Abstract
Radiation-induced mucositis is a dose-limiting concern in the treatment of head and neck cancers (HNC). This study was conducted to determine the effectiveness of the Ayurvedic drug Triphala in reducing radiation-induced mucositis and influencing tumour control when combined with providone iodine. Data from patient files of HNC patients who received Triphala in conjunction with iodine or iodine alone over the course of curative radiotherapy (> 60 Gy) from May 2013 to February 2015 were extracted for this retrospective chart based study. Data was subjected to statistical analysis, X2 and unpaired t test using the Statistical Package for Social Sciences (SPSS), version 17 (IBM, Chicago, USA). When compared to iodine alone, the group that utilised Triphala gargling was very efficient in delaying mucositis, the extent of weight loss (p = 0.038), the incidence (p = 0.03), and the number (p = 0.02) of treatment breaks. However, it had no influence on the radiation-induced tumour response. According to the observations, Triphala coupled with iodine was more successful in preventing radiation mucositis, and without affecting the killing of tumour cells than iodine gargle alone. According to the authors, this is the first observation to demonstrate the value of combining providone iodine with Triphala in preventing radiation-induced oral mucositis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiation for HNC region generally causes an inflammatory response in the mouth and pharyngeal mucosa in the treatment area [1]. Scientifically this is known as "oral mucositis" and an inflammatory state of this kind impairs the integrity of the mucosal barrier, and interferes with curative treatment plans, increases morbidity, and impacts the quality of life for the affected patient [1,2,3]. The location of the malignancy, accompanying chemotherapy, radiation dose, field size, and fractions, as well as patient-related factors such oral health, immunological condition, and comorbidities, are the main determinants of oral mucositis time of appearance, severity and incidence [4, 5]. At times, oral mucositis might be so severe that it forces the initial treatment plan to be interrupted or abandoned [3, 6].
Proper use of antibacterial, antifungal, analgesic, and nutrition administration parenterally when necessary are all parts of the management of oral mucositis and referred to as integrated supportive care [7]. Admission to an intensive care unit is made in severe cases of mucositis to prevent septicemia, and complications that can cause the person's death [4]. Clinically, Mucotrol and MuGard are used to treat oral mucositis [5, 8]. Additionally, colloidal silver solutions, antiseptics and antimicrobials, and salt-and-baking soda rinses are all widely utilized to treat oral infection and oral mucositis [9]. Additionally, biological treatments, such as recombinant human KGF-1 (palifermin), are also effective [5, 10] but expensive [9]. Due to all of these causes, a protective agent that is affordable, easy to use, effective, pleiotropic in its pharmacological action and acceptable for humans is required [11, 12].
Humans have used plants as medicinal agents to treat illness or to promote health since ancient times [13, 14]. Currently, nearly 80% of people in developing nations rely on plant-based medicines for primary care [13] due to the perception that they are non-toxic or less toxic than drugs used in modern medicine, relatively inexpensive, and socially widely accepted [13, 15]. The most often utilised plant products are decoctions, powders, infusions, and pastes [13,14,15]. Considering this, since almost three decades, researchers have been looking into the radioprotective properties of plants that are used as alternative medicines [13,14,15]. In light of this, plants that can alleviate oxidative stress, stimulate quicker wound healing and boost immune performance have been considered ideal [16, 17]. Additionally, polyherbal preparations have also been observed to be useful in reducing radiation ill effects [18, 19] and the effect are attributed to the individual constituent’s cumulative and synergistic effects [20].
Studies using the Ayurvedic drug Triphala, which contains dried fruits of Terminalia bellirica Roxb, Terminalia chebula Retz. and Phyllanthus emblica Linn. or Emblica officinalis Gaertn in equal ratio is reported to possess radioprotective effects [17,18,19]. Additionally, research with humans has demonstrated the value of Triphala mouthwash as a potential non-toxic, cost-effective chemopreventive therapy by demonstrating its ability to reverse tobacco-induced pre-cancerous lesions [21]. Triphala capsules when used on a daily basis increased HDL-C levels and concurrently decreased blood sugar, demonstrating the herb's benefit for those with dyslipidemia and prediabetes and was safe [22].
