Abstract
Central giant cell reparative granuloma (CGCRG) of the jaw is a neoformation localized in the mandible or in the maxillary bone and characterized by fibrous tissue, osteoclast-like giant cells and reactive bone formation. The CGCRG is a less frequent benign tumor but sometimes it is characterized by an aggressive behavior with a very rapid growth. It affects the young adults mainly and the children occasionally. Nowadays no medication therapy is approved for CGCRG in pediatric cases. We present a case of an aggressive form of a mandibular CGCRG in a 5-year child with Arnold-Chiari syndrome. This case is unique for the use of bisphosphonates (BPs) as neoadjuvant therapy in pediatrics. The therapy were administrated with the purpose of arresting the rapid growth of the tumor in order to avoid a demolitive surgery to the young patient. The child was without symptoms and presented an unusual swelling in the left mandible developed in a few weeks. The lesion was diagnosed by a CT scan and it was confirmed by a biopsy performed for histopathological assessment a few days later. The drug therapy consisted of seven cycles iv of Zoledronate associated to Calcium Gluconate. The child was closely observed through clinical and serological evaluations during the following months. About five months after the last cycle of BPs the child underwent CT scan and a conservative surgical treatment, consisted in a deep curettage, was programmed. Seven months after surgery the aesthetic profile of the patient improved and CT scan reported a significant calcic neoapposition in the area of the previous bone lesion. After more than one year from surgery, no relapse was observed. This case report demonstrates that BPs can be used safely in pediatric patients with CGCRG. Especially BPs could have a role as neoadjuvant therapy: If administrated before surgical treatment BPs avoid the necessity of resective surgery and reduce the risk of recurrence in pediatric CGCRG after conservative curettage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “Giant Cell Reparative Granuloma” was introduced in 1953 by Henry L. Jaffe (1896–1979) [1]. The author recommended to not use the term “tumor” as it was considered just a reactive lesion rather than a neoformation. However, nowadays, it’s known that CGCRG can assume an unpredictable aggressive behavior, so it is better classified as a benign neoplasm [2].
Histologically Central giant cell reparative granuloma (CGCRG) is a bone lesion characterized by fibrous tissue with hemosiderin deposits and hemorrhage areas and osteoclast-like giant cells. It places more frequently in the mandible and rarely in the maxilla, and accounts approximately for 7% of all benign tumors of the jaws. Patients are usually younger than 30 years old, and more frequent are female [3].
Although it is a benign tumor, sometimes, CGCRG behaves in an aggressive way until it makes necessary a resective surgical treatment, often heavily demolitive.
With the aim to avoid a highly disfiguring demolitive surgery, several drugs therapy were proposed for the treatment of aggressive CGCRG. One of them is Calcitonin, proposed in the 90’s [4]. Initially, it was used the protocol of 0.5 mg (100 IU) daily of Calcitonin subcutaneous [4], afterwards Salmon Calcitonin nasal spray (200 IU) daily was proposed [5]. However, nowadays, neither the Calcitonin s.c. nor the nasal spray one have been approved for the treatment of CGCRG.
The alpha-INF iv was also performed for the treatment of aggressive CGCRG but, since significant side effects, as drug-induced lupus erythematosus and pancreatitis, even this treatment protocol wasn’t approved [6].
The one and only medical treatments approved for CGCRG are Bisphosphonates (Zoledronate) and Denosumab [7,8,9,10,11,12], even if they have been approved for the treatment of the aggressive form of CGCRG in adult patients only and no medication protocols has ever been approved for the treatment of CGCRG in children.
There is only one work in literature, published in 2015 by Chien et al. [13], in which the authors presented four cases of CGCRG in children, age range 0.3–15 years old, treated with off-label Zoledronate (ZA). The authors reported that treatment with ZA is a reasonable option in children with CGCRG refractory to conservative surgical treatment.
Recently, intralesional Triamcinolone Hexacetonide has been used for the treatment of aggressive CGCRG in adults, apparently with promising results [14,15,16,17].
