of course inside the gland, the frontal and the zygomatic branches originate from the upper branch, while the buccal, the marginalis mandibulae and the cervical branches originate from the lower branch.
The superior branches (frontal and zygomatic) innervate the mimic muscles of the superior and middle third of the face: occipitofrontal, corrugator supercilii, depressor supercilii, procerus, orbicularis oculi, levator labii superioris (alaeque nasi), nasalis, zygomaticus major and zygomaticus minor. The inferior branches (buccal, marginalis mandibulae and cervicalis) innervate the mimic muscles of the inferior third of the face: levator anguli oris, orbicularis oris, buccinator, risorius, depressor anguli oris, depressor labii inferioris, mentalis and platisma [10].
Injuries to the CN VII occurs in the 50% of the surgical approach to parotid gland for benign lesions as temporary weakness, but just in 7% as permanent palsy [11].
Injuries of the frontal branch of CN VII are a relatively common complication of the surgical approach to the parotid gland. It results in the inability to frown and to raise the ipsilateral eyebrow.
A surgical landmark used as reference to avoid injuries to the frontal branch during parotid gland approach is the Pitanguy’s line. It was described by Pitanguy et al. [12] in 1966 as an imaginary line drawn from a point 0.5 cm below the tragus and another point sited 1.5 cm above the lateral margin of the ipsilateral eyebrow. The frontal branch of CN VII comes out from the upper edge of the parotid gland and rises upward, running inside the Temporoparietal Fascia, it passes above the lateral surface of the zygomatic arch. The point where the frontal branch, after perforating the Temporoparietal Fascia, becomes superficial and passes from a sub-SMAS to an supra-SMAS plane, is known as “dangerous zone” [12].
About the middle division of the FN, that is the zygomatic/buccal branches, the respective surgical landmark was recently described, in 2013, by Zuker et al. [13]. The Zuker’s point is in the middle of an imaginary line drawn from the root of the helix to the labial commissure.
Specific landmark for marginalis mandibulae and cervicalis branches are not described because about the marginalis is quite simple to consider the inferior border of the mandible as landmark, while about cervicalis branch the anterior margin of SCM muscle can be consider.
Another nerve at high risk of injury during surgical approach to the parotide gland is the Great Auricolar Nerve (GAN). It’s a superficial sensitive nerve which collects the sensitivity of the skin region of the auricle, the mandibular, the parotid area of the skin and the external auditory canal. It’s the largest ascending branch of the superficial plexus and arises from spinal nerves C2 and C3 following the posterior margin of the SCM muscle, until it passes on the lateral surface of the SCM heading towards the auricle and dividing into anterior and posterior branches [14, 15].
A surgical landmark to avoid GAN lesions during parotidectomy was described in 1980 by McKinney [14]. The McKinney point is 6.5 cm inferior to the external auditory canal and it’s the point in which the GAN runs in the middle portion of the lateral surface of SCM muscle and lies 0.5 cm posterior to the External Jugular Vein (EJV) [16].
Injury of GAN during parotidectomy is common, it has been assessed in the 70% of cases as short-term complication and in the 30% as long-term complication [17]. Generally, it results in hypoaesthesia of the auricle, sometimes as pain referred to the auricle and mandibular region. The hypoaesthesia is known as the main cause of QoL reduction after parotidectomy [17].
Surgical Approach to Parotid Gland and Useful of New Technologies
Surgical approach to the parotid gland started in 1845 with Weber’s description [18] and it continued over the years with a long series of modifications proposed by several authors. However, the approaches most used are two: the Blair’s incision (1920, modified in 1941 by the same author) and the Redon’s incision (1955).
The two procedures are very similar; both begin with an incision in the pre-auricolar area and, after a curve around the earlobe, they descend down the neck along the anterior margin if SCM muscle and continuing in the direction of hyoid bone. The length of the incision is variable based on surgical needs.
The only difference between the Redon’s incision and the modified Blair’s incision is the curve of the incision, which in Blair’s approach it turns around the ear, while in Redon’s incision it stops before (in retro-auricle area) [18].
The second step of the procedure is the preparation of the skin flap, through which it’s possible to expose the underlying SMAS.
The next phase is the SMAS incision and the preparation of SMAS flap, with the exposure of the parotidomasseteric fascia. Once the fascia has been opened, it’s possible to access to the underlying parotid gland.