Triphala protects healthy cells from the harmful effects of ionising radiation and does not affect the radiation's ability to kill malignant cells [18, 19]. Triphala possess anti-inflammatory, antimicrobial effects, wound-healing properties, anti-plaque, chemopreventive, cytoprotective, antimutagenic, clinically has a very high safety profile and is widely accepted [17,18,19,20,21,22]. Considering all these pharmacological effects of Triphala, study was planned to investigate how combining Triphala with providine iodine would be useful as an anti-ulcerative agent in HNC patients requiring radiotherapy along with the standard iodine gargle. Standard clinical aspects like the incidence and highest grade of mucositis, number of treatment days lost, changes in body weight and effect on tumour response, that are routinely performed in patient care, and mandatorily entered in patient care file were considered.
Patients and Methods
This study, was patient chart based and was carried out in January 2020 in the medical records department (MRD) of the Mangalore Institute of Oncology after receiving approval from the institutional ethics committee (MIO/IEC/2019/01/07). In the hospital, curative radiotherapy usually encompasses use of 60 to 70 Gy with or without cisplatin or carboplatin as per global standard practice for treating HNC. Povidone-iodine mouthwash (10 ml of the 1% Povidone-iodine) was used twice daily in the morning and once at night in the institution as per the normal protocol to prevent oral infection and radiation mucositis [23].
From May 2013 to February 2015, the hospital utilized the services of a senior Ayurvedic doctor, and together with radiation oncologists, developed a protocol of combining Triphala gargle as an adjunct treatment along with the existing iodine swish. The Ayurvedic doctor had incorporated two gargles with Triphala water (1%), one after lunch and one after tea in the evening, while keeping the povidone-iodine mouthwash constant (morning and at night), for volunteers who were willing to do so under the guidance of an oncology nurse.
As this was a retrospective study, the assistance of the head of MRD was requested. While selecting the files the inclusion criteria considered were that HNC patients were treated from May 2013 to February 2015, had Triphala gargle recommended by the Ayurvedic physician, patient had a minimum gap of 4 months when surgery preceded curative radiotherapy, patients were not on high doses of non-steroidal anti-inflammatory drugs, were not affected with uncontrolled type II diabetes or hypertension, used povidone-iodine or Triphala + povidone-iodine mouthwash throughout the time point under the supervision of the oncology nurse in the inpatient facility and all pertinent details were entered in the nursing records.
The exclusion criteria considered were that patient had neoadjuvant chemotherapy, had surgery within 4 weeks of start of radiotherapy, the current radiation treatment was for the second time and the patient was previously treated with radiotherapy for HNC region, travelled from their home for the radiation treatment, the patient used some other mouth wash and finally those who discontinued the planned treatment (left against medical advice or discharged against medical advice).
The extraction of data was conducted by one of the research assistant in January 2020 considering both inclusion and exclusion criteria specified above. Briefly, the files of HNC patients treated during the time point May 2013 to February 2015 were selected from the inpatient MRD. The patient's treatment file were inspected and scrutinized by the MRD in charge and the files that satisfied the inclusion and exclusion criteria were selected. The patient files contained information on the demographic, pathological, clinical information, radiation dose, fraction size, chemotherapy drugs used as radiation sensetizers, the frequency and severity of mucositis, weight loss, and number of treatment days lost throughout the course of the treatment due to adverse drug reaction, and treatment response evaluated using radiological and clinical methods. The data were carefully collected double checked and then entered in to Microsoft Excel 2013 by the research assistant with the assistance of the MRD head.
Preparation of Triphala
The Ayurveda physician had advised using 1% Triphala when taking it for gargling. Concisely, 100 ml of hot water and 1 gramme of Triphala (Zandu Pharmacuticals, Mumbai, India) were combined to create 1% Triphala mouthwash. The resulting solution was allowed to cool for 30 min before being filtered to remove the particle debris via a sterile linen mesh. To get a filtrate free of any particle debris that might be utilized for gargling, the filtration process was repeated twice. Every day, around 50 ml of freshly prepared Triphala filtrate was provided to the patients by the attending nurse along with the lunch. Patients were initially taught to swish their mouth with 10 ml of the 1% Triphala filtrate (three times) at the planned time points of after lunch and after tea in the evening. In accordance to the hospital protocol, the patient carried out all medical care including garglings (Triphala as well as iodine) under the supervision of the nurse and family care givers.
Radiation Therapy Treatment and Care of Patient
All patients were exposed to external radiation using a linear accelerator (Varian, Model Unique Performance, Palo Alto, CA, USA for a planned target dose of 60 to 70 Gy [30]. Whenever chemo-irradiation was intended, a weekly carboplatin (70 to 150 mg/m2/day intravenous) [24–26] or cisplatin infusion (40 to 50 mg/m2/day IV) [27, 28] was given before to the first weekly radiation exposure [29, 30]. Nutritional, medical and supportive care was conducted as described earlier [29, 30]. The orodental care, weekly grading of mucositis, and evaluation of treatment response performed four to six weeks following the end of treatment was carried out as described earlier [29, 30].