Finally, even neoadjuvant immunotherapy has been proposed to minimize surgical resection in pediatric CGCRG [18].
We present a unique case in which BPs (ZA) are used as neoadjuvant treatment in a young boy of 5 years-old with an aggressive form of CGCRG. In this case, drug-therapy was considered because of the very rapid expansion of the bone neoformation. The purpose of the administration of BPs was to arrest the progression of the tumor, avoiding a resective surgery to the young patient.
The decision to use BPs iv instead of the available oral formulations depended both on the greater efficacy of the iv administration (higher bioavailability) and on the easier handling of this method of administration in young patient (i.e. risk of esophagitis).
In our opinion this case is unique as, currently, there are no works published in which the BPs are used as neoadjuvant therapy for the treatment of aggressive CGCRG in pediatric patient.
Case Report
Patient Concerns
A.M. is a 5 year-old boy (Fig. 1) with a left mandibular Giant Cell Granuloma associated to Arnold-Chiari syndrome. In October 2020, his mother noted the presence of a not painful swelling which deformed the lower third of the left face of the child. He was not assumed any medication or underwent any therapy in the past. His family history was also negative for any kind of similar lesions, as for genetic anomalies. The lesion reached a volume clinically evident just in a few weeks and showed a very short expansion time. Child’s mother was very worried, so she suddenly brought her young son to the First Aid of the “University Hospital G. Martino” of Messina (Sicily, Italy).
Diagnostic Aids
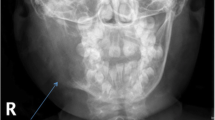
A Cranial CT scan was suddenly performed (Fig. 2). The radiological exam reported the presence of an osteolytic neoformation, with dimensions of 32 × 30x39 mm. The lesion blowed the bone and eroded the cortical of the mandible in several points (on the lower, inferior and posterior side), deforming the contour of the angle and of the ascendent branch of the left mandible. The lesion presented fine trabeculae arranged to form incomplete chambers and showed radiological features of biological aggressiveness. The neoformation involved the inferior alveolar canal and the germ of the inferior molar teeth, either were not evident in the scan.
As soon as possible, patient underwent osteomucosal incisional biopsy for histopathological assessment. The microscopic exam was diagnostic for Central Giant Cell Granuloma of the mandible.
Treatment
In consideration to the rapid growth and the short expansion time of the lesion but, above all, in consideration to the young age of the patient, our primary aim was to avoid a resective surgery of the mandible, so we proposed to the mother an alternative to the surgical treatment, consisted in BPs therapy. The mother was informed about the off-label indication of BPs in pediatric CGCRG, but she accepted the treatment proposed.
The drug therapy consisted of cycles of Zoledronic Acid iv (2 mg/50 ml NaCl solution 0.9% in 1 h of infusion) and Calcium Gluconate (15 mg/500 ml Normosol, 4 h of infusion).
Each administration of neoadjuvant protocol was preceded by the following blood chemistry assessments:
-
Cell blood count (CBC);
-
Glycemic value;
-
Creatinine;
-
Urea nitrogen;
-
Total protein;
-
Albumin;
-
Uric acid;
-
Transaminases ALT, AST and gamma-GT;
-
Alkaline phosphatase;
-
Lactate DeHydrogenase (LDH);
-
Amylase, lipase;
-
Triglyceride and cholesterol levels (LDL, HDL, Total);
-
Calcium, phosphorus, magnesium, potassium, sodium, chlorine;
-
Parathormone (PTH), vitamin D3;
-
Urinalysis.
These investigations needed us to have an overall broad-spectrum overview of the clinical conditions of the young patient, however no evidences about their actual usefulness for preventing complications are known.
Before BPs administration, the child received 300 mg of paracetamol per os.
No adverse reactions were occurred during the drugs administration, except a mild diarrhea.
If fever was occurred, it was also suggested oral paracetamol 500 mg every 6 h.
