Abstract
The purpose of this study was to investigate the effectiveness of intratympanic dexamethasone injection as a therapeutic agent against cisplatin-induced ototoxicity. Animals were randomly divided into three groups. Group one received intraperitoneal cisplatin alone, group two, received intratympanic dexamethasone after cisplatin ototoxicity had been demonstrated. Group three, which is control group, received intratympanic dexamethasone.Then we made three measurements. First we measured the baseline distortion product otoacustic emission (DPOAEs) of all the guine pigs. Second we injected cisplatin intraperitoneal group one and two the same day. Third we measured DPOAEs after 72 h of group one and two. Moreover DPOAEs were measured at the end of the first and second week only in group two. Cochleae were harvested and processed for electron microscopy after then. Values of The DPOAEs amplitudes and signal-to-noise ratio (SNR) at 1–6 kHz frequencies for group 1 after the injections significantly decreased over those before injections (P < 0.05). In group 3, there were no significant differences in DPOAE amplitude and SNR values When they are compare before and after their intratympanic dexamethasone injections (P > 0.05). In group 2, the DPOAEs measurements were close to significance at the end of the second week (P = 0.056). Intratympanic dexamethasone injection did not cause any ototoxic effect. Although intratympanic dexamethasone did not reach the statistically significant results, the measurements were close to significance. Intratympanic dexamethasone might have a significant therapeutic effect after cisplatin ototoxicity with different dose and application regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin is a potent alkylating agent that is used in the treatment of several neoplastic diseases, including head and neck cancer. Ototoxicity is a serious side effect of cisplatin. Cisplatin-induced ototoxicity is manifested by bilateral, progressive and usually irreversabl sensorineural hearing loss. The hearing loss appears to result from the destruction of outer hair cells in the organ of Corti.
Cisplatin destroys the outer hair cells (OHCs) in the cochlea in a progressive manner, from the base to the apex [1]. The mechanism of cisplatin induced ototoxicity has been intensively investigated. A large interindividual variability in ototoxicity has been well documented in experimental models and in humans. It has been shown that the accumulation of reactive oxygen species mediates cisplatin ototoxicity. Cisplatin-induced ototoxicity resulted in depletion of the cochlear antioxidant system and increased lipid peroxidation within cochlear tissues. Reactive nitrogen species have also been implicated in cisplatin-induced ototoxicity [2, 3].
Corticosteroids are widely used to treat inner ear disorders such as sudden idiopathic sensorineural hearing loss, autoimmune hearing loss, and Ménière’s disease [4]. Otolaryngologists have begun to instill steroids directly into the middle ear space, instead of oral steroids. Methylprednisolone and dexamethasone are the most widely used agents for the intratympanic administration protocols [5].
Several agents have been reported to ameliorate cisplatin ototoxicity, including sodium salicylate [6], Ginkgo Biloba Extract (EGb 761) [7], glutathione ester [8], erdosteine [9], α-tocopherol and tiopronin [10] and vitamine E [11]. Furthermore, a recent study has shown the efficacy of transtympanic N-acetylcysteine and lactate injection against cisplatin ototoxicity [12]. But these agents are not routinely used in cicplatin otoprotection. Furthermore, cancer patients usually receive therapy in otolaryngology department after cisplatin ototoxicity occurred.
Intratympanic steroids are increasingly used in the treatment of inner ear disorders, especially in patients with sudden sensorineural hearing loss and endolymphatic hydrops [13, 14]. Dexamethasone was previosly investigated as a protective agent in cisplatin-induced ototoxicity [15, 16]. Our study suggests that dexamethasone also has a theraputic effect against cisplatin-induced ototoxicity. An exhaustive review of the English-language medical literature failed to find any previous reports on the threpeutic effect of dexamethasone against cisplatin-induced ototoxicity. The aim of this study was to investigate the theraputic effects of transtympanic dexamethasone against cisplatin-induced ototoxicity.
Materials and Methods
Animals, Anesthesia and Drug Administration
The experimental animals were 24 adult, female, albino guinea pigs, that weighed 400–750 g. Guinea pigs were studied because they are similar to human beings with respect to their well-defined temporal bone anatomy, hearing physiology, and ototoxic response to drugs. This study was approved by the Institutional Review Board.
The animals were anesthetized with 30 mg/kg ketamine hydrochloride and 4 mg/kg xylazine was given as an intraperitoneal infusion before cisplatin administration and testing. The depth of anesthesia was determined with the pedal reflex. To maintain anesthesia during testing, half-doses of the xylazine/ketamine were administered as needed.