Statistical Analysis
Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS), version 17 (IBM, Chicago, USA). The X2 test was used to compare the overall incidence of the worst-ever grades of ulceration, the number of treatment days lost due to intolerable mucositis, and weight loss. The unpaired "t test" was used to compare the extent of severe mucositis score on a weekly basis, testing equality of proportion for the delay in incidence and the number of tolerable and intolerable mucositis. P values under 0.05 were regarded as significant.
Results
The records of patients who underwent conventional fractioned radiation with the goal of curative treatment from May 2013 to February 2015 and were admitted to an inpatient facility for treatment were chosen. Retrospective reviews were performed on 107 patient files treatment on an inpatient basis in the hospital for their HNC and satisfied the inclusion criteria. Of these, 3 patients had left the treatment midway and 2 had uncontrolled co-morbidities. A total of 102 patient file satisfied the inclusion criteria and had complete data and were considered. Table 1 shows the patient's age, sex, location, and stage of cancer, while Table 2 shows the type of treatment, dose, frequency of breaks, usage of painkillers, and days lost due to mucositis.
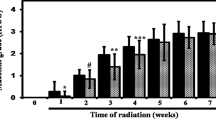
The study population for the control cohorts was 65 patients (48 men and 18 women), compared to 37 for the Triphala (24 men and 13 females). In the Triphala cohort, the mean age was 54.38 ± 12.82 while it was 54.50 ± 12.40 in the control cohort. Regarding the tumour site, both study populations contained about 25% of tongue cancer patients. Both cohorts developed mucositis as a result of radiation exposure, and both cohorts' incidence and mean of the condition rose over time (Figs. 1 and 2). However, when compared to the iodine alone control, in the Triphala cohort's both bearable and intolerable mucositis were delayed and significant at most time points (Fig. 3).
Both the incidence (29.23% vs. 10.81%; P = 0.03) and the number of days (6.78 ± 0.54 vs. 5.00 ± 3.43; P = 0.02) were lower in the Triphala group, and this difference was statistically significant (Table 2). The weight reduction in the Triphala cohorts was lower as compared to the povidone-iodine group (3.66 ± 1.70 vs 2.97 ± 1.52) and significant (p = 0.038). However there was no discernible difference in treatment response. In the Triphala gargle group; there was 75.68% (28/37) of CR, 10.81% (4/37) of PR, and 13.51% (5/37) of NR compared to 69.23% (45/64) of CR, 16.92% (11/64) of PR, and 13.85% (9/64) of NR in the control group (Table 2).
Discussion
The most significant finding is that combining Triphala rinse with iodine was more successful in preventing or postponing the onset of severe mucositis, a dreaded adverse event associated with increased morbidity, extended hospital stays, and escalating treatment costs [31–33]. However, previous studies do indicate that Triphala is useful as a radioprotective agent and can protect mice from radiation-induced sickness and mortality [34, 35], intestinal mucosal damage [36] as well as reduce radiation-induced DNA damage in both mice and cultured mammalian cells [37, 38]. The second and most crucial finding of our study was that Triphala gargling had no adverse effects on the radiation therapy response. Overall, these findings suggest that Triphala in conjunction with iodine was successful in reducing radiation mucositis without compromising response to therapy.
To substantiate this, previous studies by Sandhya and coworkers with mammalian cells have demonstrated that Triphala induced cytotoxicity in the tumour cells [barcl-95 (in a transplantable mouse thymic lymphoma) and MCF-7 (a human breast cancer cell line)], while sparing the normal cells [37]. At equivalent concentrations, Triphala induced concentration-dependent apoptosis and cell death in Capan-2 and BxPC [human pancreatic cancer cells], while sparing the normal human pancreatic ductal epithelial cells (HPDE-6) [39]. Triphala feeding also caused tumor regression in nude mice implanted with Capan-2 xenograft [39]. Studies with LNCap [human prostate cancer] and normal cells has demonstrated that Triphala and its phenolic ingredient gallic acid have distinct cytotoxicities and are more cytotoxic to the neoplastic cells at equal concentrations [40]. Triphala and its active component, chebulinic acid also suppressed phosphorylation of VEGF receptor-2 and VEGF action important for angiogenesis (VEGFR-2) [41]. These findings suggest that Triphala is safe for normal cells while exerted cytotoxic effects on the neoplastic cells and tumors.