At this point, the procedure provides to identify main trunk of FN and its bifurcation into two major branches (temporofacial and cervicofacial). Once the main trunk of FN has been identified, the procedure proceeds by the bluntly dissection of the parotid parenchyma from the nerve and its branches until the complete removal of the superficial lobe (level I and II). If the procedure of Superficial Parotidectomy is performed, the surgery ends, otherwise the procedure proceeds with the dissection of the nerve from the underlying parenchyma of the deep lobe (level III and IV) until the complete removal of the gland (Total Parotidectomy with nerve sparing).
The identification of the main trunk of FN is considered the most tricky phase of the parotidectomy and it can be provided through the anterograde or the retrograde via, but the anterograde is considered the classical procedure and it’s preferred by most surgeons [19,20,21]. Injury to the FN can affect the facial expressions, speaking, closure of the eyes and it leads to both emotional and psychological trauma to the patient. Hence, the correct identification and the meticulous dissection are absolutely necessary for anatomical and functional preservation of this nerve.
Many surgical landmarks have been described to avoid FN injuries during the procedure and the main ones are the following:
-
tragal pointer;
-
posterior belly of the digastric muscle;
-
styloid process;
-
tympanomastoid suture;
-
posterior auricular artery.
Most of surgeons use a combination of landmarks in order to safely identify the nerve, but a consensus on which is the safest landmark doesn’t exist yet.
The tragal pointer is the most used. It’s a smooth apophysis of the tragal cartilage. It’s a palpable landmark which indicates the presumable location of the facial nerve. FN main trunk should be medially and inferiorly to the pointer [22] at a variable distance of 13–20 mm (average 16.5 mm) [23].
However, some authors find the tragal pointer unsuitable for safely identification of FN, just due to its cartilaginous histology. These authors consider the tympanomastoid suture a more stable landmark thanks to its osseous nature [24].
Nowadays, technology can be very useful to facilitate surgeon for identifying FN, as well as other critical structures, during parotidectomy procedure, particularly the Microscope and the Exoscope.
Microscope-assisted parotidectomy is a procedure developed in the last 15 years [25] while use of the Exoscope is more recent and doesn’t go beyond the previous 5 years.
Both Microscopy and Exoscopy are computerized magnifying systems which allow to the surgeon a better view of the surgical field and of the closed structures. The main difference between Microscopy end Exoscopy is the tridimensional view provides by the Exoscopy (while the Microscopy simply offers a flat two-dimensional visualization of the surgical field) thanks to special 3-D lens worn by the operators and every other spectator (as training staff).
Exoscopy is widely used in Neurosurgery, first described in 1994 [26]. Some authors reported a good utility of Exoscopy for preparation of microvascular anastomosis in free flap transfer [27]. However, much less experience is in literature about the use of Exoscopy in parotid surgery.
Bartkowiak et al. [28] reported the possibility of involving all operating room staff as the main advantage of Exoscopy. The authors compared the procedure of Microscope-Assisted Parotidectomy with the Exoscopic one. They reported a superimposable quality for visualization of anatomical landmarks (Greater Auricolar Nerve, Digastric muscle, Tragal Pointer, FN main trunk and FN branches) between Microscope and Exoscope. In addition, they reported a superimposable operative times (just over 90 min) and intra-operative bleeding risk, but they reported also an higher outcomes of transient facial palsy was with Exoscopic-Assisted procedure. The authors also reported a poorer perception of the field depth with the Exoscope as, in five cases, the surgeon preferred to convert the procedure from the Exoscope-Assisted to the Microscope-Assisted one, due to a more advanced experience with the latter procedure.
Carta et al. [29] recognize to Exoscopy the considerable advantage of didactic involving for all subjects present in the operating room. Moreover, they reported shorter operating times for the Exoscopy-Assisted procedure in comparison to the Microscope-Assisted one. According to Carta et al. [29] the perception of the field depth is better for Exoscope than Microscope.
Our Experience
We report our experience conducted in Maxillofacial Unit of “G. Martino University Hospital” (Messina, Italy) with Exoscope Aesculap AEOS provided by Braun Milan (Italy).
We treated 14 patients with benign tumors of the parotid gland, since September 2023 to November 2023, 9 women and 5 men (W/M ratio 1:0.5), age 37–72 y.o. (average 56.7 y.o.).