Patient continued home therapy with 500 mg of oral Calcium Sandoz tablets two times a day, and oral 25.000 UI/2,5 ml vitamin D3 (Dibase) every 4 weeks.
The Zoledronic Acid infusion cycles were carried out with timed intervals of 28 days and were repeated for seven times.
In September 2021, the patient was reevaluated and underwent a cranial CT scan (Fig. 3). The radiological exam documented the unchanged dimensions (33 × 43x38 mm) of the osteolytic lesion of the angle and the ascending branch of the left mandible. However, compared to the previous CT, a marked calcic neoapposition was evident both in the margins of the lesion and in the intralesional area, with slight thickening of trabecular bone. Hence, BPs therapy was able to arrest the progression of the tumor. However, the volume of primary lesion was unchanged, and clinical examination relieved the persistence of the swelling of the inferior left third of the child’s face.
Once growth of the tumor was stopped we can procede with the surgical treatment, which consisted in a deep curettage of the lesion. In our opinion surgery should complete the effects of the drug therapy sterilizing the growth center of the tumor and totally avoiding the risk of recurrence. Obviously, thanks to the metabolic effects of BPs therapy, the surgical treatment could be limited to a deep curettage instead of a resective surgery.
The hospitalization of the child lasted 10 days overall, and it was without complications. Patient underwent antibiotic and corticosteroid iv therapy until the resignation. Only minimal edema of the intervention site was observed and it was considered normal.
The tissue removed during surgery was sent to the Institute of Pathological Anatomy for the final histopathological diagnosis. The microscopic examination confirmed the presence of < < fibrovascular tissue with chronic granulating inflammation without neoplastic features > > .
Outcomes
The patient was discharged 10 days after surgery. He was in good general conditions and the oral surgical wound was on the mend. However, initially, the swelling of the face consequent to surgery forbade us to assess the real improvement of the facial aesthetic profile.
Follow Up
Seven months after surgery, the patient was reviewed for control. Clinically he appears in excellent conditions, the swelling was reduced and the profile of the face was quite harmonious.
Patient underwent CT scan. The radiologist reported: < < It is evident the disappearance of the gaseous and of the fluid components presented in the preoperative CT, in addition a centripetal deposition of mineralized bone tissue is documented in the angle of the left mandible > > (Fig. 4).
The last check dates back to November 2022 (Fig. 5), more than 1 year after surgical treatment. The young patient appeared in excellent clinical condition, no swelling of the previous area was presented, no sign of recurrence was evident and the aesthetic profile of the child was quite perfect.
Discussion and Conclusion
CGCRG is a less frequent neoformation that rarely hits the children. It is a benign lesion but sometimes it behaves as an aggressive tumor. In these cases the therapeutical approach represents a true challenge for the maxillofacial surgeon, because of the high risk of recurrence after surgical curettage, and often there are no other strategies but resective surgery.
Currently, in literature, there are no works about the treatment of pediatric CGCRG with BPs, probably because of the rarity of the pathology.
Our case report shows that BPs therapy is safety and useful as neoadjuvant therapy in the aggressive forms of pediatric CGCRG preceding the conservative surgery. The treatment has been well tolerated by the child which was fully cooperative until the last day, it can reduce the risk of recurrence after non-invasive surgery and it can avoid the need of a demolitive and disfiguring resective treatment.
The limitation of the work presented is the short follow up period, that is of 13 months only but, obviously, the child will be followed until the end of his craniofacial growth.