DPOAE Measurements
Guine pigs were anesthetized as described previously. An insert earphone (Etymotic ER-2) was placed directly into the external auditory canal.
Experimental Design
The animals were randomly divided into three groups of eight Guinea Pigs. Group one, cisplatin only; group two, cisplatin and intratympanic dexamethasone; group three, dexamethasone only. In all groups, baseline DPOAEs testing preceded the administration of the drugs.
Group 1 (Cisplatin Only)
Group one was injected with cisplatin intraperitoneal only on day 0 (12 mg/kg body weight). Cisplatin 12 mg/kg (Cisplatin DBL, Faulding Pharmaceuticals, Warwickshire, UK) was administered intraperitoneally as a slow infusion. Test for DPOAE were performed before drug administration and 3 days later.
Group 2 (Cisplatin and Intratympanic Dexamethasone)
Group two was defined as a treatment group. Cisplatin was injected intraperitoneal only on day 0. Test for DPOAE were performed before drug administration and 3 days later. After 3 days under an operating microscope, an intratympanic injection of dexamethasone at 4 mg/ml (Dekort, Deva Holding, Istanbul, Turkey) was given slowly through a myringotomy in the anterosuperior quadrant (approximately 0.1–0.2 ml), with a 28-gauge dental needle to fill the middle ear cavity twice week (2 and 5 day). After keeping the animal in the same position for 30 min, the procedure was performed in the other ear. Moreover DPOAE were measured at the end of the first and second week only in group two.
Group 3 (Intratympanic Dexamethasone Only)
Group three received the standard dose of dexamethasone (4 mg/kg) which was administered through the tympanic membrane. Test for DPOAE were performed before drug administration and 3 days later. Group three was defined as control group.
Two animals (Group one) died due to systemic toxicity of cisplatin. In addition, no tympanic membrane injury, such as perforation, was observed in any test animal.
Specimen Preparation for Transmission Electron Microscopy
Guinea pigs were killed immediately after the completionof the DPOAE recordings. The temporal bones were carefully dissected. The round and oval windows and the apex of the cochlea were perforated with a small pick. Cochleas were prepared for electron microscopy as follows: Cochleas were removed and placed in 2.5% glutaraldehyde for 24 h for fixation. The tissue was post-fixed with osmium tetroxide (OsO4), dehydrated in a graded series of alcohol, and then embedded in Araldite® CY212. The thin (60–90 nm) sections were obtained with ultra-microtome (Leica), examined on a transmission electron microscope (Carl Zeiss Libra 120) and photographed.
Since only a limited number of OHCs can be seen within pathology images, capturıng mitosis was a challengig process. Therefore, we have repeted this process multiple times until we have succesfully seen mitosis.
Statistical Analysis
Comparisons between pretreatment and posttreatment results(DPOAE amplitude and SNR values) in each group were analyzed using the Wilcoxon paired 2-sample test, SPSS 15.0 for Windows. Statistical significance was accepted at a P value of less than 0.05.
Results
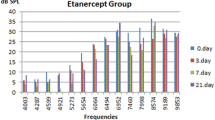
The DPOAE amplitudes and signal-to-noise ratio (SNR) values significantly decreased at 6–8 kHz frequencies for group one animals after cisplatin injections (P < 0.05). In group three, there were no significant differences in DPOAE amplitude and SNR values between before and after intratympanic dexamethasone injections, suggesting that intratympanic dexamethasone injection had no toxic effect on cochlear emissions. Considering group two, the DPOAE amplitudes and signal-to-noise ratio (SNR) values significantly decreased at 6–8 kHz frequencies after cisplatin injections. The DPOAE amplitudes and signal-to-noise ratio (SNR) values increased at the end of the first week of the IT dexamethasone injection. The measurements resulted in higher levels of DPOAE amplitudes and signal-to-noise ratio (SNR) values at the end of the second week of the application (Fig. 1). Although the improvement was not statistically significant at the end of the second week, the results were close to significance (P = 0.056).
Cochlear Morphology
Normal morphological integrity of the OHCs was observed in group three animals (Fig. 2a). There were no morphological changes and cellular degeneration in mitochondria, endoplasmic reticulum and nucleus of the OHCs in all group. Increased intracytoplasmic myelin figures and a marked intercellular adhesion complex were observed in group one animals receiving only cisplatin (Fig. 2b). Improvement of increased intracytoplasmic myelin figures and marked intercellular adhesion complex were observed at the end of the second week in group two animals (Fig. 2c).