All patients had benign tumors of the parotid gland and all patients underwent surgery after cytological examination through FNAC (but one case with cystic lesion appearance). The lesion found by the Pathologist were seven Pleomorphic Adenomas (PA), four Warthin’s Tumors and one Lipoma. In one case a suspected node was noticed in MRI but FNAC was not decisive, so patient underwent excisional biopsy without a cytological indication. In another case both MRI and CT documented the presence of a lesion with cystic appearance, so patient underwent surgery without previous cytology. In Table 1 are reported the diameters of each lesion measured in the axial and coronal planes. The surgical procedure (Enucleation, ECD, Superficial Parotidecomy, Deep Parotidectomy or Total Parotidectomy) was decided based on the measures of each lesion and on its location: small lesions (less than 25–30 mm) underwent Enucleation (7 cases) or Extra-Capsular Dissection (3 cases), larger lesions or sited between two levels of the parotid gland underwent Partial Parotidectomy (2 Superficial, 1 Deep). Just one Total Parotidectomy was performed for a patient with a large Pleomorphic Adenoma located into the deep lobe.
All the procedures were performed by the same expert surgeon which, at the end of each procedure, reported the entity of the intra-operative bleeding in comparison to traditional procedures performed without Exoscope. The bleeding could be reported as minimal, moderate, high or very high. As we can see in Table 1 the most of procedures were conducted with minimal bleeding (11 out for 14), just in three cases the entity of bleeding was moderate. No case of high, nor very high, bleeding was reported.
The follow-up period was planned in three controls at 1 month (T1), 3 months (T2) and 6 months (T3) since surgery.
The FN post-operative function was evaluated through House-Brackmann score with six grades from Normal (grade 1) to Total Paralysis (grade 6). We considered “Transient Palsy” when the weakness or the paralysis was resolved within 6 months, we considered “Persistent Palsy” when the weakness or the paralysis continued over 6 months. Today we have not awareness about possible cases of “Permanent Palsy”.
In comparison to our previous experience without Exoscope, we noticed an increase in post-operative complications. Especially for sialocele and salivary fistula that, in our experience, occurred in less than 2% of cases while four patients (almost 30%) performed with Exoscope had one of these complications.
The cases of FN palsy also increased. Generally they were ranked as “transient” (healed before 6 months), just in two cases it was “persistent”. Analyzing these outcomes, we can note that cases of “persistent” palsy meet cases performed with Total or Partial Deep Parotidectomy.
In Table 2 the outcomes of the assessments of facial motility expressed through House-Brackmann score (H-B) at control T0 (immediately post-operative time), T1 (1 month after surgery), T2 (3 months after surgery) and T3 (6 months after surgery) are reported.
The numbers of the patients listed in Table 2 correspond to the numbers of patients listed in Table 1 So, we can note that just two cases of persistent palsy are reported and they correspond to a Deep Parotidectomy (patient n. 10) and to a Total Parotidectomy (patient n. 12). Instead, the cases of transient palsy with higher H-B score correspond to the two patients performed with a Superficial Parotidectomy (patient n. 4 and n. 8).
Any case of persistent palsy is reported for the patients treated with Enucleation/ECD. Six cases of transient palsy are reported (patients n. 1, 2, 4, 5, 8 and 14) among ten patients underwent Enucleation/ECD. We can note that, in all cases, a progressive reduction of the H-B score is reported for these patients and, in all cases, an H-B score 0 is reported at 6 months control (T3). The frontal and the buccal branches of FN are equally involved (3 and 3 cases respectively).
The other complications reported are sialocele and salivary fistula (two cases and two cases). Both patients were treated with a conservative approach. One of the two patients with salivary fistula is awaiting for intra-parotid infiltration of botulinum toxin.
About “operative times”, the graphic in Fig. 1 reports a progressive reduction of the duration of the surgery procedures that proves a fast “learning curve” of the Exoscope-Assisted Parotidectomy. The graphic has been built using the ECD/Enucleation procedures because they are the most numerous procedures performed.
It’s not possible, now, to assess the learning curve regarding both Superficial and Total Parotidectomy, but it’s our opinion that even for these kind of procedures the learning curve of Exoscopy should be very fast.
Surgical Procedure
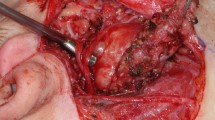
In Fig. 2 the first steps of the parotidectomy approach are reported.