Triphala has been subjected to significant scientific study, and findings published convincingly affirm its efficacy in treating a variety of conditions affecting the oral cavity [42–46]. Children who used Triphala mouthwash found it to be just as effective as chlorhexidine at reducing microbial growth, dental plaque production, and gingival inflammation [47]. Studies on people with periodontal disease have unequivocally demonstrated that Triphala mouthwash is just as effective at lowering plaque and the gingival index as 0.2% chlorhexidine is at providing oral care [48]. Triphala rinse has been demonstrated to be helpful as an endodontic irrigant against the common orodental pathogen Enterococcus faecalis [51] and to reduce plaque-induced gingivitis [49], halitosis [49], plaque accumulation [50], gingival inflammation [50], halitosis and to lessen the development of biofilms by cariogenic bacteria [52]. Importantly, a cross-over trial by Naiktari and colleagues (2018) demonstrated the enormous benefits of a single rinse with 10% Triphala mouthwash as an antibacterial agent [53].
Radiation's cytotoxic effects are known to cause a number of negative side effects including a weakening of the oral cavity's natural defense, a decrease in salivary secretion, and the development of mucositis [54]. Additionally, radiation changes the mouth's flora and encourages the growth of opportunistic pathogens, which, if left untreated, increase the risk of systemic infection, particularly in people with compromised immune systems and result in death [32]. Triphala has been shown to have immunomodulatory properties as well, and clinical studies by Phetkate and coworkers (2012) revealed that when compared to the controls, people who took Triphala had better immunostimulatory effects [55].
Radiation exposure slows the healing process, and laboratory studies in a rat model have demonstrated that applying Triphala ointment (10% w/w) improved the closure of an infected open wound. This was mediated by decreasing bacterial count, increasing collagen, hexosamine, uronic acid, superoxide dismutase, and matrix metalloproteinase levels [56]. Additionally, Staphylococcus aureus, Pseudomonas aeruginosa, and Streptococcus pyogenes were successfully eradicated by the alcoholic extract of Triphala, demonstrating its efficacy for the treatment of infected wounds [56]. Triphala has been proven to be effective against two significant orodental pathogens, Enterococcus faecalis and Streptococcus mutans [57–61]. It's probable that the observed benefit was mediated by a combination of pharmacological actions, such as boosting wound healing, exerting antibacterial properties, and stimulating the immune system.
Mechanistically, X rays, mediates it toxic effects principally by generating free radicals [62, 63]. Triphala has been examined for its free radical scavenging and antioxidant activities and has been found to be efficient against a variety of free radicals [34, 63]. In numerous xenobiotic stress-induced models, such as reducing enterotoxicity by methotrexate [64], hepatotoxicity by dimethylhydrazine dihydrochloride [65], and nephrotoxicity by bromobenzene [66], Triphala is also shown to restore the levels of the cell's primary antioxidant, glutathione [66]. Triphala has also been shown to increase antioxidants [65, 66] while concomitantly, decreasing levels of lipid peroxidation [63–72], myeloperoxidase [64], xanthine oxidase [64] and lactate dehydrogenase [65, 70]. The observed protection would have worked with a similar method.
Inflammation is a major contributing element to the development and, more crucially, the maintenance of radiation-induced oxidative stress and free radical damage [73, 74]. Investigations have demonstrated the effectiveness of Triphala in reducing gingival inflammation [44, 47–50, 75] in preventing the development of inflammatory gouty (monosodium urate crystals-induced) arthritis [70], adjuvant-induced arthritis in rats [71], and mouse ear edema brought on by ethyl phenylpropiolate [76]. According to mechanistic investigations, Triphala decreased inflammation in mice with gouty arthritis by lowering TNF-α levels [70]. Furthermore, research with rats given an adjuvant-induced arthritic condition revealed that Triphala decreased TNF-α, IL-17, IL-6, MCP-1, iNOS and COX-2; receptor activator of RANKL, NF-kB p65 and AP-1 [71, 77]. All of these findings confirm that Triphala has protective effects against a variety of organotrophic toxicants and inflammatory agents, showing that these benefits are broad-spectrum and consistent at the cellular level in a variety of cells and tissues.