Photomicrographs of the cochlea using transmission electron microscopy. Normal morphology in group three (a). Increased in intracytoplasmic myelin figures and marked intercellular adhesion complex were seen in group two and group one (b). Black arrows indicates intracytoplasmic myelin figures. Regeneration of the outer hair cell was observed in group two (c)
Discussion
Steroids, especially dexamethasone, are used to treat many conditions, including sudden hearing loss, noise-induced hearing loss, Ménière’s disease, salicylate ototoxicity, and aminoglycoside ototoxicity. Various reports on the effect of intratympanic steroid injection in sudden hearing loss have been published [14, 17–19].
Parnes et al. [20] in a well-designed animal study, compared the concentration of hydrocortisone, methylprednisolone, and dexamethasone in cochlea fluids after intravenous and topical administration. Intratympanic administration resulted in higher endolymph and perilymph levels. In addition to higher perilymphatic concentrations, intratympanic steroids avoid the risk of systemic side effects and minimize the risk of drug interactions. Additionally, the procedure is supposed to be safe, inexpensive, and easy to perform. In addition, Shirwany et al. [21] showed that intratympanic injection of steroid increase cochlear blood flow and no side effect on auditory sensitivity or cochlear histology in guinea pigs.
Dexamethasone has good round window diffusion; however, the profile may not be as beneficial as methylprednisolone. Parnes showed that methylprednisolone had a higher concentration and longer duration in perilymph after transtympanic administration than hydrocortisone or dexamethasone [21]. Despite the practicality in treating patients with a single intratympanic injection of steroids, this protocol may not be as optimal as a continuous infusion or multiple injections [22, 23].
Sockalingam et al. reported that the recording of DPOAE is a sensitive method for the evaluation of the functional state of OHCs and albino guinea pigs are the most sensitive animals in term of cisplatin ototoxicity, with alteration in DPOAE and damage to OHCs [23, 24]. Evoked OAE, especially DPOAEs due to frequency specificity, were shown to be more sensitive for evaluating OHCs than were conventional audiometry, ultra high frequency audiometry, and auditory brainstem response [25]. The most important benefits of OAEs are their non-invasive capacity and objectivity to determine the early stages of sound processing and evaluate the biomechanical activity of the outer hair cells in the cochlea [26]. So, we used DPOAE to assess cochlear functions in this experimental study. We think that higher frequencies, representing areas of the cochlea closer to the base, are very important to investigate because they have been shown to be affected by cisplatin ototoxicity first. Our study showed good results at 6 and 8 kHz.
Higher frequency measurements might be able to reveal more comprehensive information about the therapeutic role of dexamethasone against cisplatin-induced ototoxicity.
The present study evaluated ototoxicity up to 14 days after cisplatin administration. However, other single-dose studies in rats and guinea pigs suggest that hearing loss has stabilized 5–7 days after the cisplatin injection [9, 27].
Our study suggests that dexamethasone also has a therapeutic effect on cisplatin-induced ototoxicity. Before this study, there were no investigations of intratympanic dexamethasone injection as a therapeutic agent against cisplatin induced ototoxicity. An exhaustive review of the English-language literature failed to find any previous report on the therapeutic effect of dexamethasone against cisplatin-induced ototoxicity.
Conclusion
We studied the therapeutic effect of intratympanic dexamethasone against cisplatin-induced ototoxicity in guinea pigs. Intratympanic dexamethasone was close to significance on OHCs at the end of the second week (P = 0.056), which are damaged by cisplatin. In addition, intratympanic dexamethasone had no ototoxic side effects. However, well-designed, placebo-controlled human studies are needed to confirm our results and to determine the best dexamethasone regimen for recovery cisplatin-induced ototoxicity. Intratympanic dexamethasone might have a significant therapeutic effect after cisplatin ototoxicity with different dose and application regimens.