In photo 2a is the step of idrodissection. Through a long needle 21 Gauge, a Mepivacaine solution with Adrenaline (1:100.000) is infiltrated into the dermal plan. This phase has a double advantage: to avoid bleeding and to dissecate the skin from the underlying SMAS plane, that makes the next phase easier.
In photo 2b the Blair’s modification incision is shown. It’s evident the yellow superficial subcutaneous fat.
The image 2c shows the skin flap just prepared through blunt dissection into the plane of subcutaneous fat. The superficial yellow fat is evident both on the SMAS and on the skin flap.
In photo 2d the incision of the SMAS is shown.
In photo 2e is the blunt dissection of the SMAS flap performed into the deep fat layer.
The image 2f shows the SMAS flap just prepared and the overlying skin flap. It’s evident the deep fat tissue (underSMAS) both on the parotid gland and on the SMAS. The parotid fascia is evident as translucent spots that shine within the fat lobules.
In photo 2 g the surgeon touch the tragal pointer. The next phase consists in searching the FN main trunk that’s evident in image 2 h.
Once the FN has been identified the procedure can proceed in different way: for ECD/Enucleation it will be sufficient to remove the tumor (enucleation) through blunt dissection from the surrounding gland parenchyma (Fig. 3a–b) or to remove part of the parenchyma together to the tumor (ECD), while for parotidectomy it will be necessary to continue the dissection of the superficial lobe of the gland following the FN branches until the anterior margin of the gland.
In Fig. 3c–d the inferior cervicofacial branch of FN before (3c) and after (3d) Superficial Parotidectomy is shown.
The Exoscope allows to the surgeon to identify structures easier in comparison to the traditional view. In Fig. 3e is well evident the retromandibular vein.
After positioning of the drainage pipe into the subSMAS plane, the final phase is the hermetic closure of the SMAS flap through continuous suture (Fig. 3f–g). In Fig. 3h is the suture of the skin through detached stitches.
Discussion
New technologies are increasingly used in surgery. It is undeniable that they are very charming and their use is often spectacular. Especially the Exoscope is very rewarding for young surgeons in training thanks to the 3-D that provides a highly realistic view of the surgical field.
However, many doubts exist about several aspects of Exoscope-Assisted parotid surgery:
-
Is the procedure safe?
-
Is the “learning curve” acceptable?
-
Are the operative times favorable?
-
Are the complications less frequent in comparison to traditional surgery?
As we have just reported, our experience suggests that the Exoscope provides a fast “learning curve”, although the machine has been tested by an expert surgeon only.
In comparison to our experience with the traditional surgery, the Exoscopy presents operative times superimposable. During the first procedures, the operative times were longer about for 10%, but in the last the operative times were the same.
Any intra-operative bleeding were reported with Exoscope, so we can say that it’s safe enough.
Unfortunately, we must report that the incidence of the post-operative complications have been higher in our experience with Exoscopy. Although FN and its branches have been clearly and easily identified, we must report an higher incidence of facial palsy even if, in the majority of cases, a transient palsy only was reported. We explain this phenomenon with the more difficult maneuverability of the surgical tools during the finest movements, as when the thin nerve filaments were handled. It could explain how come the paralysis occurred were transient and partial.
Also for the other post-operative complications we noticed an higher frequency. In our traditional experience salivary fistula and sialocele occur for less than 2% of cases, while in 30.7% with Exoscope-assistance they occur. Even this phenomenon we can explain for a poor maneuverability in fine movements, in this case during hermetic closure of the SMAS.
Conclusions
Our experience with Exoscope can be summarized as positive. We define Exoscope a useful tool in parotid surgery, it’s a safe procedure for the patients and highly educative for the training staff. It allows to visualize even small vascular and nervous structures which could escape the naked eye. Learning curve is good and it doesn’t increase the incidence of intra-operative complications. The higher incidence of post-operative complications have been explained and justified, and it’s our opinion that they will not recur after longer use of the Exoscope. Even mild longer operative times during the first operative cases are well compensated after a few uses of the machine.