Conclusions
The current study’s findings suggest that Triphala together with povidone-iodine gargle have better protective benefits and that this combination is superior to povidone-iodine gargle alone. Triphala is well accepted, has a great safety record, and works well to prevent and treat radiation-induced mucositis. For the first time, our study demonstrates that using Triphala in addition to the usual povidone-iodine swish was successful in reducing radiation-induced mucositis. The authors recommend that future research concentrate on determining the efficacy of Triphala as a single agent as well as in conjunction with standard agent(s) in randomised, double-blinded clinical trials.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sonis ST, Elting LS, Keefe D et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Peterson DE (2006) New strategies for management of oral mucositis in cancer patients. J Support Oncol 4(2 Suppl 1):9–13
Kolokythas A (2010) Long-term surgical complications in the oral cancer patient: A comprehensive review. Part II J Oral Maxillofac Res 1(3):e2
Bossi P, Numico G, De Santis V et al (2014) Prevention and treatment of oral mucositis in patients with head and neck cancer treated with (chemo) radiation: report of an Italian survey. Support Care Cancer 22(7):1889–1896
Allison RR, Ambrad AA, Arshoun Y et al (2014) Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer 120(9):1433–1440
Rosenthal D, Trotti A (2009) Strategies for managing radiation-induced mucositis in head and neck cancer. SeminRadiatOncol 19(1):29–34
Hadjieva T, Cavallin-Ståhl E, Linden M, Tiberg F (2014) Treatment of oral mucositis pain following radiation therapy for head-and-neck cancer using a bioadhesive barrier-forming lipid solution. Support Care Cancer 22(6):1557–1562
Nicolatou-Galitis O, Sarri T, Bowen J et al (2013) Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer 21:3179–3189
Rodríguez-Caballero A, Torres-Lagares D, Robles-García M, Pachón-Ibáñez J, González-Padilla D, Gutiérrez-Pérez JL (2012) Cancer treatment-induced oral mucositis: a critical review. Int J Oral Maxillofac Surg 41:225–238
Sonis ST (2010) Efficacy of palifermin (keratinocyte growth factor-1) in the amelioration of oral mucositis. Core Evid 4:199–205
Panahi Y, Saadat A, Shadboorestan A, Ahmadi A (2016) An updated review of natural products intended to prevent or treat oral mucositis in patients undergoing radio-chemotherapy. Curr Pharm Biotechnol 17(11):949–961
Ramsay EI, Rao S, Madathil L, Hegde SK, Baliga-Rao MP, George T, Baliga MS (2019) Honey in oral health and care: a mini review. J Oral Biosci 61(1):32–36
Ong CK, Bodeker G, Grundy C, Burford G, Shein K (2005) WHO global atlas of traditional complementary and alternative medicine. World Health Organization, Kobe, Japan
Booker A, Johnston D, Heinrich M (2012) Value chains of herbal medicines–research needs and key challenges in the context of ethnopharmacology. J Ethnopharmacol 140(3):624–633
Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4:177
Fischer N, Seo EJ, Efferth T (2018) Prevention from radiation damage by natural products. Phytomedicine 47:192–200
Baliga MS (2010) Triphala, ayurvedic formulation for treating and preventing cancer: a review. J Altern Complement Med 16(12):1301–1308. https://doi.org/10.1089/acm.2009.0633. (PMID: 21138390)
Baliga MS, Meera S, Mathai B, Rai MP, Pawar V, Palatty PL (2012) Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: a review. Chin J Integr Med 18(12):946–954
Baliga MS, Meera S, Vaishnav LK, Rao S, Palatty PL (2013) Rasayana drugs from the ayurvedic system of medicine as possible radioprotective agents in cancer treatment. Integr Cancer Ther 12(6):455–463
Karole S, Shrivastava S, Thomas S, Soni B, Khan S, Dubey J et al (2019) Polyherbal formulation concept for synergic action: a review. JDDT 9:453–466
Deshpande A, Tandon S, Deshpande N (2014) Low resource screening method of pre-cancerous lesions and its reversal by Triphala in teen-age Indian population. Ayu 35(2):160–167
Phetkate P, Kummalue T, Rinthong PO, Kietinun S, Sriyakul K (2020) Study of the safety of oral Triphala aqueous extract on healthy volunteers. J Integr Med 18(1):35–40
Madan PD, Sequeira PS, Shenoy K, Shetty J (2008) The effect of three mouthwashes on radiation-induced oral mucositis in patients with head and neck malignancies: a randomized control trial. J Cancer Res Ther 4(1):3–8
Jeremic B, Zivic DJ, Djuric LJ, Mijatovic LJ. Carboplatin and radiation therapy for stage IV carcinoma of the head and neck. Preliminary results of a phase II study. J Chemother. 1992;4(3):180–4.