References
Jaffe HL (1953) Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 6(1):159–175
Ramesh V (2020) Central giant cell granuloma—an update. J Oral Maxillofac Pathol 24(3):413–415
Choe M, Smith V, Okcu MF, Wulff J, Gruner S, Huisman TAGM, Venkatramani R (2021) Treatment of central giant cell granuloma in children with denosumab. Pediatr Blood Cancer 68(3):e28778
Harris M (1993) Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg 31(12):89–94
Vered M, Shohat I, Buchner A, Dayan D, Taicher S (2007) Calcitonin nasal spray for treatment of central giant cell granuloma: clinical, radiological, and histological findings and immunohistochemical expression of calcitonin and glucocorticoid receptors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104(2):226–239
Goldman KE, Marshall MK, Alessandrini E, Bernstein ML (2005) Complication of alpha-interferon therapy for aggressive central giant cell lesion of the maxilla. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 100(3):285–291
Pogrel MA, Hossaini-Zadeh M (2021) Denosumab for the treatment of central giant cell granuloma of the jaws—a case series. Int J Oral Maxillofac Surg 50(8):1019–1022
Lipplaa A, Dijkstra S, Gelderblom H (2019) Challenges of denosumab and other giant cell-rich tumors of bone. Curr Opin Oncol 31(4):329–335
Bredell M, Rordorf T, Kroiss S, Rucker M, Zweifel DF, Rostetter C (2018) Denosumab as a treatment alternative for central giant cell granuloma: a long-term retrospective cohort study. J Oral Maxillofac Surg 76(4):775–784
Rytkonen E, Ottavainen V, Rytkonen A, Uusitalo S, Lehenkari P, Sandor JK (2018) Denosumab treatment for aggressive multiple recurrent familial central giant-cell granulomas. Ann Maxillofac Surg 8(2):265–269
Hameed M, O’Connell JE, Rogers SN (2019) Management of an aggressive giant cell granuloma of the mandible with denosumab: a case report. Br J Oralmaxillofac Surg 57(7):691–693
Mariz B, Migliorati CA, Alves FA, Penteado FM, Carvalho NP, Santos-Silva AR, Rocha AC (2021) Succesfull denosumab treatment for central giant cell granuloma in a 9 year-old child. Spec Care Dentist 41(4):519–525
Chien MC, Mascarenhas L, Hammoudeh JA, Venkatramani (2015) Zoledronic acid for the treatment of children with refractory central giant cell granuloma. J Pediatric Hematol Oncol. 37(6):399–401
Nilesh K, Dadhich A, Patil R (2020) Management of recurrent central giant cell granuloma of mandible using intralesional corticosteroid with long-term follow-up. BMJ Case Rep 13(9):e237200
de Arruda JAA, Martins AFL, Abreu LG, Mesquita RA, von Zeidler SV, Estrela C, Mendoça EF (2021) Central giant cell granuloma of the maxilla: long term follow-up of a patient treated with an adjuvant corticosteroid. Spec Care Dentist 41(3):399–407
Nogueira RLM, Osterne RLV, Lima Verde RMB, Azevedo NO, Teixeira RC, Cavalcante RB (2020) Intralesional injection of triamcinolone hexacetonide as an alternative treatment for central giant cell lesions: a prospective study. Br J Oral Maxillofacial Surg 58(10):e283–e289
de Mendonça RP, Mitre GP, Real FH, da Silva Kataoka MS, de Melo Alves Júnior S, Vianna P, da Silva Júnior NG, de Jesus Viana Pinheiro J (2020) Central giant cell granuloma treated with intralesional corticosteroid injections and bisphosphonates: a long-term follow-up case study. Head Neck Pathol 14(2):497–502
Lin J, Mecham JC, Hall SR, Oh C, Lettieri S (2020) Pediatric mandibular central giant cell granuloma: neoadjuvant immunotherapy to minimize surgical resection. J Craniofac Surg. https://doi.org/10.1097/SCS.0000000000007112
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Catalfamo, L., De Rinaldis, D., Cicchiello, S. et al. Central Giant Cell Reparative Granuloma (CGCRG) of the Jaw in Children Treated with Neoadjuvant Bisposphonates: Review and a Case Report. Indian J Otolaryngol Head Neck Surg 75, 1117–1122 (2023). https://doi.org/10.1007/s12070-022-03413-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-022-03413-6