References
Ştefănescu EH, Mogoantă CA, Căluianu EI, Predescu OI, Florou C, Chercotă V, Iovănescu G (2022) Benign tumors of the superficial lobe of the parotid gland. Rom J Morphol Embryol 63(3):563–567. https://doi.org/10.47162/RJME.63.3.11
Catalfamo L, Nava C, De Rinaldis D (2024) Use of the SMAS flap in benign parotid gland surgery: review and 5 years experience. J Maxillofac Oral Surg. https://doi.org/10.1007/s12663-024-02207-3
Kochhar A, Larian B, Azizzadeh B (2016) Facial nerve and parotid gland anatomy. Otolaryngol Clin North Am 49(2):273–284. https://doi.org/10.1016/j.otc.2015.10.002
Catalfamo L, Siniscalchi EN, De Ponte FS, De Rinaldis D (2024) Post-traumatic sinus syndrome, proposal for a new clinical entity (CDR Syndrome) as variant of the silent sinus syndrome: systematic review and case series. Indian J Otolaryngol Head Neck Surg 76(1):1378–1388. https://doi.org/10.1007/s12070-023-04112-6
Wong WK, Shetty S (2017) Classification of parotidectomy: a proposed modification to the European Salivary Gland Society classification system. Eur Arch Otorhinolaryngol 274(8):3175–3181. https://doi.org/10.1007/s00405-017-4581-0. (Epub 2017 May 11 PMID: 28497264)
Ungari C, Terenzi V, Salem YA, Filiaci F, Priore P, Della Monaca M, Battisti A, Cassoni A, Valentini V (2022) Management of benign parotid tumors. What can we learn from our experience? Ann Ital Chir 93:152–159
Liu Y, Yuan W, Sun H, Su M, Kong X, Huang X (2022) Predictors of sialocele or salivary fistula after partial superficial parotidectomy for benign parotid tumor: a retrospective study. J Oral Maxillofac Surg 80(2):327–332. https://doi.org/10.1016/j.joms.2021.09.013
Ogreden S, Ruzgar S, Alimoglu Y, Eroglu S, Taskin U, Oktay MF (2016) Comparison of Frey syndrome rates following superficial parotidectomy and partial superficial parotidectomy for pleomorphic adenoma. J Craniofac Surg 27(5):e469–e471. https://doi.org/10.1097/SCS.0000000000002746
Cristofaro MG, Cordaro R, Barca I, Giudice A (2021) Efficacy of SMAS flap technique to prevent Frey’s syndrome and aesthetic outcomes. a retrospective cohort analysis. Ann Ital Chir 92:683–690
Seneviratne SO, Patel BC. (2024) Facial Nerve Anatomy and Clinical Applications. 2023 May 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Sethi N, Tay PH, Scally A, Sood S (2014) Stratifying the risk of facial nerve palsy after benign parotid surgery. J Laryngol Otol 128(2):159–162. https://doi.org/10.1017/S0022215113003502
Pitanguy I, Ramos AS (1966) The frontal branch of the facial nerve: the importance of its variations in face lifting. Plast Reconstr Surg 38(4):352–356
Dorafshar AH, Borsuk DE, Bojovic B, Brown EN, Manktelow RT, Zuker RM, Rodriguez ED, Redett RJ (2013) Surface anatomy of the middle division of the facial nerve: Zuker’s point. Plast Reconstr Surg 131(2):253–257. https://doi.org/10.1097/PRS.0b013e3182778753
Werner C, D’Antoni AV, Iwanaga J, Watanabe K, Dumont AS, Tubbs RS (2021) A comprehensive review of the great auricular nerve graft. Neurosurg Rev 44(4):1987–1995. https://doi.org/10.1007/s10143-020-01426-9
Catalfamo L, De Rinaldis D, Cicchiello S, Scozzaro C, Nava C, De Ponte FS (2023) Central giant cell reparative granuloma (CGCRG) of the jaw in children treated with neoadjuvant bisposphonates: review and a case report. Indian J Otolaryngol Head Neck Surg 75(2):1117–1122. https://doi.org/10.1007/s12070-022-03413-6
McKinney P, Katrana DJ (1980) Prevention of injury to the great auricular nerve during rhytidectomy. Plast Reconstr Surg 66(5):675–679. https://doi.org/10.1097/00006534-198011000-00001
Plath M, Sand M, Cavaliere C, Plinkert PK, Baumann I, Zaoui K (2022) Long-term outcomes and quality of life following parotidectomy for benign disease. Acta Otorhinolaryngol Ital 42(3):215–222. https://doi.org/10.14639/0392-100X-N1728.PMID:35880361;PMCID:PMC9330751