Ackland SP, Hamilton CS, Joseph DJ, Denham JW (1993) Phase I/II study of concurrent weekly carboplatin and radiation therapy in advanced head and neck cancer. ClinOncol (R CollRadiol). 5(3):133–138. https://doi.org/10.1016/s0936-6555(05). (PMID: 8347534)
Pazdur R, Wagman LD, Camphausen K, Hoskins WJ (eds) (2009) Cancer Management: A Multidisciplinary Approach. Medical, Surgical, and Radiation Oncology, 12th edn. CMP Healthcare Media, Norwalk, CT
Reimer RR, Gahbauer R, Bukowski RM, Hewlett JS, Groppe CW Jr, Weick JK, Antunez AR (1981) Simultaneous treatment with cisplatin and radiation therapy for advanced solid tumors: a pilot study. Cancer Treat Rep 65(3–4):219–222 (PMID: 7195303)
Homma A, Inamura N, Oridate N, Suzuki S, Hatakeyama H, Mizumachi T, Kano S, Sakashita T, Onimaru R, Yasuda K, Shirato H, Fukuda S (2011) Concomitant weekly cisplatin and radiotherapy for head and neck cancer. Jpn J ClinOncol 41(8):980–986
Rao S, Hegde SK, Rao P, Dinkar C, Thilakchand KR, George T, Baliga-Rao MP, Palatty PL, Baliga MS (2017) Honey mitigates radiation-induced oral mucositis in head and neck cancer patients without affecting the tumor response. Foods 6(9):77. https://doi.org/10.3390/foods6090077.PMID:28878156;PMCID:PMC5615289
Hegde SK, Rao S, Rao P, Raghu SV, Meera S, Baliga MS. Aqueous Extract of Emblica officinalis Linn (Indian gooseberry) in Combination with Iodine is More Efficacious than Iodine Alone in Mitigating Mucositis in Head and Neck Cancer Patients Undergoing Curative Radiotherapy: Retrospective Observations. Indian J Otolaryngol Head Neck Surg. 2022. https://doi.org/10.1007/s12070-021-03059-w
Sroussi HY, Epstein JB, Bensadoun RJ et al (2017) Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med 6(12):2918–2931
Maria OM, Eliopoulos N, Muanza T (2017) Radiation-induced oral mucositis. front. Oncol 7:89. https://doi.org/10.3389/fonc.2017.00089
Elting LS, Chang YC (2019) Costs of oral complications of cancer therapies: estimates and a blueprint for future study. JNCI Monographs. https://doi.org/10.1093/jncimonographs/lgz010
Jagetia GC, Baliga MS, Malagi KJ, Sethukumar KM (2002) The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to gamma-radiation. Phytomedicine 9(2):99–108
Jagetia GC, Malagi KJ, Baliga MS, Venkatesh P, Veruva RR (2004) Triphala, an Ayurvedic rasayana drug, protects mice against radiation-induced lethality by free-radical scavenging. J Altern Complement Med 10(6):971–978
Yoon WS, Kim CY, Yang DS et al (2012) Protective effect of Triphala on radiation induced acute intestinal mucosal damage in Sprague Dawley rats. Indian J Exp Biol 50(3):195–200
Sandhya T, Lathika KM, Pandey BN et al (2006) Protection against radiation oxidative damage in mice by Triphala. Mutat Res 609(1):17–25
Takauji Y, Miki K, Mita J et al (2016) Triphala, a formulation of traditional Ayurvedic medicine, shows protective effect against X-radiation in HeLa cells. J Biosci 41(4):569–575
Shi Y, Sahu RP, Srivastava SK (2008) Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer 10(8):294. https://doi.org/10.1186/1471-2407-8-294. (PMID:18847491;PMCID:PMC2576337)
Russell LH Jr, Mazzio E, Badisa RB, Zhu ZP, Agharahimi M, Millington DJ, Goodman CB (2011) Differential cytotoxicity of Triphala and its phenolic constituent gallic acid on human prostate cancer LNCap and normal cells. Anticancer Res 31(11):3739–3745 (PMID: 22110195; PMCID: PMC3328776)
Lu K, Chakroborty D, Sarkar C, Lu T, Xie Z, Liu Z, Basu S (2012) Triphala and its active constituent chebulinic acid are natural inhibitors of vascular endothelial growth factor-a mediated angiogenesis. PLoS ONE 7(8):e43934. https://doi.org/10.1371/journal.pone.0043934
Prakash S, Shelke AU (2014) Role of Triphala in dentistry. J Indian Soc Periodontol 18(2):132–135
Shanbhag VK (2015) Triphala in prevention of dental caries and as an antimicrobial in oral cavity- a review. Infect Disord Drug Targets 15(2):89–97
Safiaghdam H, Oveissi V, Bahramsoltani R, Farzaei MH, Rahimi R (2018) Medicinal plants for gingivitis: a review of clinical trials. Iran J Basic Med Sci 21(10):978–991