References
Laurell G, Bagger-Sjoback D (1991) Dose-dependent inner ear changes after i.v. administration of cisplatin. J Otolaryngol 20:158–167
Watanabe K, Hess A, Bloch W et al (2000) Nitric oxide synthase inhibitor suppresses the ototoxic side effects of cisplatin in guinea pigs. Anticancer Drugs 11:401–406
Lee JE, Nakagawa T, Kim TS et al (2004) Role of reactive radicals in degeneration of the auditory system of mice following cisplatin treatment. Acta Otolaryngol 124:1131–1135
Choe WT, Chinosornvatana N, Chang KW (2004) Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol 25(6):910–915
Yang J, Wu H, Zhang P, Hou DM, Chen J, Zhang SG (2008) The pharmacokinetic profiles of dexamethasone and methylprednisolone concentration in perilymph and plasma following systemic and local administration. Acta Otolaryngol 128(5):496–504
Saito T, Zhang ZJ, Manabe Y, Ohtsubo T, Saito H (1997) The effect of sodium thiosulfate on ototoxicity and pharmokinetics after cisplatin treatment in guinea pig. Eur Arch Otorhinolaryngol 254:281–286
Huang X, Whitworth CA, Rybak LP (2007) Ginkgo Biloba Extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol 28:828–833
Campbell KCM, Larsen DL, Meech RP et al (2003) Glutathione ester but not glutathione protects against cisplatin-induced ototoxicity in a rat model. J Am Acad Audiol 14:124–133
Kalcioglu MT, Kizilay A, Gulec M et al (2005) The protective effect of erdosteine against ototoxicity induced by cisplatin in rats. Eur Arch Otorhinolaryngol 262:856–863
Fetoni AR, Sergi B, Ferraresi A et al (2004) Protective effects of alpha-tocopherol and tiopronin against cisplatin-induced ototoxicity. Acta Otolaryngol 124:421–426
Kalkanis JG, Whitworth C, Rybak LP (2004) Vitamin E reduces cisplatin ototoxicity. Laryngoscope 114:538–542
Choe W, Chinosornvatana N, Chang KW (2004) Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol 25:910–915
Arriaga MA, Goldman S (1998) Hearing results of intratympanic steroid treatment of endolymphatic hydrops. Laryngoscope 108(11, Part 1):1682–1685
Haynes DS, O’Malley M, Cohen S, Watford K, Labadie RF (2007) Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Triolog Soc Pap Laryngoscope 117(1):3–15
Daldal A, Odabasi O, Serbetcioglu B (2007) The protective effect of intratympanic dexamethasone on cisplatin-induced ototoxicity in guinea pigs. Otolaryngol Head Neck Surg 137:747–752
Hill GW, Morest DK, Parham K (2008) Cisplatin-induced ototoxicity: effect of intratympanic dexamethasone injections. Otol Neurotol 29(7):1005–1011
Chandrasekhar SS (2001) Intratympanic dexamethasone for sudden sensorineural hearing loss: clinical and laboratory evaluation. Otol Neurotol 22:18–23
Silverstein H, Choo D, Rosenberg SI et al (1996) Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J 75:468–471, 474, 476
Himeno C, Komeda M, Izumikawa M et al (2002) Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res 167(1–2):61–70
Parnes LS, Sun AH, Freeman DJ (1999) Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope 109:1–17
Shirwany N, Seidman MD, Tang W (1998) Effect of transtympanic injection of steroids on cochlear blood flow, auditory sensitivity, and histology in the guinea pig. Am J Otol 19:230–235
Lefebvre PP, Staecker H (2002) Steroid perfusion of the inner ear for sudden sensorineural hearing loss after failure of conventional therapy: a pilot study. Acta Otolaryngol 122:698–702
Kopke RD, Hoffer ME, Wester D, O’Leary MJ, Jackson RL (2001) Targeted topical steroid therapy in sudden sensorineural hearing loss. Otol Neurotol 22:475–479
Sockalingam R, Freeman S, Cherny TL et al (2000) Effect of high-dose cisplatin on auditory brainstem responses and otoacoustic emissions in laboratory animals. Am J Otol 21:521–527
Ress BD, Sridhar KS, Balkany TJ et al (1999) Effects of cis-platinum chemotherapy on otoacoustic emissions: the development of an objective screening protocol: third place—Resident Clinical Science Award 1998. Otolaryngol Head Neck Surg 121:693–701
Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F (2000) Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res 28:73–80
Ramirez-Camacho R, Citores MJ, Trinidad A et al (2007) HSP-70 as a nonspecific early marker in cisplatin ototoxicity. Acta Otolaryngol 127:564–567
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calli, C., Pinar, E., Oncel, S. et al. Recovery of Hearing in Cisplatin-Induced Ototoxicity in the Guinea Pig with Intratympanic Dexamethasone. Indian J Otolaryngol Head Neck Surg 64, 46–50 (2012). https://doi.org/10.1007/s12070-011-0160-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-011-0160-7