Sifuentes-Cervantes JS, Carrillo-Morales F, Castro-Núñez J, Chivukula BV, Cunningham LL, Van Sickels JE (2022) Historical evolution of surgical approaches to the face-part II: midface. Oral Maxillofac Surg 26(2):177–184. https://doi.org/10.1007/s10006-021-00956-w
Al-Qahtani KH, AlQahtani FM, Muqat MM, AlQahtani MS, Al-Qannass AM, Islam T, Alharbi J, Sebaih H, Alqarni M, Hakami H (2020) A new landmark for the identification of the facial nerve during parotid surgery: a cadaver study. Laryngoscope Investig Otolaryngol 5(4):689–693. https://doi.org/10.1002/lio2.431
Catalfamo LM, Scozzaro C, Cicchiello S, Scozzaro MP, Romeo C, De Rinaldis D, Saccà S, Nava C, Calvo A, De Ponte FS (2022) Maxillofacial injuries in padel game. J Maxillofac Oral Surg. 21(4):1393–1396. https://doi.org/10.1007/s12663-022-01725-2
Catalfamo LM, Scozzaro C, De Rinaldis D, Romeo C, Cicchiello S, Nava C, Squillacioti A, De Ponte FS (2022) The C-S approach for the management of median or paramedian frontal sinus lesion. Indian J Otolaryngol Head Neck Surg 74(Suppl 3):4598–4602. https://doi.org/10.1007/s12070-021-02896-z
Muhleman MA, Wartmann CT, Hage R, Matusz P, Shoja MM, Tubbs RS, Loukas M (2012) A review of the tragal pointer: anatomy and its importance as a landmark in surgical procedures. Folia Morphol (Warsz) 71(2):59–64
Saha S, Pal S, Sengupta M, Chowdhury K, Saha VP, Mondal L (2014) Identification of facial nerve during parotidectomy: a combined anatomical & surgical study. Indian J Otolaryngol Head Neck Surg 66(1):63–68. https://doi.org/10.1007/s12070-013-0669-z
Kaushal D, Gugliani A, Sharma V, Goyal A, Choudhury B, Soni K (2021) Identifying the facial nerve in parotid surgeries: How we do it. Iran J Otorhinolaryngol 33(115):93–96. https://doi.org/10.22038/ijorl.2020.43760.2446.PMID:33912484;PMCID:PMC8052489
Rehberg E, Schroeder HG, Kleinsasser O (1998) Chirurgie bei gutartigen Parotistumoren: individuell angepasste oder standardisierte radikale Eingriffe? [Surgery in benign parotid tumors: individually adapted or standardized radical interventions?]. Laryngorhinootologie 77(5):283–288. https://doi.org/10.1055/s-2007-996975
Gildenberg PL, Ledoux R, Cosman E, Labuz J (1994) The exoscope–a frame-based video/graphics system for intraoperative guidance of surgical resection. Stereotact Funct Neurosurg 63(1–4):23–25. https://doi.org/10.1159/000100285
Ichikawa Y, Senda D, Shingyochi Y, Mizuno H (2019) Potential advantages of using three-dimensional exoscope for microvascular anastomosis in free flap transfer. Plast Reconstr Surg 144(4):726e–727e. https://doi.org/10.1097/PRS.0000000000006088
Bartkowiak E, Łuczewski Ł, Chou JT, Wierzbicka M (2022) Is the 3D exoscope better than the surgical microscope in parotid surgery: a prospective, randomized single-center study. Eur Arch Otorhinolaryngol 279(2):1029–1034. https://doi.org/10.1007/s00405-021-06876-5
Carta F, Mariani C, Marrosu V, Gerosa C, Puxeddu R (2020) Three-dimensional, high-definition exoscopic parotidectomy: a valid alternative to magnified-assisted surgery. Br J Oral Maxillofac Surg 58(9):1128–1132. https://doi.org/10.1016/j.bjoms.2020.06.015
Funding
Any found has been received by the authors for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each author disclose interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Rinaldis, D., Scozzaro, C., Calvo, A. et al. New 3-D Technologies in Salivary Gland Surgery: The Exoscope-Assisted Surgery for Treatment of Benign Tumors of Parotid Gland. Indian J Otolaryngol Head Neck Surg (2024). https://doi.org/10.1007/s12070-024-04898-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12070-024-04898-z