Prasad S, Srivastava SK (2020) Oxidative stress and cancer: chemopreventive and therapeutic role of Triphala. Antioxidants (Basel). 9(1):72. https://doi.org/10.3390/antiox9010072
Al Jameel AH, Almalki SA (2020) Effect of Triphalamouthrinse on plaque and gingival inflammation: a systematic review and meta-analysis of randomized controlled trials. Int J Dent Hyg. https://doi.org/10.1111/idh.12444.10.1111/idh.12444
Bajaj N, Tandon S (2011) The effect of Triphala and Chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth. Int J Ayurveda Res 2(1):29–36
Naiktari RS, Gaonkar P, Gurav AN, Khiste SV (2014) A randomized clinical trial to evaluate and compare the efficacy of Triphala mouthwash with 0.2% chlorhexidine in hospitalized patients with periodontal diseases. J Periodontal Implant Sci. 44(3):134–140
Mamgain P, Kandwal A, Mamgain RK (2017) Comparative evaluation of Triphala and ela decoction With 0.2% chlorhexidine as mouthwash in the treatment of plaque-induced gingivitis and halitosis: a randomized controlled clinical trial. J Evid Based Complementary Altern Med. 22(3):468–472
Baratakke SU, Raju R, Kadanakuppe S, Savanur NR, Gubbihal R, Kousalaya PS (2017) Efficacy of Triphala extract and chlorhexidine mouth rinse against plaque accumulation and gingival inflammation among female undergraduates: a randomized controlled trial. Indian J Dent Res 28(1):49–54
Divia AR, Nair MG, Varughese JM, Kurien S (2018) A comparative evaluation of Morindacitrifolia, green tea polyphenols, and Triphala with 5% sodium hypochlorite as an endodontic irrigant against Enterococcus faecalis: an in vitro study. Dent Res J (Isfahan). 15(2):117–122
Ramalingam K, Amaechi BT (2018) Antimicrobial effect of herbal extract of Acacia arabica with Triphala on the biofilm forming cariogenic microorganisms. J Ayurveda Integr Med. S0975-9476(17):30459–X
Naiktari RS, Dharmadhikari C, Gurav AN, Kakade S (2018) Determining the antibacterial substantivity of Triphala mouthwash and comparing it with 0.2% chlorhexidine gluconate after a single oral rinse: a crossover clinical trial. J Indian Soc Periodontol. 22(6):498–502
Mazzola R, Fiorentino A, Ricchetti F, Gregucci F, Corradini S, Alongi F (2018) An update on radiation therapy in head and neck cancers. Expert Rev Anticancer Ther 18(4):359–364
Phetkate P, Kummalue T, U-Pratya Y, Kietinun S (2012) Significant increase in cytotoxic T lymphocytes and natural killer cells by Triphala: a clinical phase I study. Evid Based Complement Alternat Med. https://doi.org/10.1155/2012/239856
Kumar MS, Kirubanandan S, Sripriya R, Sehgal PK (2008) Triphala promotes healing of infected full-thickness dermal wound. J Surg Res 144(1):94–101
Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG (2010) Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: an in vitro study. J Endod 36(1):83–86
Saxena D, Saha SG, Saha MK, Dubey S, Khatri M (2015) An in vitro evaluation of antimicrobial activity of five herbal extracts and comparison of their activity with 2.5% sodium hypochlorite against Enterococcus faecalis. Indian J Dent Res 26(5):524–527
Srinagesh J, Pushpanjali K (2011) Assessment of antibacterial efficacy of Triphala against mutans streptococci: a randomised control trial. Oral Health Prev Dent 9(4):387–393
Prabhakar J, Balagopal S, Priya MS, Selvi S, Senthilkumar M (2014) Evaluation of antimicrobial efficacy of Triphala (an Indian Ayurvedic herbal formulation) and 0.2% chlorhexidine against Streptococcus mutans biofilm formed on tooth substrate: an in vitro study. Indian J Dent Res 25(4):475–479
Padiyar B, Marwah N, Gupta S, Padiyar N (2018) Comparative evaluation of effects of Triphala, garlic extracts, and chlorhexidine mouthwashes on salivary Streptococcus mutans counts and oral hygiene status. Int J ClinPediatr Dent 11(4):299–306
Alizadeh E, Orlando TM, Sanche L (2015) Biomolecular damage induced by ionizing radiation: the direct and indirect effects of low-energy electrons on DNA. Annu Rev Phys Chem 66:379–398
Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mishra KP, Banavalikar MM, Mohan H (2005) In vitro antioxidant studies and free radical reactions of Triphala, an ayurvedic formulation and its constituents. Phytother Res 19(7):582–586
Nariya M, Shukla V, Jain S, Ravishankar B. Comparison of enteroprotective efficacy of triphala formulations (Indian Herbal Drug) on methotrexate-induced small intestinal damage in rats. Phytother Res. 2009;23(8):1092–8. https://doi.org/10.1002/ptr.2744. PMID:19170156
Sharma A, Sharma KK (2011) Chemoprotective role of Triphala against 1, 2-dimethylhydrazine dihydrochloride induced carcinogenic damage to mouse liver. Indian J ClinBiochem 26(3):290–295. https://doi.org/10.1007/s12291-011-0138-y
Baskaran UL, Martin SJ, Mahaboobkhan R, Prince SE (2015) Protective role of Triphala, an Indian traditional herbal formulation, against the nephrotoxic effects of bromobenzene in Wistar albino rats. J Integr Med 13(2):115–121
Deep G, Dhiman M, Rao AR, Kale RK (2005) Chemopreventive potential of Triphala (a composite Indian drug) on benzo(a)pyrene induced forestomach tumorigenesis in murine tumor model system. J Exp Clin Cancer Res 24(4):555–563
Srikumar R, Parthasarathy NJ, Manikandan S, Narayanan GS, Sheeladevi R (2006) Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem 283(1–2):67–74
Dhanalakshmi S, Devi RS, Srikumar R, Manikandan S, Thangaraj R (2007) Protective effect of Triphala on cold stress-induced behavioral and biochemical abnormalities in rats. Yakugaku Zasshi 127(11):1863–1867
Sabina EP, Rasool M (2008) An in vivo and in vitro potential of Indian ayurvedic herbal formulation Triphala on experimental gouty arthritis in mice. Vascul Pharmacol 48(1):14–20
Kalaiselvan S, Rasool M (2015) Triphala exhibits anti-arthritic effect by ameliorating bone and cartilage degradation in adjuvant-induced arthritic rats. Immunol Invest 44(4):411–426
Ingale DR, Kulkarni PG, Koppikar SJ, Harsulkar AM, Moghe AS, Jagtap SD (2018) Reduced synovial inflammation and inhibition of matrix metalloproteinases explicates anti-osteoarthritis activity of polyherbal formulations. Indian J Pharmacol 50(1):22–29
Sprung CN, Ivashkevich A, Forrester HB, Redon CE, Georgakilas A, Martin OA (2015) Oxidative DNA damage caused by inflammation may link to stress-induced non-targeted effects. Cancer Lett 356(1):72–81
Khodamoradi E, Hoseini-Ghahfarokhi M, Amini P et al (2020) Targets for protection and mitigation of radiation injury. Cell Mol Life Sci 77(16):3129–3159
Penmetsa GS, B V, Bhupathi AP, Rani P S, B V S, M V R (2019) Comparative evaluation of Triphala, aloe vera, and chlorhexidine mouthwash on gingivitis: a randomized controlled clinical trial. ContempClin Dent 10(2):333–337
Sireeratawong S, Jaijoy K, Soonthornchareonnon N (2012) Evaluation of anti-inflammatory and antinociceptive activity of Triphala recipe. Afr J Tradit Complement Altern Med 10(2):246–250
Belapurkar P, Goyal P, Tiwari-Barua P (2014) Immunomodulatory effects of Triphala and its individual constituents: a review. Indian J Pharm Sci 76(6):467–475
Acknowledgements
The authors grateful to Dr LK Vaishnav, the Ayurvedic physician for initiating of combining Ayurveda with conventional care.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose with respect to the research, authorship, and/or publication of this article.
Consent for Publication
All authors consented to the submission of the article for publication.
Ethical Approval
This was a retrospective study ad was conducted after obtaining clearance from Institutional Ethics Committee (MIO/IEC/2019/01/07).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rao, S., Kini, V., Hegde, S.K. et al. Ayurvedic Drug Triphala in Combination with Providone Iodine Mitigates Radiation-Induced Mucositis in Head and Neck Cancer Patients without Affecting the Tumor Response. Indian J Otolaryngol Head Neck Surg 75, 1480–1489 (2023). https://doi.org/10.1007/s12070-023-03516-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-03516-8